Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
Abstract
The structure and transition behavior of crosslinked thermo-responsive poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) (P(MEO2MA-co-EGMA360)) gel film on a flat cellulosic-based substrate were investigated. The regenerated cellulose (RC) film was prepared by spin-coating with trimethylsilyl cellulose (TMSC), followed by etching with hydrochloric acid vapor on a treated silicon wafer, then crosslinked polymer gel film was obtained by spin-coating, drying, and baking with a pre-crosslinked solution containing polymers. Fourier transform infrared spectroscopy, X-ray photoelectron spectrometer, and atomic force microscopy results show that a RC film with a thickness of 25 nm is generated in the upper layer of TMSC film on the silicon wafer. The cross-linking induces closer arrangement and hinders the extension of chain segments, leading to less prominent phase transition behaviors of polymer gel films. By quartz crystal microbalance measurement and 3D microscopes, a phase transition hysteresis is discovered, the hydrated and loose structure of crosslinked polymer gel film switches to dehydrated and compact structure in initial heating process, which subsequently recovers during the following cooling process. However, the degrees of rehydration and flexibility of film could not reach the initial value because of the insufficient transition time and steric hindrance caused by crosslinking.
1 Introduction
In recent decades, a class of nonlinear polyethylene glycol (PEG) with unique properties has gained considerable attention (1,2,3,4). Different from common linear PEG, their side chain contains a poly(oligo(ethylene glycol) (POE) structure that exhibits a similar phase transition mechanism to common thermo-responsive polymer poly(N-isopropylacrylamide) (PNIPAM) (5,6,7,8). As a combination of main chain, polyacrylates, and side chain, POE, POE acrylates possess significant advantages over PNIPAM in terms of flexibility and adjustable transition temperature (TT). For flexibility, their glass TTs (T g, –60°C to 90°C) are significantly lower than PNIPAM (∼32°C) (6). For instance, POE acrylate containing two ethoxy groups have a T g around −40°C (9), it can be expected that the flexibility of substrate materials will not be negatively affected after combining with it. For TT, they can be adjusted in a large range by applying POE side chains with various lengths.
POE acrylates are amphiphilic polymers, their main chains are hydrophobic C–C skeleton structure, and the side chains contain hydrophilic C–C–O groups. When the temperature is lower than TT, the ethoxy groups bind to the surrounding water molecules with intermolecular hydrogen bonds, which is dominated by hydrophilic groups, so the chain segments present an extended coil structure and dissolve in water, resulting in a homogeneous phase. While above TT, the hydrogen bonds between ethoxy groups and water molecule are broken, the hydrophobic groups release the water molecules, and the chain segments shrink, aggregate, and precipitate out of the solutions, resulting in phase separation. Considering the sensitive, drastic, and reversible phase transition behaviors of thermo-responsive POE acrylates, various fabrication processes have been utilized to construct thermo-responsive materials with complex topological structures, such as polymer gels, polymer brushes, surface micelles, nanoparticles, metal organic framework materials, and so on, which have enriched their phase transition forms for application in related fields (10,11,12,13). With the stimulation of temperature around TT, the above structures will undergo sol/gel, hydrophilic/hydrophobic, swelling/collapse, chain segments stretching/aggregation, etc. So they can be applied to cell separation/therapy (14,15,16,17), drug release (18), thermochromic discoloration (10), membrane separation (19), intelligent cleaning (20,21), microclimate regulation (22), and other systems (23–25).
In our previous work (26), POE acrylates have been combined with textiles with a cross-linking method for smart textiles, achieving the microclimate regulation of textiles. However, given the application of polymer, compared with well-studied silicon-based polymer films (27–30), the effects of textile as substrate on structure and transition behavior of polymer gel films remain unknown. Besides, the properties of crosslinked thermo-responsive polymer gel films are rarely reported in the literature. Due to the interwoven structure of the yarns, the microscopic surface of textiles possess massive micropores and exhibit high unevenness, which hinders the observation and investigation of the micro-structure of polymer films.
Based on discussions above, we first synthesized trimethylsilyl cellulose (TMSC) and studied its structure and solubility. Then, it was dissolved in an organic solvent with low boiling point and was spin-coated onto silicon wafers. With the treatment of concentrated hydrochloric acid, homogeneous regenerated cellulose (RC) films on silicon wafer were obtained. Then, pre-crosslinked solutions containing thermo-responsive polymer were spin-coated on the cellulosic-based substrate, the crosslinked polymer gel films were fixed onto the RC films after the treatment of drying and baking, which is expected to simulate the binding state of the polymer and textiles, and structure and transition behavior of polymers were conveniently investigated. To understand the application principles and methods of composite cellulosic textiles based on thermo-responsive polymers, the changes in crosslinked film with respect to morphology, structure, and thicknesses induced by temperature were further investigated.
2 Materials and methods
2.1 Materials
The random thermo-responsive copolymer P(MEO2MA-co-EGMA360) (M n = 28,700) was prepared by ATRP polymerization as reported previously (20). Silicon wafer (single side polished, orientation index 111) was purchased from Kaihua Lijing Electronic Co., Ltd. microcrystalline cellulose (MCC, M n = 36,000), N,N-dimethylacetamide (DMA, purity 99%), lithium chloride (LiCl, purity 99.9%), hexamethyl disilazane (HMDS, purity 98%) and trimethylchlorosilane (TMSCl, purity 99%) were bought from Aladdin, and used as received. Hydrofluoric acid (HF, 5% aq) and concentrated hydrochloric acid (HCl, 36–38% v/v) were from Huadong Medicine Co., Ltd.
2.2 Hydrophobic treatment of silicon wafer surface
Generally, the surface of fresh silicon wafer is easy to be oxidized in air and forms a layer of hydrophilic silicon dioxide (SiO2) film, resulting in a hydrophilic surface. If the hydroxyl of cellulose is replaced by trimethylsilyl, the hydrophilic SiO2 film will greatly reduce the binding fastness of TMSC film on silicon wafer. In order to improve the binding fastness of TMSC on silicon wafer, hydrophobic treatment is needed. Herein, HF is applied to etch the SiO2 film on the surface of silicon wafer, recovering initial hydrophobic state of silicon wafer. The reaction mechanism is illustrated in Figure 1, the contact angle (CA) of the initial silicon wafer is 26°, which is in a hydrophilic state. After etching, the CA rises to 98° and changes into hydrophobic state. The specific preparation process is presented as follows:

Schematic illustration of the preparation of cellulosic substrate.
The precut Si wafers with a size of 2 × 2 cm2 were successively rinsed by acetone and ethanol to remove the organic and water-soluble impurities on their surface. Then, the primary treated Si wafers were placed on a teflon platform with several grooves and warily immersed in 5% HF solution to react at room temperature for 1 min, followed by an immediate rinse with deionized water. The Si wafers were blow-dried with nitrogen and stored in a vacuum drying oven.
2.3 Preparation of TMSC
Typical preparation of TMSC with a substitution degree of 2.21 is as follows: MCC (0.4 g, 2.21 mmol) and 15 mL of DMA were mixed in a reaction bottle and stirred at 150°C for 1 h, a cellulose suspension was obtained. Then, 1.2 g LiCl were added to the former suspension. The mixture became transparent during cooling to room temperature under a constant stirring. HMDS (1.29 mL, 6.18 mmol) and TMSCl (32 μL, 0.25 mmol) were added to the above solution. After stirring at 80°C for 1 h and cooling to room temperature, white flocs were isolated with the addition of deionized water. The products were filtrated and dried to constant weight.
2.4 Immobilization of RC film on silicon wafer
TMSC 10% (w/v) chloroform solution was dissolved and filtered by a syringe-driven filter, which was spin-coated on a silicon wafer with a spin coater (KW-4A, SETCAS, China) at a speed of 1,000 rpm for 30 min. Then, the silicon wafer containing TMSC film was embedded in a teflon platform carved with several grooves and placed in a hydrochloric acid solution (36–38%). The TMSC film was etched by the HCl vapor when the solution was heated up to 60°C for 20 s. After immersing in deionized water and drying, a RC film was obtained on silicon wafer.
2.5 Preparation of crosslinked films
A typical pre-crosslinked solution including 8% polymer, 2% citric acid, 3% sodium hypophosphite, and 87% deionized water was prepared. Then, 2 mL solution was spin-coated on the silicon wafer loaded with a RC film. After pre-drying at 80°C for 30 min and baking at 130°C for 2 min, the wafer was rinsed with deionized water and dried at 70°C for 1 h, and stored in a vacuum chamber.
2.6 Spectroscopies
The functional groups of MCC and TMSC were characterized by Fourier transform infrared (FTIR) spectroscopy (Nicolet 5700, Thermo Fisher Scientific, USA). The scanned wavelength ranged from 4,000 to 600 cm−1 with a resolution of 4 cm−1.
The surface elemental compositions of the films were analyzed by X-ray photoelectron spectrometer (XPS, K-Alpha, Thermo Fisher Scientific, USA). The Al-Kα X-ray (1486.6 eV, width 0.85 eV, scope 100 μm2) was selected as the photoexcitation source. The binding energy of C 1s = 285 eV was used as a reference (31).
2.7 Microscopes
The surface morphology of thin films was probed by noncontact atomic force microscopy (AFM, NC-AFM, XE-100E, PSIA, Korea). The measurement was carried out with a scan rate of 0.5 Hz and set point of −0.65 µm. The scan size is 2 × 2 µm2.
The surface morphologies of the films were observed by an upright metallurgical microscope (DM2700 M, Leica, Germany). The transition behaviors of crosslinked films were measured after placing in specified atmospheric environment (30°C and 40°C) for 2 h.
2.8 White light interferometry
The thickness of film was determined by a white light interferometer (Filmetrics F20, Filmetrics Inc., USA) equipped with a thermo scientific HAAKE bath (Julabo F12, Julabo, Germany). The refractive indexes were set as n(TMSC) = 1.404 and n(RC) = 1.505, as previously reported (31–33). For probing the thicknesses of TMSC and RC films, the thickness of upper layer (RC) was first set to a fixed value, then that of lower layer (TMSC) was fitted until reaching a R-Square above 0.95.
2.9 CA measurement
CA of water droplet on the film was measured by a video tension CA tester (DSA20, Kruss, Germany). The measurements were carried out by dropping 2 μL water on the film, followed by calculating the CAs based on shape of the water droplets. The mean value was calculated by selecting five points for each sample in order to minimize errors.
2.10 Quartz crystal microbalance measurement
The dissipation and frequency factors of crosslinked films were probed by a quartz crystal microbalance (AWS A20 RP, AWsensors, Spain) equipped with a dissipation monitoring (QCM-D) (34–38). The cross-linked film was generated on the quartz oscillator by spin-coating. According to Sauerbrey equation (34),
where ρ and l represent the specific density and thickness of the quartz crystal, respectively, n = 1, 3, 5, …. The dissipation factor is defined by Eq. 2.
where E d and E s are the dissipated energy and stored energy during one oscillation. Dissipation change (Δf) and frequency change (ΔD) are obtained by switching the driving voltage on and off periodically.
3 Results
3.1 Preparation of TMSC
Figure 2 shows the Fourier transform infrared spectra of MCC and TMSC. As the characteristic peaks of cellulose, ν(–OH) (3,780–3,030 cm−1), ν(C–H) (3,000–2,800), and ν(C–O–C) appear in both spectrograms. However, by integrating the peak area, the –OH peak area of TMSC is significantly smaller than that of MCC, indicating that –H of TMSC was replaced by –Si(CH3)3. Meanwhile, a shoulder peak at 2,900 cm−1 arises in the spectrogram of TMSC, attributing to the deformation vibration of Si–C–H. In addition, three sharp peaks caused by δ(Si–C) were found at 1,250, 870, and 752 cm−1. By contrastive analysis, it can be concluded that partial –H of MCC were substituted by –Si(CH3)3, resulting in the enrichment of hydrophobic alkyl groups on six-membered pyran ring of cellulose, which benefits the dissolution of TMSC in some organic solvents.

FTIR spectra ranging from 4,000 to 600 cm−1 of MCC and TMSC. The curves are shifted vertically for clarity.
Since the solubility of polymer has a great influence on the homogeneity of the film, the degree of substitution (DS) and solvent of TMSC were selected and optimized to obtain a uniform TMSC solution. Table 1 shows the solubility of TMSC prepared with various feeding ratios and dissolved in two commonly used organic solvents with different polarities. The DS of TMSC is only 0.58 when the feeding ratio of HMDS is 1. By increasing the feeding ratio of HMDS to 1.5, the DS of TMSC rises to 1.29. Further increasing the feeding ratio, it can be found that the improvement rate of the DS of TMSC becomes less prominent. The reason may be that the increasing substitution of –Si(CH3)3 induces the steric hindrance effects between d-glucoses of cellulose and reduces the reactivity of nucleophilic substitution (Si(CH3)3 → H). Hence, the molar ratio of MCC:HMDS:TMSCl is determined as 1:2.5:0.1 in subsequent experiments, and the substitution degree of the synthesized product TMSC is 2.21.
Solubility of TMSC depending on DS and solvent
| Reaction conditions | Product | ||
|---|---|---|---|
| Molar ratio MCC/HMDS/TMSCl | DSa | Solubilityb | |
| THF | CHCl3 | ||
| 1:1:0.1 | 0.58 | + + | − |
| 1:1.5:0.1 | 1.29 | + + | + |
| 1:2:0.1 | 1.84 | + | + + |
| 1:2.5:0.1 | 2.21 | − | + + |
| 1:3:0.1 | 2.34 | − | − |
aDegree of substitution determined gravimetrically; b+ + easily soluble, + soluble, − insoluble. The concentration of polymer dissolved in THF or CHCl3 is 1 mg/mL.
On the contrary, due to the substitution of –Si(CH3)3 for –H, the increase in DS will inevitably lead to enhanced hydrophobicity of TMSC. TMSC with DS of 0.58 and 1.29 are both dissolved easily in tetrahydrofuran (THF). With the increased DS, the solubility of TMSC in THF reduces. When DS reaches 2.21, TMSC will only swell in THF. Conversely, TMSC with low DS (0.58) has poor solubility, and the higher one will dissolve in CHCl3, attributing to the weak polarity of CHCl3. Table 1 presents that TMSC with DS of 2.21 dissolve easily in CHCl3. In addition, the weak polarity of CHCl3 endows lower surface tension of solution, promoting the spread ability of the TMSC solution on the hydrophobic silicon substrate, which was conducive to the homogeneity of the spin-coated film.
In conclusion, through the optimization and analysis of reaction conditions and solvents, the TMSC chloroform solution with DS = 2.21 was selected for spin-coating to prepare homogeneous TMSC films.
3.2 Characterization of RC film
The chemical composition of TMSC undergoes significant changes after treating with HCl vapor. XPS analysis can be employed to acquire surface composition and content information, enabling the inference of alterations in chemical structure and verification of the reaction process between TMSC and RC.
Figure 3 illustrates the wind-scan spectra of XPS for TMSC (180 nm) and RC (25 nm) films on silicon wafers. According to the binding energy of TMSC group, the peaks located at 533, 285, 153, and 102 eV are assigned to O 1s, C 1s, and Si 2s and Si 2p, respectively. By comparison, the corresponding binding energies of Si 2s and Si 2p for RC spectra notably drop, indicating that –Si(CH3)3 groups are removed after the acid etching. Besides, the relative higher intensity binding energy of O 1s for RC reveals the increased content of oxygen elements, attributing to the substitution of –OH for –OSi(CH3)3.

XPS patterns of wide-scan of TMSC and RC film on the silicon wafer.
To further understand the quantitative changes of functional groups in the substitution reaction, C 1s detail spectra of TMSC and RC were analyzed. Figure 4 shows the chemical state changes of C in TMSC and RC. The C 1s spectra could be split into three curve-fitted peaks at binding energies of 284.7, 287, and 288.6 eV, which are successively ascribed to –Si(CH3)3, –C–O–, and –O–C–O–. As mentioned previously, the substitution degree of TMSC is 2.21. As each –Si(CH3)3 contains three C atoms, the contents of C in a structural unit for TMSC can be identified as 2.21 × 3. On the other hand, the number of –C–O– and –O–C–O– on each original cellulose structural units is widely known as 6. Thus, the ratio of C contents ((–C–O– + –O–C–O–):–Si(CH3)3) = 6:(2.21 × 3) ≈ 9:10, which is in qualitative agreement with the areas of peaks for C 1s spectra. The details of area values can be found in Table S1. Additionally, it is found that the intensity of –Si(CH3)3 for RC decreases significantly, but does not disappear yet, indicating –Si(CH3)3 traces on the surface of RC, which is negligible in the following experiments.

High-resolution XPS spectra of C 1s for (a) TMSC and (b) RC film on silicon wafer. The fitted peaks were conducted with Origin.
AFM was employed to observe the surface characteristics of the films. Figure 5 presents the surface morphologies including 2D, 3D morphologies and cross sections views of TMSC (180 nm) and RC (25 nm) films. The spinning coating process for the TMSC film used in this experiment, which is relatively large with a thickness of 180 nm, may result in hole formation due to solvent evaporation. The depth of micropores on TMSC film is 4.8 nm by measuring the longitudinal depth (Figure 5e), and the roughness characterized by R q is 2.01 nm. By contrast, the depth of the micropores for RC film increases to 12.2 nm, and the corresponding R q = 3.59 nm. It is worth noting that the number of micropores per area on the surface of RC film increases significantly. This is due to the treatment with concentrated HCl vapor, acid etch occurs on the film surface. Therefore, compared with TMSC, the quantities and depths of micropores on RC film are obviously enhanced, which further confirms the substitution reaction of –H for –Si(CH3)3.

(a) 2D and (c) 3D AFM images of TMSC film, (b) 2D and (d) 3D AFM images of RC film with area of 2 × 2 μm2, and (e) curves plotted with blue lines corresponding to cross-sectional data of films.
In order to investigate the influence of solution concentrations on film thickness, different concentrations of TMSC solutions were first prepared. Considering that the subsequent treatment of acid etching will remarkedly reduce the thickness of films, the solution concentrations of TMSC are controlled at 10–50 mg/mL to ensure that the film thickness is more than 100 nm. As shown in Figure 6, the thickness of TMSC film is 181 nm when the concentration is 10 mg/mL. By increasing the solution concentrations, the film thickness increases. However, the thickness of film increases faster than that of common polymer films that have a linear relation with solution concentrations (39–41). The reason is that chloroform selected as the solvent has a higher density. With the increase in concentration, the viscosity of the solution system increases exponentially, hindering the polymer separating from solvent in spin-coating. Therefore, the concentration of TMSC solution increases to five times (50 mg/mL), while the film thickness eventually reached more than ten times (2.2 μm).
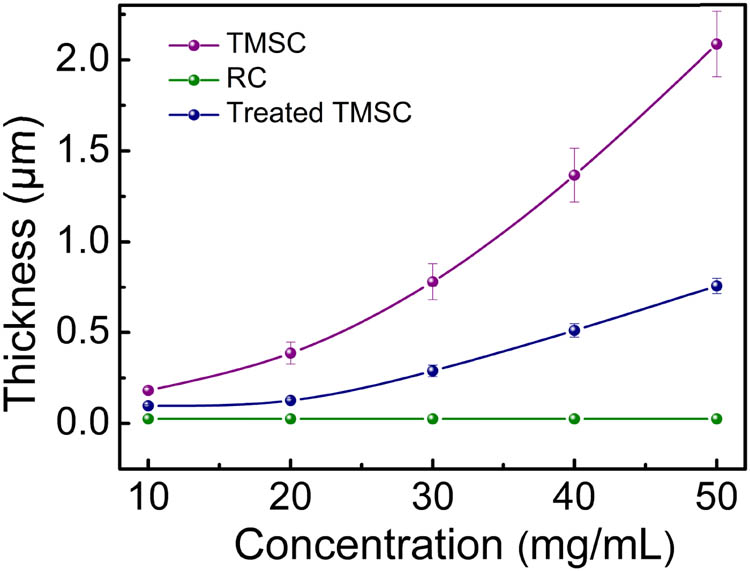
The thickness of TMSC, RC, and treated TMSC film as a function of concentrations.
Since the substitution reaction only takes place on the surface, the double-layer film including upper RC film and lower TMSC film model is proposed to measure the film thickness. Considering that TMSC films with varied thicknesses have undergone the same treatment, the thicknesses of RC films on their upper layer are assumed to be equal, which is confirmed by the acceptable R-square of thickness for corresponding TMSC (Table S2). By multiple fitting, the thicknesses of RC films are determined to be 25 nm. As expected, the thickness of TMSC film drops in half. This is because that HCl vapor not only induces the substitution reaction of –H for –Si(CH3)3 on upper layer of TMSC film, but also carbonizes the structure of TMSC film that is removed by the following rinsing treatment, resulting in the sharply reduced thickness of TMSC film.
3.3 Transition behaviors of crosslinked polymer gel film
CA is widely perceived as one of the commonly used methods to characterize the hydrophilicity-hydrophobicity of material surface. The changes in water CA of crosslinked films with different percentage concentrations in the range of 25–45°C were measured, and non-crosslinked RC film was applied as a reference sample. The results are shown in Figure 7. The CA of RC film decrease from the initial value of 35° to the final value 32° when the temperature rises from 25°C to 45°C, presenting a slight downward trend. This is due to the fact that with the increase in the temperature, the evaporation rate of water increases, and the water droplets are more likely to spread on the surface of the film, exhibiting a decrease in CA. In contrast, the CAs of 2% crosslinked polymer gel film are 48° and 45° at 25°C and 30°C, respectively, indicating that the surface of the fabric is in a hydrophilic state. However, when the temperature continues rising to 35°C, the CA increases from 45° to 62°, showing an upward trend. After that, the CA changes little within the testing temperature range. This phenomenon indicates that the surface properties of the film have changed between 30°C and 35°C, and the hydrophobicity is enhanced. We can include that the crosslinked polymer gel film has undergone phase transition behaviors between 30°C and 35°C. In addition, it can be found that with the increase in the polymer concentration, the crosslinked films exhibit more prominent phase transition from hydrophilicity to hydrophobicity. In particular, when the polymer concentration reaches 6%, the CA of the film increases from 49° (hydrophilic) to 102° (hydrophobic) after phase transition. Further increasing the concentration to 8%, the CAs basically remain unchanged, revealing that 6% crosslinked P(MEO2MA-co-EGMA360) is sufficient for the phase transition of gel films.

(a) The CAs of RC film and P(MEO2MA-co-EGMA360) crosslinked gel films as a function of temperature. (b) The CAs of 6% P(MEO2MA-co-EGMA360) crosslinked gel films in five temperature switch cycles. The applied temperatures are 30°C (blue point) and 40°C (red point).
In addition, the CAs of cross-linked thermo-responsive polymer gel films under cycles of swelling and transition were measured within five temperature switch cycles (Figure 7b). The 6% crosslinked polymer film presents a prominent change in CAs in the switching of the temperature (30–40°C). This transition behavior remains unchanged after five cycles, which demonstrates the perdurability of reversible phase transition of crosslinked P(MEO2MA-co-EGMA360) gel films.
Thermo-responsive polymers possess the properties that they absorb water molecules to swell below TT, and repel water molecules to collapse above TT. Hence, the phase transition behaviors of crosslinked polymer gel films can be characterized by the changes in film thickness. As seen in Figure 8a, the films rapidly absorb water and expand within the first 10 min, then the thicknesses rise slowly and finally reach equilibrium within 2 h. For swelling process, saturated NaCl solution is added to the testing chamber, which maintains the rapidly increasing humidity around films. So hydrophilic groups of the polymer chain combine with water vapor in the chamber to form hydrogen bonds. Simultaneously, due to the good hydrophilicity of the cellulosic substrate, the initial moisture absorbed on top of the film continues to penetrate into the interior of films, resulting in the rapid expansion of the film. After a period of time, the relative humidity in the chamber increases slowly until it is saturated (30,39), and the film thickness correspondingly shows a slight increasing trend. After 2 h, the film thickness stays unchanged due to the achievement of maximal absorption. It is shown that the expansion rate of the film has a certain synchronization with the ambient humidity, which is similar to the film expansion process mentioned in the literature (29,40,41). In addition, it is found that maximum expansion rates of films decrease with the increase in the film thickness. This is because the thicker films weaken the hydrophilic force between cellulosic-based substrate and free water molecules on top of film, leading to less water absorption and smaller swelling ratio.

The (a) swelling (25°C, below TT) and (b) collapse (transition behaviors) of P(MEO2MA-co-EGMA360) crosslinked polymer gel films.
For collapse process, the thickness changes in films can be divided into three stages. In the first stage, for 25°C < T < 31°C, the thicknesses of films decrease slightly, attributing to the evaporation of free water molecules in films induced by the increase in the temperature. The second stage for 31°C < T< 39°C is the phase transition region of the film. The hydrogen bonds break and the hydrophobic force dominates the process. Meanwhile, the polymer gel films repel water molecules and collapse, resulting in a significantly reduced film thickness. In the third stage of 39°C< T <45°C, the film thickness remained almost constant with the increase in the temperature.
Compared with the reports in the literature (40), the crosslinked polymer gel films possess much broader phase transition region comparing with polymer films. This is because the cross-linking hinders the movement of chain segments, resulting in limited hydration and a smaller increase in film thickness. Besides, in the process of phase transition, the “zipper effect” (42,43) is also hindered by the existence of cross-linking points, leading to residual water molecule in crosslinked polymer gel films and a less prominent phase transition behaviors.
Differing from polymer film on silicon wafer, the crosslinked polymer is directly fixed on RC film by covalent bonds instead of hydrogen bonds or van der Waals force, which worsens the difficulty of movement and rearrangement of polymer chains. Hence, this strong chemical bond will unavoidably limit the phase transition behavior of crosslinked polymer. Nevertheless, on the other hand, it is benefit for the long-term application of thermo-responsive polymer in textiles.
3.4 Structure of crosslinked film
Because the polymer was fixed onto the cellulosic-based substrate with a cross-linking strategy in application of smart textiles, similarly, the frequency and dissipation factors of the polymer gel film crosslinked to the surface of the oscillator were measured by QCM-D in one heating and cooling cycle. In previous studies (37,38), Δf is mainly caused by the hydration and dehydration, whereas ΔD is due to the viscoelastic and conformational change. In Figure 9a, −Δf decreases with the increase in the temperature during heating process. Below TT, thermo-responsive polymer gel film exhibits good hydrophilicity, and strongly interact with surrounding water. Whereas above TT, the polymer chain collapses accompanied by dehydration, leading to the decrease in −Δf. Inversely, −Δf increases with the cooling process, revealing the rehydration of the polymer gel film. ΔD of polymer gel film as a function of temperature was measured (Figure 9b). Similarly, ΔD decreases with the increase in the temperature, showing that the polymer gel film collapses to a dense and rigid structure when heated above TT. During the cooling process, ΔD gradually increases, revealing that the collapsed film swells into a loose and flexible structure (Figure 9b) (44–47).

Temperature dependence of the shifts in (a) frequency (Δf) and (b) dissipation (ΔD) of P(MEO2MA-co-EGMA360) crosslinked films at n = 3; The optical images of crosslinked gel films at (c) 30°C and (d) 40°C in ambient conditions.
It should be noted that neither −Δf nor ΔD of the polymer gel film can return to the initial value after the heating and cooling process, this result differs from grafting thermo-responsive polymer (48). Usually, the crosslinked polymer gel film keeps a swelling equilibrium state in initial experiment, and relatively easily repels water and collapse in the heating process, whereas in cooling process, the time is not sufficient for chains of polymer absorbing water and stretching in the time scale of the experiment. Actually, it took about 48 h or more for polymer gel film to accomplish the swelling in the atmosphere. Besides, the crosslinked structure with steric hindrance will further limit the structure transformation. Figure 9c and d illustrates the surface morphology of polymer gel films at 30°C and 40°C. The gel film presents a relatively homogeneous state at 30°C (below TT). However, abundant micropores are observable on the film surface at 40°C (above TT). When increasing the temperature to 40°C, the polymer chains undergo a conformational change and the water molecules are repelled out of the film, leading to the formation of micropores. This further confirms the phase transition of crosslinked polymer gel film around TT. By the way, the continuity of film is not destroyed in phase transition process, showing a good stability of crosslinked thermo-responsive polymer gel film. Based on the discussions above, the application principles for smart textiles mentioned earlier can be explained. For textile crosslinked with thermo-responsive polymers, the space between the fibers can be enlarged or narrowed under the temperature stimulation, hence, the function of micro-climate control of smart textile is finally realized.
4 Conclusion
With the treatment of concentrated HCl, a homogeneous RC film with a thickness of 25 nm was effectively fabricated on silicon wafer. The crosslinked thermo-responsive copolymer P(MEO2MA-co-EGMA360) films were generated on silicon wafers by spin-coated pre-crosslinked solutions that are subsequently dried and baked. The crosslinked polymer gel films were found to undergo phase transition behaviors between 30°C and 35°C by CA measurements. With the increase in the polymer concentrations, the crosslinked films exhibited more prominent phase transition of hydrophilicity to hydrophobicity. Because the cross-linking limits the movement of chain segments, comparing with non-crosslinked polymer films, crosslinked polymer gel films present much broader phase transition region and less prominent phase transition behaviors. The hydrated and loose structure will switch to dehydrated compact structure in a heating process, and will recover in a cooling process. However, the degrees of rehydration and flexibility of film could not reach the initial value because of the insufficient transition time and steric hindrance induced by crosslinking. Finally, the micro-climate control of smart textile has been successfully demonstrated.
-
Funding information: The authors are grateful to the financial support provided by Fujian External Cooperation project of Natural Science Foundation (No. 2022I0042).
-
Author contributions: Yangyi Chen: writing – original draft, writing – review and editing, methodology, and formal analysis; Tong Su, Shihang Zhou, and Chendi Xie: formal analysis and visualization; Huan Qi: writing – review and editing, methodology, and formal analysis; Zaisheng Cai and Liqun Chen: methodology and formal analysis. All authors have given approval to the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
References
(1) Han S, Hagiwara M, Ishizone T. Synthesis of thermally sensitive water-soluble polymethacrylates by living anionic polymerizations of oligo(ethylene glycol) methyl ether methacrylates. Macromolecules. 2003;36(22):8312–9. 10.1021/ma0347971.Search in Google Scholar
(2) Lutz JF, Weichenhan K, Akdemir Ö, Hoth A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules. 2007;40(7):2503–8. 10.1021/ma062925q.Search in Google Scholar
(3) Lutz JF. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J Polym Sci Part A: Polym Chem. 2008;46(11):3459–70. 10.1002/pola.22706.Search in Google Scholar
(4) Hu Z, Cai T, Chi C. Thermoresponsive oligo(ethylene glycol)-methacrylate-based polymers and microgels. Soft Matter. 2010;6(10):2115. 10.1039/B921150K.Search in Google Scholar
(5) Haq MA, Su Y, Wang D. Mechanical properties of PNIPAM based hydrogels: A review. Mater Sci Eng C. 2017;70:842–55. 10.1016/j.msec.2016.09.081.Search in Google Scholar
(6) Wang Z, Wu J, Zhao P, Dai N, Zhai Z, Ai T. Improving cracking resistance of cement mortar by thermo-sensitive poly n-isopropyl acrylamide (PNIPAM) gels. J Clean Prod. 2018;176:1292–303. 10.1016/j.jclepro.2017.11.242.Search in Google Scholar
(7) Khang MK, Zhou J, Huang Y, Hakamivala A, Tang L. Preparation of a novel injectable in situ-gelling nanoparticle with applications in controlled protein release and cancer cell entrapment. RSC Adv. 2018;8(60):34625–33. 10.1039/C8RA06589F.Search in Google Scholar
(8) Cao M, Wang Y, Hu X, Gong H, Li R, Cox H, et al. Reversible thermoresponsive peptide–PNIPAM hydrogels for controlled drug delivery. Biomacromolecules. 2019;20(9):3601–10. 10.1021/acs.biomac.9b01009.Search in Google Scholar
(9) Aseyev V, Tenhu H, Winnik FM. Non-ionic thermoresponsive polymers in water. Berlin: Springer Berlin Heidelberg; 2011. p. 29–89.Search in Google Scholar
(10) Cai T, Wang G, Thompson S, Marquez M, Hu Z. Photonic hydrogels with poly (ethylene glycol) derivative colloidal spheres as building blocks. Macromolecules. 2008;41(24):9508–12. 10.1021/ma802035d.Search in Google Scholar
(11) Wellert S, Kesal D, Schön S, Von Klitzing R, Gawlitza K. Ethylene glycol-based microgels at solid surfaces: Swelling behavior and control of particle number density. Langmuir. 2015;31(7):2202–10. 10.1021/la504556m.Search in Google Scholar
(12) Zou XN, Han X, Zhang Q, Yin J-J, Chu L-Q. Preparation and antibacterial activity of silver-loaded poly (oligo (ethylene glycol) methacrylate) brush. J Biomater Sci Polym Ed. 2019;30(9):756–68. 10.1080/09205063.2019.1603066.Search in Google Scholar
(13) Ly J, Li Y, Vu MN, Moffat BA, Jack KS, Quinn JF, et al. Nano-assemblies of cationic mPEG brush block copolymers with gadolinium polyoxotungstate [Gd(W5O18)2]9− form stable, high relaxivity MRI contrast agents. Nanoscale. 2018;10(15):7270–80. 10.1039/C8NR01544A.Search in Google Scholar
(14) Ooya T, Lee J, Park K. Effects of ethylene glycol-based graft, star-shaped, and dendritic polymers on solubilization and controlled release of paclitaxel. J Controlled Rel. 2003;93(2):121–7. 10.1016/j.jconrel.2003.07.001.Search in Google Scholar
(15) Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly (ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjugate Chem. 2000;11(6):910–7. 10.1021/bc0000583.Search in Google Scholar
(16) Najafi F, Sarbolouki MN. Biodegradable micelles/polymersomes from fumaric/sebacic acids and poly (ethylene glycol). Biomaterials. 2003;24(7):1175–82. 10.1016/S0142-9612(02)00487-8.Search in Google Scholar
(17) Gao M, Yang Y, Bergfel A, Huang L, Zheng L, Bowden TM. Self-assembly of cholesterol end-capped polymer micelles for controlled drug delivery. J Nanobiotechnology. 2020;18(1):1–10. 10.1186/s12951-020-0575-y.Search in Google Scholar
(18) Liu C, Li Y, Li Y, Duan Q. Preparation of a star-shaped copolymer with porphyrin core and four PNIPAM-b-POEGMA arms for photodynamic therapy. Mater Sci Eng C. 2019;98:74–82. 10.1016/j.msec.2018.12.121.Search in Google Scholar
(19) Ye Y, Huang J, Wang X. Fabrication of a self-cleaning surface via the thermosensitive copolymer brush of P(NIPAAm-PEGMA). ACS Appl Mater Interfaces. 2015;7(40):22128–36. 10.1021/acsami.5b07336.Search in Google Scholar
(20) Zhong Q, Chen Y, Guan S, Fang Q, Chen T, Müller-Buschbaum P, et al. Smart cleaning cotton fabrics cross-linked with thermo-responsive and flexible poly (2-(2-methoxyethoxy) ethoxyethyl methacrylate-co-ethylene glycol methacrylate). RSC Adv. 2015;5(48):38382–90. 10.1039/C5RA03256C.Search in Google Scholar
(21) Zhu X, Hu N, Xu Z, Cai X, Müller-Buschbaum P, Zhong Q. Easy care of silk fabrics realized by crosslinking thermo-responsive copolymer film on its surface. J Polym Sci. 2023;61(11):1015–25. 10.1002/pol.20220421.Search in Google Scholar
(22) Gu P, Fan N, Wang Y, Wang J, MüLler-Buschbaum P, Zhong Q. Linear control of moisture permeability and anti-adhesion of bacteria in a broad temperature region realized by cross-linking thermoresponsive microgels onto cotton fabrics. ACS Appl Mater Interfaces. 2019;11(33):30269–77. 10.1021/acsami.9b09294.Search in Google Scholar
(23) Zhang X, Zhang P, Lu M, Qi D, Müller-Buschbaum P, Zhong Q. Synergistic stain removal achieved by controlling the fractions of light and thermo responsive components in the dual-responsive copolymer immobilized on cotton fabrics by cross-linker. ACS Appl Mater Interfaces. 2021;13(23):27372–81. org/10.1021/acsami.1c03290.Search in Google Scholar
(24) Zhang P, Zhong Q, Qi D, Müller-Buschbaum P. Facile preparation of silk fabrics with enhanced UV radiation shielding and wrinkle resistance by cross-linking light-responsive copolymers. ACS Appl Mater Interfaces. 2022;14(23):27187–94. 10.1021/acsami.2c05726.Search in Google Scholar
(25) Zhong Q, Lu M, Nieuwenhuis S, Wu B-S, Wu G-P, Xu Z-K, et al. Enhanced stain removal and comfort control achieved by cross-linking light and thermo dual-responsive copolymer onto cotton fabrics. ACS Appl Mater Interfaces. 2019;11(5):5414–26. 10.1021/acsami.8b19908.Search in Google Scholar
(26) Wang J, Chen Y, An J, Xu K, Chen T, Müller-Buschbaum P, et al. Intelligent textiles with comfort regulation and inhibition of bacterial adhesion realized by cross-linking poly(n-isopropylacrylamide-co-ethylene glycol methacrylate) to cotton fabrics. ACS Appl Mater Interfaces. 2017;9(15):13647–56. 10.1021/acsami.7b01922.Search in Google Scholar
(27) Hu N, Mi L, Metwalli E, Bießmann L, Herold C, Cubitt R, et al. Effect of thermal stimulus on kinetic rehydration of thermoresponsive poly(diethylene glycol monomethyl ether methacrylate)-block-poly(poly(ethylene glycol) methyl ether methacrylate) thin films probed by in situ neutron reflectivity. Langmuir. 2022;38(26):8094–103. 10.1021/acs.langmuir.2c00940.Search in Google Scholar
(28) Hu N, Lin L, Metwalli E, Bießmann L, Philipp M, Hildebrand V, et al. Kinetics of water transfer between the LCST and UCST thermoresponsive blocks in diblock copolymer thin films monitored by in situ neutron reflectivity. Adv Mater Interfaces. 2023;10(3):2201913. 10.1002/admi.202201913.Search in Google Scholar
(29) Zhong Q, Chen C, Mi L, Wang JP, Yang J, Wu GP, et al. Thermoresponsive diblock copolymer films with a linear shrinkage behavior and its potential application in temperature sensors. Langmuir. 2020;36(3):742–53. 10.1021/acs.langmuir.9b03462.Search in Google Scholar
(30) Magerl D, Philipp M, Qiu X-P, Winnik FM, Müller-Buschbaum P. Swelling and thermoresponsive behavior of linear versus cyclic poly(N-isopropylacrylamide) thin films. Macromolecules. 2015;48(9):3104–11. 10.1021/acs.macromol.5b00436.Search in Google Scholar
(31) Tanaka M, Kaufmann S, Nissen J, Hochrein M. Orientation selective immobilization of human erythrocyte membranes on ultrathin cellulose films. Phys Chem Chem Phys. 2001;3(18):4091–5. 10.1039/B105007A.Search in Google Scholar
(32) Wei Y, Cheng F, Hou G, Sun S. Amphiphilic cellulose: Surface activity and aqueous self-assembly into nano-sized polymeric micelles. Reactive Funct Polym. 2008;68(5):981–9. 10.1016/j.reactfunctpolym.2008.02.004.Search in Google Scholar
(33) Rehfeldt F, Tanaka M. Hydration forces in ultrathin films of cellulose. Langmuir. 2003;19(5):1467–73. 10.1021/la0261702.Search in Google Scholar
(34) Chen Q, Xu S, Liu Q, Masliyah J, Xu Z. QCM-D study of nanoparticle interactions. Adv Colloid Interface Sci. 2016;233:94–114. 10.1016/j.cis.2015.10.004.Search in Google Scholar
(35) Raudino M, Giamblanco N, Montis C, Berti D, Marletta G, Baglioni P. Probing the cleaning of polymeric coatings by nanostructured fluids: A QCM-D study. Langmuir. 2017;33(23):5675–84. 10.1021/acs.langmuir.7b00968.Search in Google Scholar
(36) Su H, Liu H-Y, Pappa A-M, Hidalgo TC, Cavassin P, Inal S, et al. Facile generation of biomimetic-supported lipid bilayers on conducting polymer surfaces for membrane biosensing. ACS Appl Mater Interfaces. 2019;11(47):43799–810. 10.1021/acsami.9b10303.Search in Google Scholar
(37) Chang GX, Ren KF, Zhao YX, Sun YX, Ji J. Modulation of cell behaviors by electrochemically active polyelectrolyte multilayers. e-Polymers. 2014;14(5):297–304. 10.1515/epoly-2014-0075.Search in Google Scholar
(38) Lin CH, Luo SC. Combination of AFM and electrochemical QCM-D for probing Zwitterionic polymer brushes in water: Visualization of ionic strength and surface potential effects. Langmuir. 2021;37(42):12476–86. 10.1021/acs.langmuir.1c02230.Search in Google Scholar
(39) Zhong Q, Wang W, Adelsberger J, Golosova A, Bivigou Koumba AM, Laschewsky A, et al. Collapse transition in thin films of poly(methoxydiethylenglycol acrylate). Colloid Polym Sci. 2011;289(5-6):569–81. 10.1007/s00396-011-2384-1.Search in Google Scholar
(40) Zhong Q, Metwalli E, Kaune G, Rawolle M, Bivigou-Koumba AM, Laschewsky A, et al. Switching kinetics of thin thermo-responsive hydrogel films of poly(monomethoxy-diethyleneglycol-acrylate) probed with in situ neutron reflectivity. Soft Matter. 2012;8(19):5241. 10.1039/C2SM25401H.Search in Google Scholar
(41) Zhong Q, Adelsberger J, Niedermeier MA, Golosova A, Bivigou-Koumba AM, Laschewsky A, et al. The influence of selective solvents on the transition behavior of poly(styrene-b-monomethoxydiethylenglycol-acrylate-b-styrene) thick films. Colloid Polym Sci. 2012;291(6):1439–51. 10.1007/s00396-012-2879-4.Search in Google Scholar
(42) Xiao XC, Chu LY, Chen WM, Wang S, Li Y. Positively thermo-sensitive monodisperse core–shell microspheres. Adv Funct Mater. 2003;13(11):847–52. 10.1002/adfm.200304513.Search in Google Scholar
(43) Li S, Ge Y, Piletsky SA, Turner APF. A zipper-like on/off-switchable molecularly imprinted polymer. Adv Funct Mater. 2011;21(17):3344–9. 10.1002/adfm.201100593.Search in Google Scholar
(44) Ishida N, Biggs S. Direct observation of the phase transition for a poly(N-isopropylacryamide) layer grafted onto a solid surface by AFM and QCM-D. Langmuir. 2007;23(22):11083–8. 10.1021/la701461b.Search in Google Scholar
(45) Rudolph-Schöpping G, Schagerlöf H, Jönsson A-S, Lipnizki F. Comparison of membrane fouling during ultrafiltration with adsorption studied by quartz crystal microbalance with dissipation monitoring (QCM-D). J Membr Sci. 2023;672:121313. 10.1016/j.memsci.2022.121313.Search in Google Scholar
(46) Ying X, He J, Li X. Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid. e-Polymers. 2021;21(1):500–10. 10.1515/epoly-2021-0049.Search in Google Scholar
(47) Xiong W, Qiu X, Zhong R, Yang D. Characterization of the adsorption properties of a phosphorylated kraft lignin-based polymer at the solid/liquid interface by the QCM-D approach. Holzforschung. 2016;70(10):937–45. 10.1515/hf-2015-0226.Search in Google Scholar
(48) Navarro LA, Shah TP, Zauscher S. Grafting to of bottlebrush polymers: Conformation and kinetics. Langmuir. 2020;36(17):4745–56. 10.1021/acs.langmuir.9b03620.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

