Abstract
Polyvinyl alcohol (PVA) is well-known for its excellent mechanical properties and eco-friendliness in food packaging. Recently, PVA has gained significant attention in research due to its potential as antibacterial substrates. However, because of its high hydrophilicity, practical application of pure PVA film has been limited by low water resistance and rapid bacterial growth in humid conditions. Diverse effective strategies have been developed to decrease hydrophilicity and endow films with antibacterial properties. This review provides an overview of universal antibacterial agents and their functions in PVA-based packaging films. It also introduces the changes in the mechanical and physical properties of PVA-based films after adding antibacterial agents. Additionally, the release behavior of antibacterial agents in PVA-based film materials and their practical applications in the food industry is discussed in fresh food packaging. Biodegradability of PVA-based films is also mentioned, with a promising future for more effective and eco-friendly food packaging materials.
Abbreviations
- AgNPs
-
silver nanoparticles
- AHSG
-
alyssum homolocarpum seed gum
- BRS
-
boiled rice starch
- CA
-
citric acid
- CD
-
cynodon dactylon
- CEO
-
cinnamon essential oil
- CIN
-
cinnamaldehyde
- CMC
-
carboxymethylcellulose
- CNC
-
cellulose nanocrystals
- CNP
-
chitosan nanoparticle
- CO
-
clove oil
- CS
-
chitosan
- D P
-
diffusion coefficient
- EB
-
elongation at break
- GD
-
glutaraldehyde
- HNT
-
halloysite nanotube
- LNP
-
lignin nanoparticles
- MC
-
moisture content
- MFC
-
microfilamentous cellulose
- MMT
-
montmorillonite
- mBNCs
-
modified bacterial nanocelluloses
- NAC
-
N-acetylcysteine
- OEO
-
oregano essential oil
- PC
-
proanthocyanidins
- PVA
-
polyvinyl alcohol
- RPE
-
rose petal extract
- ST
-
starch
- TS
-
tensile strength
- WVP
-
water vapor permeability
- XG
-
xanthan gum
- YM
-
Young’s modulus
- ZIF-8
-
zeolite imidazole skeleton-8
- 4-HR
-
4-hexylresorcinol
1 Introduction
Food products are extremely susceptible to microbial contamination and spoilage under certain temperature and humidity, which not only leads to the wastage of resources but can also do harm to human health. Food packaging mainly separates the food inside from internal and external environmental factors (such as microorganisms, oxygen, temperature, and humidity) to maximize the protection, ensuring the quality and shelf life. With the development of food industry, food safety and quality have become more significant, which could be solved by antimicrobial food packaging to a great extent (1).
Antimicrobial food packaging presents as food packaging composite films with the addition of antimicrobial substances induced or on the surfaces, which are in contact with food to achieve a preservative function for the products inside the packaging (2,3). Among many film materials, polyvinyl alcohol (PVA) has been reported as an excellent candidate as antibacterial packaging polymer material (4,5). PVA is a non-toxic, environmentally friendly polymer film material utilized in food packaging for its good barrier properties, flexibility, transparency, and biodegradability (6). With the addition of appropriate antimicrobial agents and non-toxic plasticizers, PVA-based films can be endued with improved performance, including mechanical properties, barrier properties, and other packaging physical properties stable with antimicrobial properties at the same time. For example, silver nanoparticles (AgNPs) were added to PVA/chitosan (CS) to prepare food packaging films with excellent mechanical properties. The inclusion of AgNPs reduced water vapor permeability (WVP) and solubility, enhancing food preservation. These composite films, known for their mechanical strength, antibacterial properties, flexibility, and transparency, hold significant potential for fruit storage and transportation (7). In food contact applications, the use of PVA has been defined by European Food Safety Authority (EFSA (2005)) (8). PVA can be used as a covering material for a variety of foods without raising health concerns.
PVA was discovered in 1924 by German chemists Dr. W. O. Herrmann and W. Hacehnel. PVA is a water-soluble polymer obtained by the alcoholysis of polyvinyl acetate (9). Its molecular structure is simple and linearly arranged. There are several free hydroxyl groups on PVA molecules, which have strong hydrophilicity. Compared with biopolymers, petroleum-based polymer material PVA is suited for food packaging applications because of its low cost, good physical strength, and excellent gas barrier properties. PVA is recognized as a promising sustainable biopolymer with the potential to replace plastic-based packaging materials due to its biocompatibility, degradability, and excellent film-forming properties. Recently, research on developing antibacterial food packaging films using PVA has been actively conducted. Several antibacterial food packaging films based on PVA have been developed and used for actual food storage. There are varied degrees of increased food shelf life, ranging from PVA-based antimicrobial packaging to packaging such as strawberries (10), fresh beef (11), and cheese (12), among other foodstuff. Therefore, there is a need to develop a PVA-based film as food packaging with growth potential.
This review provides the recent research progress of PVA-based antimicrobial packaging film materials. In this work, we discuss the preparation and properties of PVA-based film materials with a variety of properties and types of antimicrobial agents in PVA-based films, including mechanical properties, water resistance and antibacterial effect. It also introduces the release behavior of antimicrobial agents in PVA-based film materials and their practical applications in the food industry. For the future development of PVA-based antimicrobial food packaging, the new thought which includes the categories of antimicrobial agents and their antimicrobial effects, the study of antimicrobial agent release, and the development of PVA-based antimicrobial packaging film applications in food packaging should be taken into consideration.
2 Film preparation
The methodology of manufacturing PVA packaging films involves various techniques including solution casting (13), extrusion (14,15), electrostatic spinning (16), coating (17), layer-by-layer assembly (18), hydrogels (19), etc. These different preparation methods have influences on the physical, structural, and mechanical properties of the films (20). Among these preparation techniques, solution casting, coating, and electrostatic spinning are the methods most commonly used due to simple process, low cost, and good controllability.
Solution casting method is a common method of film preparation, based on the principle of dissolving the desired material in a suitable solvent, then pouring the solution into a mold and leaving the solution to dry before peeling off to obtain the film. The advantage of the solution casting method is that a high purity and homogeneous film material is obtained (Figure 1a).
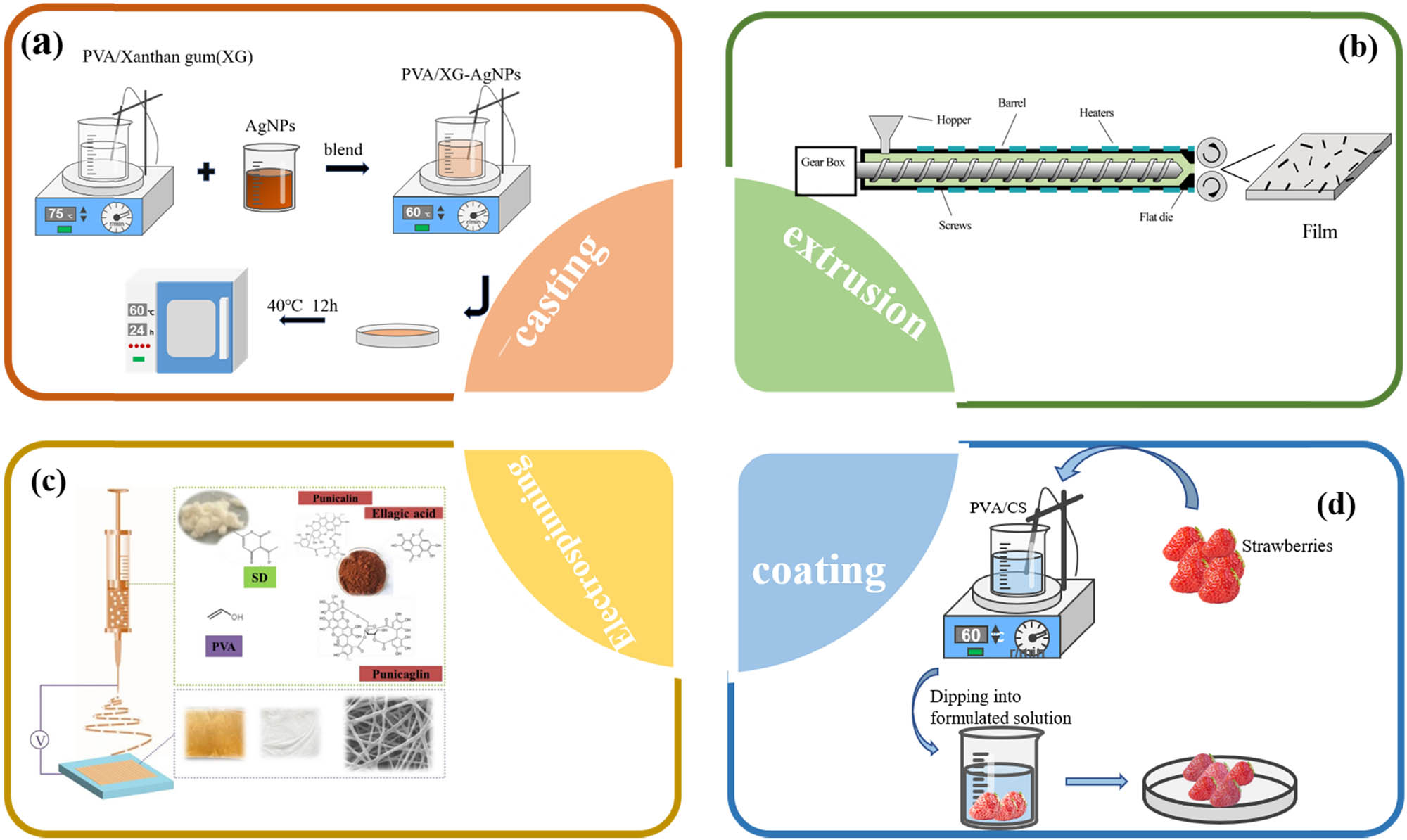
Common preparation methods in PVA based (a) casting, (b) extrusion, (c) electrospinning (31), and (d) coating.
The extrusion method for PVA involves using an extruder machine to process and shape PVA into various forms, such as films, sheets, or filaments. Extrusion is a common manufacturing technique for thermoplastic polymers like PVA (Figure 1b). A twin-screw extruder was used to prepare PVA/starch/montmorillonite (MMT) nanocomposite films for food packaging (14). In the same study, Wang et al. added clay to PVA/starch and produced PVA/starch/clay nanocomposite films with high mechanical and barrier properties using extrusion blowing (15).
Electrospinning is currently one of the simplest, most efficient, and cost-effective methods of preparing nanofibers. Nanofibers prepared by electrospinning methods have high specific surface area, high loading rate, high porosity, easy modification, and adjustable structures, which allows nanofibers to be used as delivery or controlled release carriers for antimicrobial agents and other active compounds, effectively extending the shelf life of food products (21,22). These unique advantages have promoted the development and application of nanofibers in various fields such as biomaterials, sensing, protective materials, energy, and food packaging. Nanofibers as food packaging materials are a very promising method for the preparation of antimicrobial food packaging films (Figure 1c).
Coating is the process of immersing the packaged material in a film-forming solution to obtain a dense film that evenly wraps the contents. Film coating is a new and simple method of storage and preservation introduced in recent years. The results of film coating depend on the choice of film materials and the method of film formation (Figure 1d). For example, strawberries coated with the edible PVA/cashew gum polysaccharide (23).
In these methods, various other substances such as starch (24), CS (16), soy protein (25), carboxymethyl cellulose (26), and β-cyclodextrin (27) can be blended with PVA to fabricate food film substrates. To improve the barrier properties, and mechanical properties of the film, plasticizers such as glycerol, sorbitol, and citric acid (CA) are usually added to the film solution. Additionally, cross-linking agents such as glutaraldehyde (GD) (28), boric acid (29), and glyoxal (30) are used to further enhance the film’s properties. GD was used to crosslink PVA composite films containing cellulose nanocrystals (CNC) and lignin nanoparticles (LNPs) (28). Due to the powerful interactions between LNP, CNC, and PVA in the presence of GD, the thermodynamic study revealed better thermal stability. Meanwhile, the tensile strength (TS) of PVA/CNC/LNP increased from 26 for neat PVA to 35.4 MPa, and PVA/CNC/LNP films crosslinked by GD showed excellent barrier ability to the WVP. Furthermore, the selection and addition of antimicrobial agents play crucial roles in the performance and effectiveness of PVA-based film materials. It is essential to consider various factors such as compatibility with PVA, dispersibility, stability, and release when choosing the appropriate antimicrobial agents.
3 Properties of PVA-based film
PVA is a water-soluble polymer synthesized by polymerization alcoholysis of vinyl acetate (9). PVA molecules contain a substantial hydrogen bonding, tight structure, with good mechanical properties. While there are also a large number of hydrophilic hydroxyl groups, resulting in the water absorption rupture of PVA film when packaging products with high water content (Figure 2). Thus, it is necessary to add additives and active substances to enhance the application of PVA composite film (32). Moreover, the addition of antimicrobial agents not only endues the film antibacterial properties but also certain mechanical properties and additional barrier properties (20,33,34).
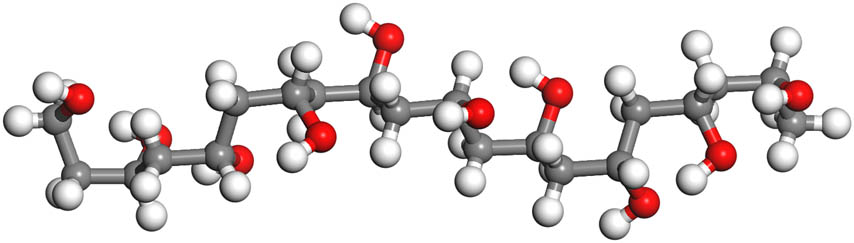
PVA chain.
3.1 Mechanical properties
Mechanical properties play important roles in food packaging, which ensure the food quality by influencing the resistance to deformation or breakage. The elongation at break (EB) and TS show the toughness and strength of the film, respectively. Measurement of the mechanical properties of PVA-based films can evaluate the feasibility of their practical use as food packaging materials. As shown in Table 1, mechanical properties are remarkable parameters for developing food packaging materials combined with different antimicrobials.
Effect of PVA-based packaging with different antimicrobial agents on microbial inhibition and the performance of film materials
| Film substrates | Antimicrobial agent | Tested microorganisms | Mechanical property | Antimicrobial film diameter (mm) | Max. diameter of inhibition circle (mm) | Ref. |
|---|---|---|---|---|---|---|
| PVA | Calcium propionate + ethylparaben | E. coli, S. aureus | TS↑, EB↓, WVP↑ | 6 | 8.5, 13.1 | (98) |
| PVA | Quaternary ammonium salt of CS | E. coli, S. aureus | TS↑, EB↓, WVP↑ | 6 | 13.4, 13.3 | (98) |
| PVA | Quaternary ammonium salt of CS + tea polyphenols | E. coli, S. aureus | TS↑, EB↓, WVP↑ | 6 | 14.1, 10.5 | (98) |
| PVA | Tea polyphenols | E. coli, S. aureus | TS↓, EB↓, WVP↑ | 6 | 14.4, 12.3 | (98) |
| PVA | Calcium propionate | Bacillus cereus, E. coli | TS↑, EB↓, WVP↑ | 6 | 7.5, 6.8 | (35) |
| PVA/carboxymethyl CS | CA | S. aureus | TS↑, YM↑, WVP↓ | 8 | 12 | (100) |
| PVA | Piperic acid | Fusarium solani, Penicillium, Aspergillus | TS↑, EB↓, WVP↑, OP↑ | 20 | 5.3, 20.2, 50.5 | (37) |
| PVA | AgNPs | E. coli, S. aureus | TS↑ | 6 | 14, 13.6 | (62) |
| PVA | AgNPs | Aspergillus niger | TS↓, EB↓, WVP↓ | 10 | 20.1 | (40) |
| PVA | AgNPs | P. fluorescens | TS↑ | 10 | 10.9 | (63) |
| PVA/CS | TiO2 | E. coli, Salmonella enterica, L. monocytogenes, Staphylococcus aureus | TS↑, EB↑, WVP↓, OP↓ | 6 | 36.0, 47.5, 51.5, 26.0 | (41) |
| PVA/CS | ZnO-SiO2 | E.coli, S.aureus | TS↑, WVP↓, OP↓ | 10 | 35.0, 37.0 | (72) |
| PVA (single coated paper) | Thymus vulgaris L. essential oil + Ocimum gratissimum L. essential oil | Aspergillus cinerea, Rhizopus, Streptospora actinomyces, Penicillium | NA | 20 | 25.0, 27.0, 14.0, 21.0 | (85) |
| PVA (Double coated paper) | Thymus vulgaris L. essential oil + Ocimum gratissimum L. essential oil | Aspergillus cinerea, Rhizopus, Streptospora actinomyces, Penicillium | NA | 20 | 44.0, 46.0, 25.0, 48.0 | (85) |
| PVA/starch | CIN | S. putrefaciens | TS↓, EB↑, WVP↓, OP↑ | 6 | 10.8 | (87) |
| PVA/starch/MFC | CIN | S. putrefaciens | TS↑, EB↑, WVP↓, OP↑ | 6 | 10.2 | (87) |
| PVA/AHSG | Nisin | L. innocua | 7-9 | 13.3 | (96) | |
| PVA | CA | E. coli, S. aureus | WVP↓ | 7 | 20.0, 13.0 | (43) |
| PVA/β-cyclodextrin | CEO | L. monocytogenes, S. enteritidis | NA | 6 | 13.6, 11.4 | (99) |
| PVA/β-cyclodextrin | CEO | A. niger, Penicillium | NA | 6 | 19.1, 40.6 | (99) |
| PVA/β-cyclodextrin | CEO + lysozyme | A. niger, Penicillium | NA | 6 | 15.2, 13.7 | (99) |
| PVA/β-cyclodextrin | CEO + lysozyme | A. niger, Penicillium | NA | 6 | 19.2, 40.7 | (99) |
| PVA | Lysozyme | L. monocytogenes, S. enteritidis | NA | 6 | 15.1, 7.9 | (99) |
| PVA/corn starch | Natamycin | Aspergillus niger, penicillium, yeast | TS↑, EB↓ | 15 | 31.2, 28.5, 23.2 | (100) |
| PVA | CNC | Colletotrichum gloeosporioides, Lasiodiplodia theobromae | TS↑, EB↑, | 20 | 34.0, 28.0 | (101) |
| PVA | Chitosan nanoparticle (CNP) | Colletotrichum gloeosporioides, Lasiodiplodia theobromae | TS↑, EB↑, | 20 | 25.7, 25.0 | (101) |
| PVA | CNC + CNP | Colletotrichum gloeosporioides, Lasiodiplodia theobromae | TS↑, EB↑, | 20 | 27.8, 27.2 | (101) |
| PVA | Poria coco | E. coli, S. aureus, Bacillus subtilis | TS↓, EB↑ | 10 | 16.8, 14.5, 12.0 | (102) |
Mechanical strength of the PVA film was highly improved after the incorporation of 2.5 wt% calcium propionate (35) compared with pure PVA film (31). This may be due to the uniform distribution of calcium propionate in the film and the interaction with PVA molecular chains, which enhanced the strength and toughness of the film. Among all the films prepared, the PVA/carboxymethyl CS composite film with CA showed highly improved mechanical properties (36). In another study, the EB of PVA/CS films was 223.89 ± 7.50%, and it increased to 281.10 ± 24.69% upon the addition of modified bacterial nanocelluloses (mBNCs) and 4-hexylresorcinol (4-HR) (11). EB raises as the concentration of mBNCs and 4-HR increases, but the composite films show a decrease in TS. Likewise, the solution casting method for the preparation of PVA films containing cinnamic and ferulic acids showed enhanced TS and elastic modulus compared to the pure PVA films, and EB was significantly enhanced for the films after thermo-processing. Moreover, the solution casting method was used to prepare PVA films containing cinnamic and ferulic acids, which demonstrated enhanced TS and elastic modulus compared to pure PVA films. Furthermore, EB was significantly improved for the films after thermo-processing. In addition, the TS of the films increased, while the EB tended to decrease with the addition of piperic acid, and the results indicated the suitability of the composite films for food packaging (37). The TS of composite films prepared from PVA/ST was increased up to 31.83 ± 0.17 MPa compared to neat films, which is 18.05 ± 0.04 MPa, depending on the nanoparticle filler concentration (38).
3.2 WVP
WVP is the main parameter to evaluate water transfer between the internal and external atmosphere of food packaging, which is considered to be an important parameter of food packaging materials. The difference between the relative humidity in the external environment and the water activity of the food itself can lead to moisture or dehydration during the storage of food, so it is necessary to explore the effect of WVP of the film on food packaging. The WVP of PVA-based films containing different antimicrobial agents are shown in Table 1.
The moisture content (MC) and WVP of the PVA/CS composite films were improved with the addition of mBNCs and 4-HR, while water solubility was reduced (12). The WVP and oxygen permeability of the PVA films containing cinnamic and ferulic acids were significantly reduced (39). The incorporation of AgNPs decreased the WVP of the PVA films, which is in line with that reported in previous studies, suggesting that the addition of nanoparticles enhances the vapor barrier properties of the films (40). This is also consistent with another study which found that the WVP of PVA/CS nanocomposite films incorporated with TiO2 were observed to decrease the WVP property ranging from 6.57 to 4.36 × 10−12 g·cm·cm−2·s−1·Pa−1 (41). The addition of CA into PVA/carboxymethyl CS composite films can induce a decrease in WVP values (36). The presence of nanoparticles reduced the intermolecular spacing within the films, thus reducing the WVP through the film (42). Another study also confirmed that the incorporation of CA into the PVA for the fabrication of food packaging films can decrease the WVP up to 1.28 × 10−14 g·m−1·s−1·Pa−1 compared to control composites without CA having WVP of 4.02 × 10−14 g·m−1·s−1·Pa−1 (43).
According to Chen et al. (44), the WVP significantly decreased from 3.13 × 10–14 to 1.43 × 10−14 g·(cm⋅s⋅Pa)−1 for PVA/ST film with the addition of clove oil (CO). The addition of CO would structure a zigzag route at the hydrophilic hydrophobic interface, which decreased the diffusion capability of WVP.
3.3 Transparency
The transparency of PVA-based food packaging is of great importance. Transparent PVA-based packaging offers several benefits, including expanded product visibility, increased safety assurance, improved food quality assessment, and a platform for promoting eco-friendly materials and sustainability initiatives by manufacturers. PVA-based transparent packaging can contain environmentally friendly packaging materials and promote sustainable development efforts of manufacturers.
The addition of 2–6 wt% of CNC to PVA improved interfacial interactions, yielding clear micro-structures. The bio-nanocomposite films maintained high transparency (90% transmittance in UV-vis), indicating good CNC dispersion. These eco-friendly composites are promising for food packaging (45). The light transmittance of the PVA/starch/glycerol/halloysite nanotube (HNT) nanocomposite films decreased linearly by 26.90% as the HNT content increased from 0 to 5 wt%, making them suitable for sustainable food packaging, especially for lipophilic and acidic foods (46). The transparency of PVA-based films decreased with higher loading of TiO2 and MMT nanoparticles in the nanocomposite films prepared using the solution casting method (47). Pure PVA had better light transmittance than PVA/xanthan gum (XG) composite films, suggesting better transmittance in pure PVA due to the composite film’s internal structure affecting light transmission and scattering. It is worth mentioning that all samples exhibited over 85% transmittance, demonstrating good uniformity and transparency (48).
In summary, the transparency of PVA-based food packaging not only meets practical goals such as product visibility and safety, but also plays a key role in marketing, branding, sustainability, and overall consumer satisfaction. This meets consumers’ desire for transparency and helps build trust in food products and their packaging.
4 Types of antimicrobial agents and their antimicrobial effects
There are diverse antibacterial agents and some of them have significant effects on Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), etc. Conventional antibacterial agents can be classified into three categories: organic antibacterial agents, inorganic antibacterial agents, and natural antibacterial agents. PVA-based antibacterial packaging typically involves a range of organic acids and salts, plant essential oils or their extracts, enzymes, CS, bacteriocins, and inorganic metal antibacterial agents to obtain antibacterial properties.
4.1 Organic antimicrobial agents
Organic antimicrobial agents largely disrupt cell membranes by releasing antimicrobial substances, which causes sulfhydryl acidification and protein denaturation. It blocks DNA synthesis and cellular metabolism and inhibits the reproduction of bacteria and fungi. The antimicrobial agent not only has a superior antimicrobial ability, abundant source, fast bactericidal speed, wide antibacterial range, and good bactericidal effect but also has poor safety, poor stability, common drug resistance, and relatively weak effect on bacteria (49). The organic antibacterial agents typically added in PVA substrates includes potassium sorbate (50), calcium propionate (35), CA (36), sodium lactate (51), and caffeic acid (52).
The film material of PVA with calcium propionate additive of 2.5 wt% produced certain antimicrobial effects on Bacillus cereus, E. coli, and Aspergillus oryzae (35). CA was discovered to have a substantial inhibitory impact on S. aureus at a certain dose in PVA/carboxymethyl CS packaging film (36). A study (52) of solid caffeic acid and β- or γ-cyclodextrin inclusions embedded in PVA nanofibers demonstrated high antibacterial activity against both E. coli and S. aureus. This study used a sodium lactate-loaded PVA/CS/MMT composite film with excellent inhibition activity against E. coli. PVA/piperic acid composite films demonstrated strong antimicrobial action against the Fusarium solani, Penicillium, and Aspergillus foodborne pathogens (33). Food can be shielded from browning and exposed to less UV light thanks to antibacterial films containing PVA/N-acetylcysteine (NAC) ability (45). At 85°C and when exposed to moisture, the released NAC significantly slowed the growth of S. aureus and E. coli.
Meanwhile there are some lipid compounds, halides, and organometallics added as antibacterial agents in food packaging film materials. The growth of four major food pathogens, including Campylobacter jejuni, E. coli, Listeria monocytogenes (L. monocytogenes), and Salmonella typhimurium, was inhibited by PVA/CS conjugate films (34), and the best antibacterial effect was seen for films with 5% and 10% ethyl lauroyl arginate content. Additionally, food packaging films with strong antibacterial activity and severe sensitivity to E. coli and L. monocytogenes were made using PVA grafted ethylene radical alteration and N-haloamines (53). The zeolite imidazole skeleton-8 (ZIF-8) metal-organic skeleton, along with PVA and quaternary ammonium CS, constructs ZIF-8 films. These composite films possess high bacterial filtering capabilities and demonstrate effectiveness in eradicating both E. coli and S. aureus (54).
4.2 Inorganic antimicrobial agent
Inorganic antimicrobial agents are broadly used in medical materials, agricultural antimicrobial agents, optical materials, and semiconductor materials. It is one of the top research hotspots for antibacterial materials because of its high antibacterial efficiency and durability, low drug resistance, high safety, and UV-barrier properties. Pursuant to the inverse antibacterial mechanism, inorganic antibacterial agents can be categorized into two types: dissolution type and photocatalytic type. The soluble type is loaded with inorganic carriers such as Ag, Cu, and Zn; the photocatalytic type consists of inorganic oxides such as TiO2 and ZnO, which have relatively high heat resistance, but have bactericidal effects only in the presence of UV light, oxygen, or water. A wide range of antibacterial agents have been reported in the literature as metal nanoparticles (e.g., Ag, Cu, etc.) (55,56), metal oxide nanoparticles (e.g., TiO2, ZnO, CuO, Cu2O, etc.) (54,57,58,59).
Inorganic metal nanometer antimicrobial agents treated with nanotechnology have a wide range of highly effective antibacterial bactericidal properties, AgNPs are most widely used among them. PVA-based film materials enriched with AgNPs exhibited significant inhibition of Salmonella typhi (60), E. coli (53), S. aureus (61), and Aspergillus niger (40). Salmonella typhi and S. aureus growth was greatly slowed down by PVA/boiled rice starch (BRS)/AgNPs composite films. In comparison with the control PVA/BRS composite films, the nanocomposite showed improved mechanical and optical properties and had a lower water sensitivity. Moreover, PVA/BRS/AgNP films function better against environmental microbes (60). Pure PVA film containing AgNPs showed significant inhibition of Aspergillus niger (40). In the alternative, the addition of nanocellulose also enhanced the inhibition of E. coli and S. aureus by PVA/AgNPs film materials (62).
Plant extracts majorly contain polysaccharides, polyphenols, alkaloids, aldehydes, proteins and amino acids, which can be used as reducing and stabilizing agents for the biosynthesis of AgNPs by reaction with silver nitrate solution, and have significant effects on the biosynthesis of AgNPs. AgNPs synthesized by the reaction of cynodon dactylon (CD) leaves extract with silver nitrate were added to PVA film-forming solution to prepare antibacterial film materials, which showed significant inhibition of Pseudomonas fluorescens. The addition of biosorbed-AgNPs improved the physical and antibacterial properties of the films (63). Ginger extract was reacted with silver nitrate to synthesize AgNPs and the prepared PVA/MMT/AgNPs composite film materials showed strong antibacterial effect against pathogenic bacteria S. typhimurium and S. aureus (64). The significant increase in TS of the nanocomposite films could be attributed to the high compatibility between nanofillers (MMT and AgNPs) and PVA. The effective dispersion of nanofillers in the polymer matrix helps to control the stress transfer at the interface between the filler and matrix, which ultimately leads to the increase in TS of the nanocomposites. The increase in Young’s modulus (YM) of the nanocomposite blended films may be due to the interaction between the filler and matrix, resulting in the limitation of matrix motion and stiffness. On the other hand, the EB of pure PVA films was found to decrease after blending with ginger extract, MMT, and AgNPs. Similarly, AgNPs synthesized by the reaction of capparis zeylanica leaf extract with silver nitrate were added to PVA/polyethylene glycol film-forming solution to make films, and the composite films showed better inhibition of Pseudomonas aeruginosa (65). Other AgNPs such as periwinkle (59,66), sodium citrate dihydrate (67), oregano essential oil (OEO) (42), morinda citrifolia leaf extract (68), CS (69), and glucose (70) synthesized by reaction with silver nitrate were added to PVA film-forming solution to prepare antibacterial film materials.
The metal oxides that have been reported for antibacterial packaging of PVA substrates are TiO2, ZnO, CuO, Cu2O, etc. E. coli, Salmonella enterica, L. monocytogenes, and S. aureus were all significantly inhibited by PVA/CS composite films containing TiO2, and the inhibition of the first three bacteria by the composite film materials increased with the increase in the TiO2 concentration (41). Other studies discovered that film materials made of PVA/corn starch and PVA/carboxymethylcellulose (CMC) that contained ZnO nanoparticles both demonstrated strong antibacterial activity against E. coli and S. aureus (24,71), while PVA/corn starch film with outstanding UV-shielding capability meanwhile retaining highly optical transparency (approximately 90%) (24).
The antibacterial activity of PVA/CS was further enhanced by the addition of Cu2O@NCs, with 2% Cu2O@NCs achieving 72% and 66% inhibition against S. aureus and E. coli, respectively (59). Similarly, it was found that the introduction of CuO@ZIF-8 nanoparticles into the PVA/quaternary ammonium CS as the inner layer exhibited better antibacterial ability against S. aureus and E. coli (54). Meanwhile, the films provided low WVP and strong UV-barrier ability. There were also PVA/CS/ZnO-SiO2 composite film materials made by adding different ratios (0.50%, 1.0%, 3.0%, and 5.0%) of ZnO-SiO2 (72) to the PVA/CS mixture, which exhibited better antibacterial properties against E. coli and S. aureus. The addition of ZnO-SiO2 nanocomposites substantially reduced the WVP of the films, with values of 954.19, 890.38, 890.38, and 500.60 g·(m2·day)−1, respectively, compared to 980.86 g·(m2·day)−1 for PVA/CS films (72).
4.2.1 Toxicity of the inorganic antibacterial agent
Ensuring the absence of toxicity is crucial in food packaging. Consideration of the toxicity of the inorganic antibacterial agent is of utmost importance, as it necessitates a comprehensive assessment and evaluation to ensure the safety and well-being of individuals and the environment when these particles are employed or present in food packaging.
Toxicity testing on the PVA/CS blend and various PVA/CS/ZnO-SiO2 nanocomposites films with different ZnO-SiO2 ratios demonstrated no toxicity towards the marine luminescent bacterium Vibrio fischeri (72). The cell viability of NIH 3T3 cells treated with PVA/quaternary ammonium CS composite films remained above 90%, and this trend persisted regardless of the concentration of CuO@ZIF-8 NPs (54).
4.3 Natural antimicrobial agent
Natural antimicrobial agents can be grouped into three main categories in terms of their main sources: natural antimicrobial agents of plant origin, natural antimicrobial agents of microbial origin (73), bacteriocins (nisin, Lantibiotics, antibiotics, etc.) (74,75,76), biopolymers (CS) (77,78), and enzymes (lysozyme) (79). Section 4.3.1 focuses on the more studied plant-derived antimicrobial agent, streptococin lactate.
4.3.1 Antimicrobial agents of plant origin
Natural antimicrobial agents of plant origin are predominantly extracted from natural substances (10,20,23,80,81), such as plant extracts (essential oils or extracts) (74,82). The essential oils spiced in PVA-based film materials are mainly lauric essential oil, iterative essential oil, OEO, cinnamon essential oil (CEO), thyme essential oil, and clove essential oil (44). The electrostatic spinning method was used for preparing PVA film materials containing essential oils of laurel and iterative. These materials significantly reduced the number of L. monocytogenes during refrigeration (20). The combined film materials prepared by adding emulsifiers made of OEO or CEO to PVA film materials showed better inhibition of both E. coli and S. aureus, with E. coli being more sensitive to CEO (83).The combined film materials made of PVA and clove essential oil had better antifungal (melon fruit rot) activity and lower MC and film solubility, which indicated their suitability for modified atmosphere packaging applications (84). In PVA-based coated papers, addition of thyme essential oil and clove basil essential oil showed good antimicrobial properties against gray mold, root mold, cross-streptomycosis, and penicillium (85).
Cinnamaldehyde (CIN) is a natural plant extract that has the ability to inhibit the growth of bacteria and fungi (84,86). CIN-encapsulated PVA/granular gum nanofiber films exhibited excellent antibacterial properties against S. aureus and Pseudomonas aeruginosa (22). Addition of CIN to PVA/maize starch/microfilamentous cellulose (MFC) composite film materials by using the co-blending technique created to considerably suppress the growth of S. putrefaciens (87).
Sodium trimetaphosphate and boric acid (88) were used to double cross-link the PVA/potato starch polymer, and further addition of anthocyanin and limonene prepared composite film materials could achieve both colorimetric indication and antimicrobial activity, especially satisfactory antimicrobial activity against three microorganisms, Bacillus subtilis, Aspergillus niger, and S. aureus. It was found (89) that PVA packaging film containing 20% tea polyphenols had a 95.5 ± 4.2% and 91.8 ± 3.7% inhibition against S. aureus and E. coli. It should be mentioned that the film treated with ultrasound had better bacterial inhibition. It was also found that the PVA/tapioca starch composite films with the addition of red pitaya (Hylocereus polyrhizus) peel extract (betaine) had antibacterial effects on S. aureus, L. monocytogenes, E. coli, and Salmonella, with stronger inhibition of the first two microorganisms (90). The PVA/tea polyphenol electrostatic spinning nanofiber membrane also showed inhibition of E. coli and S. aureus with a maximum inhibition activity up to 82.48% and 86.25% (91). The PVA/tannin composite films containing different tannin contents showed good antibacterial ability against E. coli, S. aureus, and S. epidermidis, and the antibacterial activity increased with the increase in tannin content (92). The PVA/algae biomass cellulose nanocrystal composite film containing basil leaf extract had good antibacterial properties, and the addition of 20% basil leaf extract significantly inhibited Bacillus cereus (93). The antibacterial films were prepared by impregnating curcumin emulsion with PVA and corn starch as substrates, and it was found that curcumin significantly inhibited S. aureus and Bacillus subtilis, while E. coli was less affected by curcumin (94).
4.3.2 Antimicrobials of microbial origin
Nisin as a natural food preservative, has an inhibitory effect mainly against most Gram-positive bacteria and their budding embrace, addition of nisin to PVA/CS effectively inhibited S. aureus colonization (95), and addition of Alyssum homolocarpum seed gum (AHSG) to PVA/CS nisin effectively inhibited the multiplication of harmless L. monocytogenes (96). Lipopeptides, a class of non-toxic and highly stable antimicrobial agents of microbial origin, can cause damage to microbial cell membranes through electrostatic interactions with negatively charged membranes. The lipopeptides were cultured, isolated, and purified from bacillus sp. for column purification and added to a composite film material of PVA/MMT for excellent inhibition of Salmonella typhimurium (97).
In a nutshell, PVA-based antimicrobial food packaging film materials have a notable inhibitory effect on the growth of food microorganisms. Table 1 summarizes the inhibition effect of PVA-based packaging containing different antimicrobial agents on microorganisms such as E. coli, S. aureus, B. cereus, Streptococcus spoilage, and the effect of antimicrobial agent addition on the film performance in recent years.
5 Study on the release of antibacterial agent in PVA-based antibacterial film
The process and mechanism of antimicrobial agent release is crucial for assessing the effectiveness and safety of PVA-based antimicrobial packaging materials, and in practical applications, the focus is usually on the antimicrobial agent exerting antimicrobial/antibacterial effects in long-term use, thus ensuring food quality. The key factor determining the antimicrobial effect of antimicrobial agents is the regulation of their rate of release in packaging film materials (103,104,105,106,107). The type, content, distribution, structure, and morphology of the antimicrobial agent, as well as the degree of cross-linking, molecular weight, crystallinity, hydrolysis, and porosity of the PVA matrix, all affect the release factors of PVA-based antimicrobial packaging materials. Figure 3 shows the release of antibacterial agents in the packaging system.
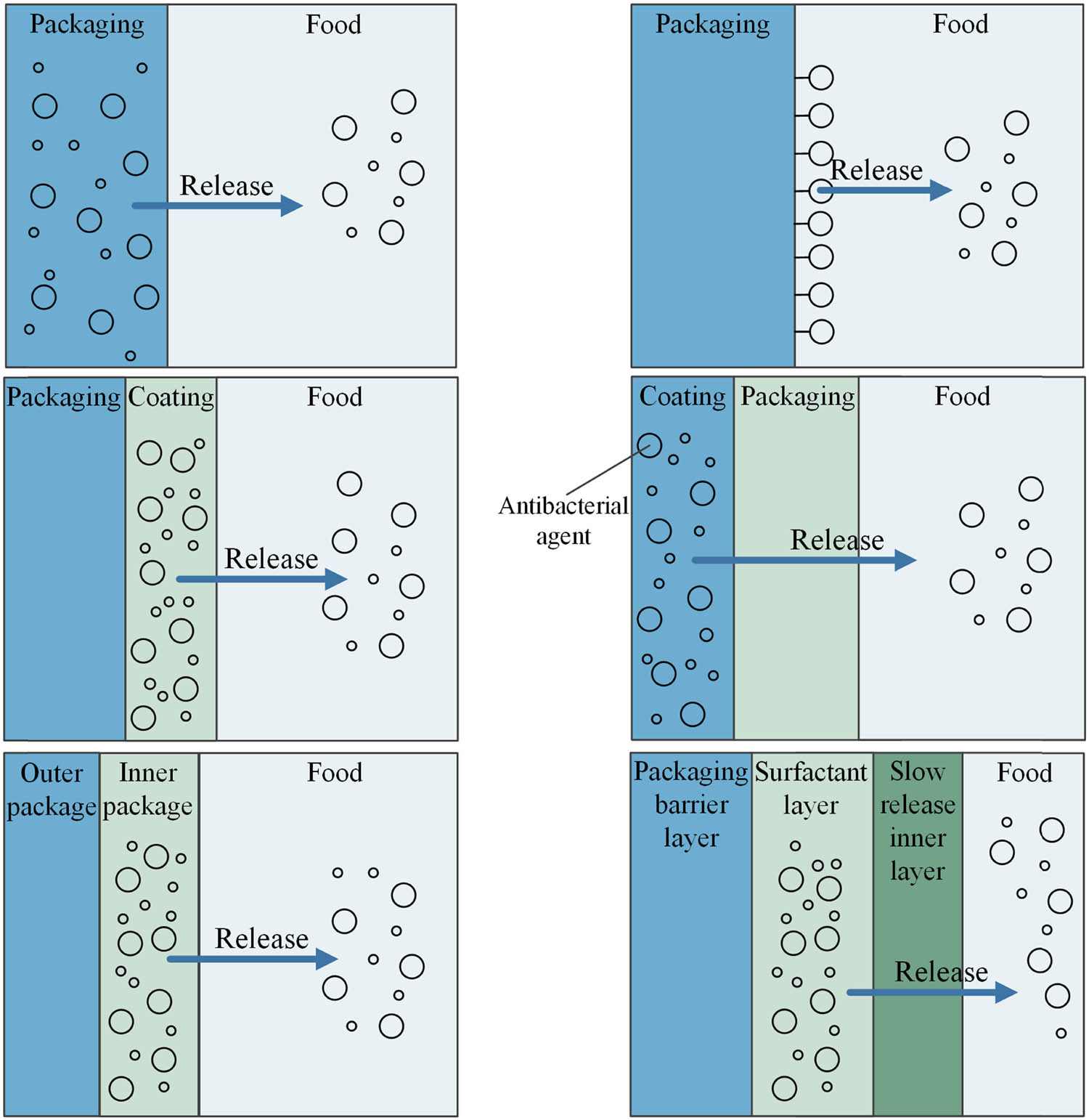
Release of antibacterial agents in the packaging system.
5.1 Release factors
By altering the environmental conditions such as temperature and humidity or by adjusting the concentration of antimicrobial agents, researchers have changed the release ability of antimicrobial agents. Figure 4 shows the release of antibacterial agents in the packaging system. For illustration, the release process of thymol from PVA-based films was investigated at different temperatures (4°C, 25°C, and 40°C) using thymol as an antimicrobial agent (108). The release process of CEO at three different temperatures made it clear that the release rate of CEO increases proportionally with the increase in temperature and relative humidity (109). Nisin was added to PVA/AHSG films and its release rate in 50% ethanol food simulants was regulated by varying the concentration or temperature (4°C, 25°C, and 40°C) and time of nisin release, and the results showed that the migration of nisin from PVA/AHSG films to food simulants accelerated with the increasing temperature (110). When the release of sodium lactate from active antimicrobial packaging with PVA and CS as substrates was also explored, its diffusion from the membrane was found to be influenced by the pH and ionic strength of the aqueous solution (51).
In another study (95), nisin in the PVA/CS film material increased from 0% to 10%, the relative concentration of S. aureus decreased sharply from 100% to 11.65%, and then gradually decreased. With the increase in nisin content in the film, the release of nisin in the bacterial suspension increased, thus effectively inhibiting the growth of S. aureus. The increase in nisin content had no significant effect on the antimicrobial activity when the nisin content was higher than 10%. The release rate during the release of NAC was proportional to the square of the amount of NAC in the membrane at each moment (111). Other researchers (71) investigated the release process of ZnO nanoparticles in CMC/PVA films, and the release of nanoparticles in the simulants ethanol and acetic acid was related to the concentration of Zn2+ in the film. The PVA/MMT/potassium sorbate active packaging materials were prepared by flow casting method, and it was found that adjusting the MMT concentration changed the release effect of potassium sorbate in fatty food simulants. The release process of OEO and CEO in 10% ethanol and 50% ethanol food simulants within PVA composite films at room temperature was found to be higher than OEO release for the same concentration of CEO (83). Antimicrobial food packaging films were synthesized by mixing PVA, starch, and glycerol with embedded CuO and ZnO nanoparticles, and the release of Cu2+ and Zn2+ metal ions from the films with embedded nanoparticles was explored (38).
Parameters such as pH, temperature, and antimicrobial agent concentration affect the antimicrobial activity of the film, and each of these parameters has a strong influence on the bioactivity, compatibility, and stability of the antimicrobial agent and its rate of release from the film matrix. Regulation of the release of antimicrobial agents from packaging films under different circumstances is therefore necessary.
5.2 Release kinetics
Modeling the release of antimicrobial agents is useful to further explore and predict the release behavior of antimicrobial agents in film materials and to evaluate the diffusion ability and distribution behavior of antimicrobial agents within the film materials to the food products inside, so as to optimize the antimicrobial packaging system and reduce the experimental cost. In the process of exploring the kinetics of antimicrobial agent release, the commonly used model in PVA substrates is the Fickian model (112).
The expression of Fickian’s second law is as follows:
where D P is the diffusion coefficient of antimicrobial agent in the packaging material (cm2·s−1); C is the concentration of active substance (mg·cm−3); l is the distance in the direction of release (cm); and t is the active substance release time (s).
The limited packaging-limited food system considers a limited volume of packaging and a limited volume of food. At this time
where M F,t is the amount of active substance released into the food/simulation fluid at time t, mg; M F,∞ is the amount of active substance released into the food/simulation fluid at equilibrium, mg; q n is the non-zero positive root of the equation tan q n = −αq n
The diffusion coefficient (D P) during the release of potassium sorbate to 95% ethanol in PVA/MMT films ranged from 0.08 × 10–11 to 30.3 × 10–11 cm2·s−1 when the ambient temperature was increased from 4°C to 60°C (50). The D P of lactobacillus peptides in membranes was determined by adding lactobacillus peptides to PVA/AHSG films, and the D P increased from 2.04 × 10–13 cm2·s−1 to 1.51 × 10–12 cm2·s−1 as the temperature increased from 4°C to 40°C (110). Fickian equation was used to describe the D P of sodium lactate in PVA and CS-based active antimicrobial packaging in aqueous solutions with different pH and NaCl concentrations, and it was found that D P increased with the increase in pH, while higher NaCl concentrations inhibited the movement of ions, especially at pH 7 and NaCl concentration of 0, where D P was maximum at 12.52 × 10–7 cm2·s−1, while the D P is minimal at a NaCl concentration of 0.10, 2.47 × 10–7 cm2·s−1 (51). In kinetic studies of silver ion release from starch/PVA films into polar food simulants distilled water, ethanol, and acetic acid, the D P of silver ions was 3.37 × 10–11 cm2·s−1 in distilled water, 3.2 × 10–11 cm2·s−1 in ethanol, and 2.88 × 10–11 cm2·s−1 in acetic acid (113). A study to investigate the release kinetics of rosemary polyphenols from PVA electrospun nanofibers in three food simulants revealed that the D P of polyphenols showed similar results in hydrophilic and acidic media (114). The diffusion rate of polyphenols was similar in the PVA base layer, while the D P in the lipophilic simulant was the largest at 1.37 × 10–14 cm2·s−1 containing 4% tea polyphenols in a polypropylene/PVA/polypropylene multilayer active film, the D P increased from 2.06 × 10–11 to 8.06 × 10–11 cm2·s−1 with the increase in the film pore size in 50% ethanol (115). Table 2 summarizes the release effects and diffusion coefficients of some different antimicrobial agents in food simulants.
Release effect and diffusion coefficient of PVA-based packages of different antibacterial agents
| Membrane substrate | Antimicrobial agents | Food/food simulator | Condition | D P (cm2·s−1) | Ref. |
|---|---|---|---|---|---|
| PVA | Potassium sorbate | 95% ethanol | 4–60°C | 0.08 × 10–11–30.3 × 10–11 | (50) |
| PVA/CS (1:3) | Nisin | Distilled water (pH 6.5) | 5–45°C | 8.1 × 10–9–2.7 × 10–8 | (95) |
| PVA/CS (1:2) | Nisin | Distilled water (pH 6.5) | 5–45°C | 4.3 × 10–9–1.9 × 10–8 | (95) |
| PVA/CS (1:1) | Nisin | Distilled water (pH 6.5) | 5–45°C | 2.5 × 10–9–1.6 × 10–8 | (95) |
| PVA | Rosemary polyphenols | 10% ethanol, 3% acetic acid, 50% ethanol | 6.83 × 10–16, 6.7 × 10–16, 1.37 × 10−14 | (114) | |
| PVA/polypropylene | Tea polyphenols | 50% ethanol | 25°C | 2.06 × 10–11–8.06 × 10–11 | (115) |
6 Application in food packaging
6.1 Application in fruits and vegetables packaging
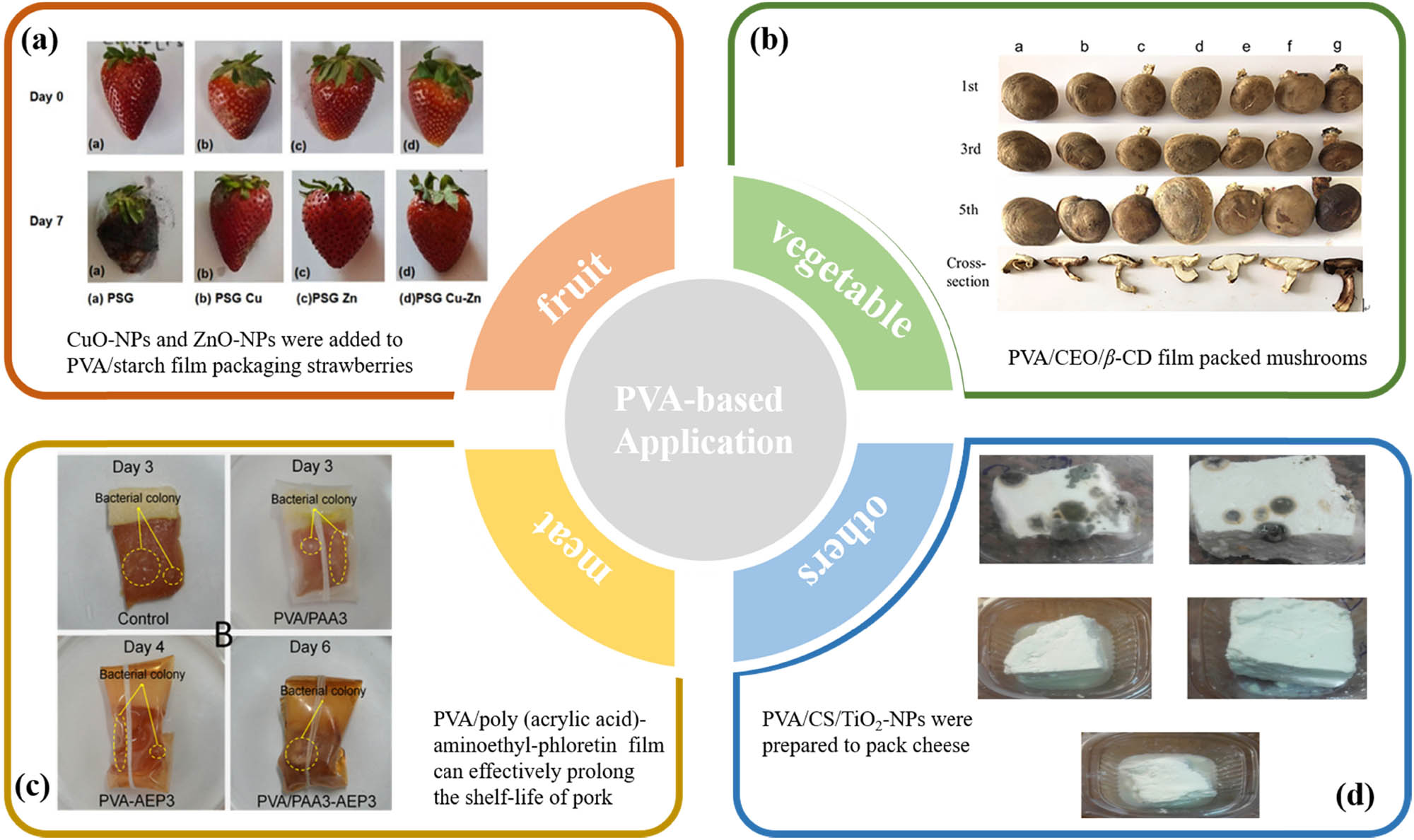
Antimicrobial films or coatings formulated by adding antimicrobial agents to PVA substrates were used to package fruits and vegetables to elongate the shelf life of strawberries (10,23,38,116,117), cherry tomato (59,118), bananas (17), grapes (40), lemons (21), mushrooms (119), etc. According to Wen et al. (10), PVA/β-cyclodextrin/CEO nanofiber films used for strawberry preservation not only increased the shelf life of strawberries but also preserved their color and look while being stored. Similar to this, strawberries that were wrapped in PVA/starch films with CuO and ZnO nanoparticles demonstrated improved shelf life and kept their fruit’s nutritional value (38). As reported by Shao et al. (117), PVA/permutite fibrous/CEO electrostatic spinning films increased the shelf-life of packaged strawberries.
An active nanocomposite coating made by co-blending PVA, agar, and maltodextrin, along with AgNPs, sprayed onto the surface of bananas, significantly extended the shelf life of bananas (17). The novel antimicrobial agent CuO@ZIF-8 was introduced into a composite bilayer film with poly (lactic acid) and PVA-quaternary ammonium CS as the outer and inner substrates, and the bilayer film had good antimicrobial activity and the composite film inhibited the growth of harmful microorganisms on the surface of sainfoin fruits (54). It was also found that the addition of Cu2O@NCs prolonged the shelf life of sainfoin fruits (59). When compared to PE cling film, PVA/CEO/β-cyclodextrin electrostatically spun nanofiber films improved the hardness and retained a better color for the mushrooms, but the rate of weight loss was slower (119).
6.2 Application in fresh meat packaging
Microbial contamination and protein oxidation are the main problems in food safety and quality deterioration of fresh meat products. The packaging materials and technologies have a significant impact on the meat quality and safety characteristics. Therefore, it is highly justified to conducting further research in this area. PVA-based films or coatings prepared by adding antimicrobial agents were used for packing chicken (120), pork (121), beef (11), lamb, fish (122), and shrimp (107) to the extension of their shelf life.
Active food packaging materials containing essential oils were prepared by electrostatic spinning from two commonly used spices (laurel and iteranum Laurus nobilis and Rosmarinus officinalis) and applied to chicken breasts, which improved the shelf life, decreased lipid oxidation, and reduced the number of L. monocytogenes during refrigeration (20). In a study, the combination of 4-HR and mBNC in the PVA/CS composite film was found to effectively suppress spoilage bacteria in vacuum-packed chilled raw beef (9). This innovative approach holds promise for enhancing meat preservation and preventing bacterial growth, thus contributing to the extension of its shelf life (11).
Changes induced by rose petal extract (RPE) have been effectively studied in films using PVA/CMC, the study indicated that the total bacterial count, pH, total volatile alkaline nitrogen, and thiobarbituric acid reactive substances of fish were higher in PVA/CMC composite film compared to the RPE film. This finding suggests that the RPE film exhibits better performance in terms of controlling bacterial growth and maintaining the quality of the fish by reducing the accumulation of volatile alkaline nitrogen and thiobarbituric acid reactive substances (122). Antimicrobial food packaging films prepared from PVA/polyacrylic acid combined with aminoethyl radicicicin aminoethyl-phloretin showed significant inhibition of L. monocytogenes and S. aureus and potently extended the shelf life of pork by 4 days at 25°C (121).
Proanthocyanidins (PC) as an active ingredient have also exhibited potent antibacterial properties in food packaging materials. However, it is important to note that PC is susceptible to acid hydrolysis. The CS-graft-PVA film containing PC was used for the preservation of salmon muscle and showed the potential ability to prevent microbial contamination and texture deterioration within 10 days. It is suggested that CS-grafted PVA films had good prospects for future food packaging applications (123).
6.3 Application in other types of food packaging
The physical appearance of bread packed with this bio-nanocomposite film was significantly improved when ZnO-SiO2 were utilized in PVA/CS nanocomposites (72). The presence of anthocyanins and limonene was added to the composite of PVA/potato starch, which showed good color development and antibacterial activity against pasteurized milk (88). In other study, the effect of TiO2 on PVA/CS film antimicrobial properties were investigated. The results showed that the weight loss of the cheese was reduced, the sensory properties was enhanced, and the shelf life was extended due to the nanomaterials during a storage period of 25 days. Among the different compositions tested, the composite containing 3% TiO2NPs exhibited the highest effectiveness in terms of preserving the quality and prolonging the shelf life of the cheese (12). When PVA/20% sorbitol film-forming solution containing Salmonella enterica phage PBSE191 was applied to the surface of eggshells, the PVA coating containing PBSE191 on the eggshell surface showed significant anti-Salmonella activity within 24 h (124).
Figure 4 presents the application of PVA-based food package film in different fields. Table 3 summarizes the antimicrobial activity of PVA-based packages of different antibacterial agents.
Antibacterial activity of PVA-based packages of different antibacterial agents
| Membrane substrate | Antibacterial agent | Preparation method | Food test | Testing duration | Effect | Ref. |
|---|---|---|---|---|---|---|
| PVA/carboxymethyl CS | CA | Solvent casting | Strawberries, cherry tomatoes | 5 days, 33 days | Delayed the water loss, reduced the growth of bacteria, and extended the shelf life | (36) |
| PVA | CEO | Electrospinning | Strawberries | 18 days | Higher hardness value, the highest sensory score, no discernible impact on strawberry flavor, extend the shelf life of strawberry | (10) |
| PVA | Nano-silver | Solvent casting | Grape | 10 days | Lighter moisture loss and decay rate, significantly mass loss reduction, and lowest loss of VC content | (40) |
| PVA/MMT | AgNPs | Solvent casting | Chicken sausages | 4 days | Less bacterial growth | (64) |
| PVA | Laurus nobilis–LEO, Rosmarinus officinalis-REO-) | Electrospinning | Chicken breast fillets | 7 days | The pH of the packaged samples did not change significantly or even decrease slightly during storage | (20) |
| PVA/agar/maltodextrin | AgNPs | Coating | Banana | 5 days | Decreased the respiration rate in bananas and delayed their ripening, reduction in the weight loss, acidity loss, pH, total soluble solids, and softening of bananas with the coating during storage period | (17) |
| PVA/CS | TiO2 | Coating | Karish cheese | 25 days | The rate of weight loss was lower, and the acidity of the cheeses increased significantly and the pH decreased during storage | (64) |
| PVA/β-cyclodextrin | CEO | Electrospun | Mushroom | 5 days | Reached a minimum weight loss, after 5 days mushrooms were intact and edible | (119) |
6.4 Degradation of the PVA-based films
PVA is a water-soluble synthetic polymer that can degrade under various environmental conditions. The dominant factor is the water solution of the film in the initial stage of degradation, the phenomenon might result from the hydrophily of the main component, then the moisture in the soil comes in contact with the surface of films which results in the solution, swelling, and the further osmosis of moisture into the polymer network. As PVA chains break, smaller, water-soluble PVA fragments are formed. These fragments dissolve in water. Microorganisms absorb these water-soluble fragments and use them as a carbon and energy source for growth and metabolism. As long as suitable conditions exist, microorganisms continue producing PVA enzymes and breaking down PVA, allowing them to grow and reproduce. The rate and efficiency of degradation depend on factors like the type of microorganisms, environmental conditions, and the specific PVA material (125).
The PVA/starch/glycerol/HNT films become less degradable and less transparent as the HNT content goes up from 0 to 5 wt% as a biodegradable material (46). PVA/XG composite films can be fully decomposed in soil and water within 12 h, much faster than regular plastic bags and other biodegradable materials (48). This makes it a promising option for eco-friendly, renewable, and sustainable food packaging, potentially replacing traditional plastic bags.
7 Conclusion and prospect
This review summarizes the results of recently published studies on categories of antimicrobial agents, functional and physical properties enhancement of PVA-based films. We discussed the advantages and challenges of some strategies used to improve the performance of PVA-based films, including mechanical properties, water resistance, UV-barrier properties, and antibacterial effect. It also presented the release behavior of antimicrobial agents in PVA-based film materials and their practical applications in the food industry. We also considered the issue of toxicity with the addition of inorganic antimicrobial agents, and discussed the biodegradability of PVA, with a view to producing highly effective antimicrobial, biodegradable PVA-based films with excellent properties in the future.
PVA-based films have an important role to play in the development of more environmentally-friendly, economical, and sustainable food packaging materials in the future. The current limitations are the low water resistance and rapid multiplication of different bacteria in a humid environment of PVA as food packaging films. In order to solve this problem, a variety of antimicrobial agents such as organic acids and salts, plant essential oils or their extracts, and inorganic metals have been used in PVA packaging films, and numerous studies have confirmed the feasibility of PVA-based antimicrobial films for use in the food industry. However, several challenges need to be addressed. First, antimicrobial agents in the PVA substrate dispersion homogeneity, stability, and compatibility issues should be investigated further in order to prepare outstanding mechanical characteristics and antibacterial impact of food packaging. In order to enhance the mechanical properties and antibacterial effectiveness of food packaging, further investigation is needed into the homogeneity, stability, and compatibility of antimicrobial agents in the PVA substrate dispersion. Second, the stability and rate of release of antimicrobial agents from the PVA packing film material, as well as their concentration on the food surface, are important determinants in the performance of their antibacterial activities. Further research should be conducted to develop a set of standard protocols for evaluating the safety of antimicrobial compounds in order to ensure food quality, safety, and sensory qualities.
-
Funding information: This work was supported by the Scientific Research Fund of Hunan Provincial Education Department (23A0415), Hunan Provincial Natural Science Foundation (2024JJ7159), Science and Technology Innovation Program of Hunan Province (2021RC4065) and Key Research and Development Program of Hunan Province (2022GK2037).
-
Author contributions: Jingxian Jiang: writing – original draft, writing – review & editing, methodology, formal Analysis; Wenxi Yu: writing – review and editing, conceptualization, and funding acquisition; Weiran Zhang: writing – review and editing and funding acquisition. Xijian Yi: supervision; Qin Lei: project; Yuru Liao: project administration.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data are available only upon request to the authors.
References
(1) Bhargava N, Sharanagat VS, Mor RS, Kumar K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci Technol. 2020;105:385–401. 10.1016/j.tifs.2020.09.015.Search in Google Scholar
(2) Topuz F, Uyar T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res Int. 2020;130:108927. 10.1016/j.foodres.2019.108927.Search in Google Scholar PubMed
(3) Lavoine N, Desloges I, Manship B, Bras J. Antibacterial paperboard packaging using microfibrillated cellulose. J Food Sci Technol. 2015;52(9):5590–600. 10.1007/s13197-014-1675-1.Search in Google Scholar PubMed PubMed Central
(4) Min T, Sun X, Zhou L, Du H, Zhu Z, Wen Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal-organic framework for antibacterial food packaging. Carbohydr Polym. 2021;270:118391. 10.1016/j.carbpol.2021.118391.Search in Google Scholar PubMed
(5) Suganthi S, Vignesh S, Kalyana Sundar J, Raj V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl Water Sci. 2020;10(4):100. 10.1007/s13201-020-1162-y.Search in Google Scholar
(6) Oun AA, Shin GH, Rhim JW, Kim JT. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag Shelf Life. 2022;34:100991. 10.1016/j.fpsl.2022.100991.Search in Google Scholar
(7) Yang D, Liu Q, Gao Y, Wan S, Meng F, Weng W, Zhang Y. Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. Food Hydrocoll. 2023;136:108305. 10.1016/j.foodhyd.2022.108305.Search in Google Scholar
(8) Demerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41(3):319–26. 10.1016/S0278-6915(2)00258-2.Search in Google Scholar
(9) Gautam L, Warkar SG, Ahmad SI, Kant R, Jain M. A review on carboxylic acid cross‐linked polyvinyl alcohol: Properties and applications. Polym Eng Sci. 2021;62(2):225–46. 10.1002/pen.25849.Search in Google Scholar
(10) Wen P, Zhu D-H, Wu H, Zong M-H, Jing Y-R, Han S-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control. 2016;59:366–76. 10.1016/j.foodcont.2015.06.005.Search in Google Scholar
(11) Choo KW, Dhital R, Mao L, Lin M, Mustapha A. Development of polyvinyl alcohol/chitosan/modified bacterial nanocellulose films incorporated with 4-hexylresorcinol for food packaging applications. Food Packag Shelf Life. 2021;30:100769. 10.1016/j.fpsl.2021.100769.Search in Google Scholar
(12) Youssef AM, El-Sayed SM, El-Sayed HS, Salama HH, Assem FM, Abd El-Salam MH. Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish). Int J Biol Macromol. 2018;115:1002–11. 10.1016/j.ijbiomac.2018.04.165.Search in Google Scholar PubMed
(13) Song M, Yu H, Gu J, Ye S, Zhou Y. Chemical cross-linked polyvinyl alcohol/cellulose nanocrystal composite films with high structural stability by spraying Fenton reagent as initiator. Int J Biol Macromol. 2018;113:171–8. 10.1016/j.ijbiomac.2018.02.117.Search in Google Scholar PubMed
(14) Navarchian AH, Jalalian M, Pirooz M. Characterization of starch/poly(vinyl alcohol)/clay nanocomposite films prepared in twin-screw extruder for food packaging application. J Plast Film Sheet. 2015;31(3):309–36. 10.1177/8756087914568904.Search in Google Scholar
(15) Wang W, Zhang H, Jia R, Dai Y, Dong H, Hou H, et al. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018;79:534–43. 10.1016/j.foodhyd.2017.12.013.Search in Google Scholar
(16) Ebrahimzadeh S, Bari MR, Hamishehkar H, Kafil HS, Lim LT. Essential oils-loaded electrospun chitosan-poly(vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. Lwt. 2021;144:111217. 10.1016/j.lwt.2021.111217.Search in Google Scholar
(17) Nguyen TT, Huynh Nguyen TT, Tran Pham BT, Van Tran T, Bach LG, Bui Thi PQ, et al. Development of poly (vinyl alcohol)/agar/maltodextrin coating containing silver nanoparticles for banana (Musa acuminate) preservation. Food Packag Shelf Life. 2021;29:100740. 10.1016/j.fpsl.2021.100740.Search in Google Scholar
(18) Hou Q, Wang X, Ragauskas AJ. Preparation and characterization of nanocellulose–polyvinyl alcohol multilayer film by layer-by-layer method. Cellulose. 2019;26(8):4787–98. 10.1007/s10570-019-02413-0.Search in Google Scholar
(19) Thanyacharoen T, Chuysinuan P, Techasakul S, Nooeaid P, Ummartyotin S. Development of a gallic acid-loaded chitosan and polyvinyl alcohol hydrogel composite: Release characteristics and antioxidant activity. Int J Biol Macromol. 2018;107(Pt A):363–70. 10.1016/j.ijbiomac.2017.09.002.Search in Google Scholar PubMed
(20) Göksen G, Fabra MJ, Pérez-Cataluña A, Ekiz HI, Sanchez G, López-Rubio A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag Shelf Life. 2021;27:100613. 10.1016/j.fpsl.2020.100613.Search in Google Scholar
(21) Kowsalya E, MosaChristas K, Balashanmugam P, Tamil Selvi A. Biocompatible silver nanoparticles/poly(vinyl alcohol) electrospun nanofibers for potential antimicrobial food packaging applications. Food Packag Shelf Life. 2019;21:100379. 10.1016/j.fpsl.2019.100379.Search in Google Scholar
(22) Mishra P, Gupta P, Pruthi V. Cinnamaldehyde incorporated gellan/PVA electrospun nanofibers for eradicating Candida biofilm. Mater Sci Eng C Mater Biol Appl. 2021;119:111450. 10.1016/j.msec.2020.111450.Search in Google Scholar PubMed
(23) Moreira BR, Pereira-Júnior MA, Fernandes KF, Batista KA. An ecofriendly edible coating using cashew gum polysaccharide and polyvinyl alcohol. Food Biosci. 2020;37:100722. 10.1016/j.fbio.2020.100722.Search in Google Scholar
(24) Hu W, Zou Z, Li H, Zhang Z, Yu J, Tang Q. Fabrication of highly transparent and multifunctional polyvinyl alcohol/starch based nanocomposite films using zinc oxide nanoparticles as compatibilizers. Int J Biol Macromol. 2022;204:284–92. 10.1016/j.ijbiomac.2022.02.020.Search in Google Scholar PubMed
(25) Tian X, Chen Z, Lu X, Mu J, Ma Q, Li X. Soy protein/polyvinyl-alcohol (PVA)-based packaging films reinforced by nano-TiO2. Polymers (Basel). 2023;15(7):1764. 10.3390/polym15071764.Search in Google Scholar PubMed PubMed Central
(26) Allafchian A, Hosseini H, Ghoreishi SM. Electrospinning of PVA-carboxymethyl cellulose nanofibers for flufenamic acid drug delivery. Int J Biol Macromol. 2020;163:1780–6. 10.1016/j.ijbiomac.2020.09.129.Search in Google Scholar PubMed
(27) Kharazmi F, Hosseini FS, Ebrahimzadeh H. Polyvinyl alcohol/citric acid/beta-cyclodextrin/CuONP composite nanofibers as an effective and green absorbent for the simultaneous extraction of three antidepressant drugs in biological fluids prior to GC-FID analysis. Mikrochim Acta. 2023;190(6):218. 10.1007/s00604-023-05800-4.Search in Google Scholar PubMed
(28) Yang W, Qi G, Kenny JM, Puglia D, Ma P. Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers (Basel). 2020;12(6):1364. 10.3390/polym12061364.Search in Google Scholar PubMed PubMed Central
(29) Woo JH, Kim NH, Kim SI, Park O-K, Lee JH. Effects of the addition of boric acid on the physical properties of MXene/polyvinyl alcohol (PVA) nanocomposite. Compos Part B: Eng. 2020;199:108205. 10.1016/j.compositesb.2020.108205.Search in Google Scholar
(30) Panigrahi D, Kumar S, Dhar A. Modulating chain conformations of polyvinyl alcohol through low cost and nontoxic glyoxal crosslinker: Application in high performance organic transistors. Org Electron. 2019;65:193–200. 10.1016/j.orgel.2018.11.017.Search in Google Scholar
(31) He L, Lan W, Ahmed S, Qin W, Liu Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag Shelf Life. 2019;22:100390. 10.1016/j.fpsl.2019.100390.Search in Google Scholar
(32) Zhang R, Wang Y, Ma D, Ahmed S, Qin W, Liu Y. Effects of ultrasonication duration and graphene oxide and nano-zinc oxide contents on the properties of polyvinyl alcohol nanocomposites. Ultrason Sonochem. 2019;59:104731. 10.1016/j.ultsonch.2019.104731.Search in Google Scholar PubMed
(33) Wu F, Zhou Z, Li N, Chen Y, Zhong L, Law WC, et al. Development of poly(vinyl alcohol)/starch/ethyl lauroyl arginate blend films with enhanced antimicrobial and physical properties for active packaging. Int J Biol Macromol. 2021;192:389–97. 10.1016/j.ijbiomac.2021.09.208.Search in Google Scholar PubMed
(34) Haghighi H, Leugoue SK, Pfeifer F, Siesler HW, Licciardello F, Fava P, et al. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020;100:105419. 10.1016/j.foodhyd.2019.105419.Search in Google Scholar
(35) Jiang S, Yang FX, Zhang Y, Huang ZY, Ou LJ, Yang H. Study on properties of calcium propionate modified polyvinyl alcohol packaging films (in Chinese). Food Ind Sci Technol. 2015;36(2):308–12.Search in Google Scholar
(36) Wen L, Liang Y, Lin Z, Xie D, Zheng Z, Xu C, et al. Design of multifunctional food packaging films based on carboxymethyl chitosan/polyvinyl alcohol crosslinked network by using citric acid as crosslinker. Polymer. 2021;230:124048. 10.1016/j.polymer.2021.124048.Search in Google Scholar
(37) Gowsia I, Mir FA, Banday JA. Preparation and characterization of polyvinyl alcohol-piperic acid composite film for potential food packaging applications. Prog Biomater. 2022;11(3):281–95. 10.1007/s40204-022-00195-6.Search in Google Scholar PubMed PubMed Central
(38) Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel). 2010;1(3):413–26. 10.3390/genes1030413.Search in Google Scholar PubMed PubMed Central
(39) Andrade J, González-Martínez C, Chiralt A. Physical and active properties of poly (vinyl alcohol) films with phenolic acids as affected by the processing method. Food Packag Shelf Life. 2022;33:100855. 10.1016/j.fpsl.2022.100855.Search in Google Scholar
(40) Deng J, Chen QJ, Peng ZY, Wang JH, Li W, Ding QM, et al. Nano-silver-containing polyvinyl alcohol composite film for grape fresh-keeping. Mater Express. 2019;9(9):985–92. 10.1166/mex.2019.1592.Search in Google Scholar
(41) Lian Z, Zhang Y, Zhao Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov Food Sci Emerg. 2016;33:145–53. 10.1016/j.ifset.2015.10.008.Search in Google Scholar
(42) Srikhao N, Kasemsiri P, Ounkaew A, Lorwanishpaisarn N, Okhawilai M, Pongsa U, et al. Bioactive nanocomposite film based on cassava starch/polyvinyl alcohol containing green synthesized silver nanoparticles. J Polym Environ. 2020;29(2):672–84. 10.1007/s10924-020-01909-2.Search in Google Scholar
(43) Zhang Y, Yang F, Jiang S, Yang H, Li N, Guan Y. Study on the performance of citric acid/PVA antibacterial films (in Chinese). Packag Eng. 2014;35(21):10–4. 10.19554/j.cnki.1001-3563.2014.21.003.Search in Google Scholar
(44) Chen C, Xu Z, Ma Y, Liu J, Zhang Q, Tang Z, et al. Properties, vapour-phase antimicrobial and antioxidant activities of active poly(vinyl alcohol) packaging films incorporated with clove oil. Food Control. 2018;88:105–12. 10.1016/j.foodcont.2017.12.039.Search in Google Scholar
(45) Ulaganathan RK, Senusi NAM, Amin MAM, Razab M, Ismardi A, Abdullah NH, editors. Effect of cellulose nanocrystals (CNC) on PVA/CNC bio-nanocomposite film as potential food packaging application. 14th AUN/SEED-Net Regional Conference on Materials (RCM)/4th International Postgraduate Conference on Materials, Minerals and Polymer (MAMIP); 2021 Dec 01-02. Electr Network 2022.Search in Google Scholar
(46) Abdullah ZW, Dong Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front Mater. 2019;6:58. 10.3389/fmats.2019.00058.Search in Google Scholar
(47) Zamanian M, Sadrnia H, Khojastehpour M, Hosseini F, Kruczek B, Thibault J. Barrier properties of PVA/TiO2/MMT mixed-matrix membranes for food packaging. J Polym Environ. 2021;29(5):1396–411. 10.1007/s10924-020-01965-8.Search in Google Scholar
(48) Chen J, Zheng M, Tan KB, Lin J, Chen M, Zhu Y. Polyvinyl alcohol/xanthan gum composite film with excellent food packaging, storage and biodegradation capability as potential environmentally-friendly alternative to commercial plastic bag. Int J Biol Macromol. 2022;212:402–11. 10.1016/j.ijbiomac.2022.05.119.Search in Google Scholar PubMed
(49) Luo SY, Zhao JY, Zhang XL, Xu WC. Research development of antibacterial plastic packaging. Adv Mater Res. 2011;380:226–9. 10.4028/www.scientific.net/AMR.380.226.Search in Google Scholar
(50) Yu WX, Yan J, Li Z, Lu L, Chen Y, Chen J, et al. Preparation and release of polyvinyl alcohol/montmorillonite/potassium sorbate activated packaging films (in Chinese). Packag Eng. 2022;43(5):1–7. 10.19554/j.cnki.1001-3563.2022.05.001.Search in Google Scholar
(51) Zhang L, Wang H, Jin C, Zhang R, Li L, Li X, et al. Sodium lactate loaded chitosan-polyvinyl alcohol/montmorillonite composite film towards active food packaging. Innov Food Sci Emerg. 2017;42:101–8. 10.1016/j.ifset.2017.06.007.Search in Google Scholar
(52) Narayanan V, Alam M, Ahmad N, Balakrishnan SB, Ganesan V, Shanmugasundaram E, et al. Electrospun poly (vinyl alcohol) nanofibers incorporating caffeic acid/cyclodextrins through the supramolecular assembly for antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2021;249:119308. 10.1016/j.saa.2020.119308.Search in Google Scholar PubMed
(53) Ma Y, Li J, Si Y, Huang K, Nitin N, Sun G. Rechargeable antibacterial N-halamine films with antifouling function for food packaging applications. ACS Appl Mater Interfaces. 2019;11(19):17814–22. 10.1021/acsami.9b03464.Search in Google Scholar PubMed
(54) Fu D, Ding Y, Guo R, Zhang J, Wang H, Niu B, et al. Polylactic acid/polyvinyl alcohol-quaternary ammonium chitosan double-layer films doped with novel antimicrobial agent CuO@ZIF-8 NPs for fruit preservation. Int J Biol Macromol. 2022;195:538–46. 10.1016/j.ijbiomac.2021.12.022.Search in Google Scholar PubMed
(55) Arfat YA, Ejaz M, Jacob H, Ahmed J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr Polym. 2017;157:65–71. 10.1016/j.carbpol.2016.09.069.Search in Google Scholar PubMed
(56) Pica A, Guran C, Andronescu E, Oprea O, Ficai D, Ficai A. Antimicrobial performances of some film forming materials based on silver nanoparticles. J Optoelectron Adv Mater. 2012;14(9/10):863–8. 10.1007/s00340-012-5160-0.Search in Google Scholar
(57) Siripatrawan U, Kaewklin P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018;84:125–34. 10.1016/j.foodhyd.2018.04.049.Search in Google Scholar
(58) Vasile BS, Oprea O, Voicu G, Ficai A, Andronescu E, Teodorescu A, et al. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin-chitosan composite with potential applications in wounds care. Int J Pharm. 2014;463(2):161–9. 10.1016/j.ijpharm.2013.11.035.Search in Google Scholar PubMed
(59) Yan J, Li M, Wang H, Lian X, Fan Y, Xie Z, et al. Preparation and property studies of chitosan-PVA biodegradable antibacterial multilayer films doped with Cu2O and nano-chitosan composites. Food Control. 2021;126:108049. 10.1016/j.foodcont.2021.108049.Search in Google Scholar
(60) Mathew S, Jayakumar A, Kumar VP, Mathew J, Radhakrishnan EK. One-step synthesis of eco-friendly boiled rice starch blended polyvinyl alcohol bionanocomposite films decorated with in situ generated silver nanoparticles for food packaging purpose. Int J Biol Macromol. 2019;139:475–85. 10.1016/j.ijbiomac.2019.07.187.Search in Google Scholar PubMed
(61) Yahia R, Owda ME, Abou‐Zeid RE, Abdelhai F, Gad ES, Saleh AK, et al. Synthesis and characterization of thermoplastic starch/PVA/cardanol oil composites loaded with in‐situ silver nanoparticles. J Appl Polym Sci. 2021;139(3):51511. 10.1002/app.51511.Search in Google Scholar
(62) Sarwar MS, Niazi MBK, Jahan Z, Ahmad T, Hussain A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr Polym. 2018;184:453–64. 10.1016/j.carbpol.2017.12.068.Search in Google Scholar PubMed
(63) Wang L, Periyasami G, Aldalbahi A, Fogliano V. The antimicrobial activity of silver nanoparticles biocomposite films depends on the silver ions release behaviour. Food Chem. 2021;359:129859. 10.1016/j.foodchem.2021.129859.Search in Google Scholar PubMed
(64) Mathew S, Snigdha S, Mathew J, Radhakrishnan E. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag Shelf Life. 2019;19:155–66. 10.1016/j.fpsl.2018.12.009.Search in Google Scholar
(65) Ernest Ravindran RS, Subha V, Ilangovan R. Silver nanoparticles blended PEG/PVA nanocomposites synthesis and characterization for food packaging. Arab J Chem. 2020;13(7):6056–60. 10.1016/j.arabjc.2020.05.005.Search in Google Scholar
(66) Desphande DS, Bajpai AK. Green synthesis of colloidal silver nanoparticles reinforced PVA-corn starch hydrogel films. American Institute of Physics Conference Series. 2100, AIP Publishing LLC AIP Publishing; 2019. p. 020160. 10.1063/1.5098714.Search in Google Scholar
(67) Cyriac V, Molakalu Padre S, Ismayil I, Sangam Chandrashekar G, Chavan C, Fakeerappa Bhajantri R, et al. Tuning the ionic conductivity of flexible polyvinyl alcohol/sodium bromide polymer electrolyte films by incorporating silver nanoparticles for energy storage device applications. J Appl Polym Sci. 2022;139(28):52525. 10.1002/app.52525.Search in Google Scholar
(68) Abdelhamid AE, Yousif EAA, El-Saidi MMT, El-Sayed AA. Polyvinyl alcohol food packaging system comprising green synthesized silver nanoparticles. Indones J Chem. 2020;21(2):350. 10.22146/ijc.55483.Search in Google Scholar
(69) Pandey VK, Upadhyay SN, Niranjan K, Mishra PK. Antimicrobial biodegradable chitosan-based composite nano-layers for food packaging. Int J Biol Macromol. 2020;157:212–9. 10.1016/j.ijbiomac.2020.04.149.Search in Google Scholar PubMed
(70) Fan L, Zhang H, Gao M, Zhang M, Liu P, Liu X. Cellulose nanocrystals/silver nanoparticles: In-situ preparation and application in PVA films. Holzforschung. 2020;74(5):523–8. 10.1515/hf-2018-0251.Search in Google Scholar
(71) Helmiyati H, Hidayat ZSZ, Sitanggang IFR, Liftyawati D. Antimicrobial packaging of ZnO–Nps infused into CMC–PVA nanocomposite films effectively enhances the physicochemical properties. Polym Test. 2021;104:107412. 10.1016/j.polymertesting.2021.107412.Search in Google Scholar
(72) Al-Tayyar NA, Youssef AM, Al-Hindi RR. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag Shelf Life. 2020;25:100523. 10.1016/j.fpsl.2020.100523.Search in Google Scholar
(73) Guo J, Zhang J, Wang JM, Zhu QS. Natural antibacterial agents and their application in food packaging: A review (in Chinese). Food Sci. 2021;42(9):336–46.Search in Google Scholar
(74) Wan Yahaya WA, Abu Yazid N, Mohd Azman NA, Almajano MP. Antioxidant activities and total phenolic content of Malaysian herbs as components of active packaging film in beef patties. Antioxid (Basel). 2019;8(7):204. 10.3390/antiox8070204.Search in Google Scholar PubMed PubMed Central
(75) Yu HH, Kim YJ, Park YJ, Shin DM, Choi YS, Lee NK, et al. Application of mixed natural preservatives to improve the quality of vacuum skin packaged beef during refrigerated storage. Meat Sci. 2020;169:108219. 10.1016/j.meatsci.2020.108219.Search in Google Scholar PubMed
(76) Huang T, Qian Y, Wei J, Zhou C. Polymeric antimicrobial food packaging and its applications. Polym (Basel). 2019;11(3):560. 10.3390/polym11030560.Search in Google Scholar PubMed PubMed Central
(77) Nguyen TT, Thi Dao UT, Thi Bui QP, Bach GL, Ha Thuc CN, Ha Thuc H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog Org Coat. 2020;140:105487. 10.1016/j.porgcoat.2019.105487.Search in Google Scholar
(78) Gingasu D, Mindru I, Patron L, Ianculescu A, Vasile E, Marinescu G, et al. Synthesis and characterization of chitosan-coated cobalt ferrite nanoparticles and their antimicrobial activity. J Inorg Organomet P. 2018;28(5):1932–41. 10.1007/s10904-018-0870-3.Search in Google Scholar
(79) Sofi SA, Singh J, Rafiq S, Ashraf U, Dar BN, Nayik GA. A comprehensive review on antimicrobial packaging and its use in food packaging. Curr Nutr Food Sci. 2018;14(4):305–12. 10.2174/1573401313666170609095732.Search in Google Scholar
(80) Ma Q, Ren Y, Wang L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017;70:286–92. 10.1016/j.foodhyd.2017.04.018.Search in Google Scholar
(81) Liu Q, Jing Y, Han C, Zhang H, Tian Y. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019;93:432–42. 10.1016/j.foodhyd.2019.02.003.Search in Google Scholar
(82) Lacatusu I, Badea N, Badea G, Brasoveanu L, Stan R, Ott C, et al. Ivy leaves extract based – lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities. RSC Adv. 2016;6(81):77243–55. 10.1039/c6ra12016d.Search in Google Scholar
(83) Oun AA, Shin GH, Kim JT. Multifunctional poly(vinyl alcohol) films using cellulose nanocrystals/oregano and cellulose nanocrystals/cinnamon Pickering emulsions: Effect of oil type and concentration. Int J Biol Macromol. 2022;194:736–45. 10.1016/j.ijbiomac.2021.11.119.Search in Google Scholar PubMed
(84) Jose A, Anitha Sasidharan S, Chacko C, Mukkumkal Jacob D, Edayileveettil Krishnankutty R. Activity of clove oil and chitosan nanoparticles incorporated PVA nanocomposite against Pythium aphanidermatum. Appl Biochem Biotechnol. 2022;194(4):1442–57. 10.1007/s12010-021-03709-3.Search in Google Scholar PubMed
(85) Liu G, Song H, Luo W, Tang X, Zhang Z, Zhu Z. Antimicrobial paper with thyme-clove basil essential oil for preservative and freshness preservation of strawberries (in Chinese). Packag Eng. 2018;39(19):91–7. 10.19554/j.cnki.1001-3563.2018.19.017.Search in Google Scholar
(86) Srisa A, Harnkarnsujarit N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020;333:127537. 10.1016/j.foodchem.2020.127537.Search in Google Scholar PubMed
(87) Chen C, Zong L, Wang J, Xie J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr Polym. 2021;272:118448. 10.1016/j.carbpol.2021.118448.Search in Google Scholar PubMed
(88) Liu B, Xu H, Zhao H, Liu W, Zhao L, Li Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr Polym. 2017;157:842–9. 10.1016/j.carbpol.2016.10.067.Search in Google Scholar PubMed
(89) Liu Y, Wang S, Lan W, Qin W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason Sonochem. 2019;51:386–94. 10.1016/j.ultsonch.2018.07.043.Search in Google Scholar PubMed
(90) Qin Y, Liu Y, Zhang X, Liu J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020;100:105410. 10.1016/j.foodhyd.2019.105410.Search in Google Scholar
(91) Luo J, Zuo D, Deng Z, Ji A, Xia G. Effects of heat treatment and tea polyphenols on the structure and properties of polyvinyl alcohol nanofiber films for food packaging. Coatings. 2020;10(1):49. 10.3390/coatings10010049.Search in Google Scholar
(92) Yang FL, Yao JR, Chen X, Shao ZZ. Preparation and antibacterial properties of polyvinyl alcohol/tannin blends (in Chinese). J Polym. 2012;2:125–30. 10.3724/SP.J.1105.2012.11092.Search in Google Scholar
(93) Singh S, Gaikwad KK, Lee YS. Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int J Biol Macromol. 2018;107(Pt B):1879–87. 10.1016/j.ijbiomac.2017.10.057.Search in Google Scholar PubMed
(94) Liu D, Dang S, Zhang L, Munsop K, Li X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int J Biol Macromol. 2021;188:974–82. 10.1016/j.ijbiomac.2021.08.080.Search in Google Scholar PubMed
(95) Wang H, Zhang R, Zhang H, Jiang S, Liu H, Sun M, et al. Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly(vinyl alcohol). Carbohydr Polym. 2015;127:64–71. 10.1016/j.carbpol.2015.03.058.Search in Google Scholar PubMed
(96) Marvdashti LM, Yavarmanesh M, Koocheki A. Controlled release of nisin from polyvinyl alcohol - Alyssum homolocarpum seed gum composite films: Nisin kinetics. Food Biosci. 2019;28:133–9. 10.1016/j.fbio.2019.01.010.Search in Google Scholar
(97) Jayakumar A, Radoor S, Nair IC, Siengchin S, Parameswaranpillai J, Radhakrishnan EK. Polyvinyl alcohol-nanocomposite films incorporated with clay nanoparticles and lipopeptides as active food wraps against food spoilage microbes. Food Packag Shelf Life. 2021;30:100727. 10.1016/j.fpsl.2021.100727.Search in Google Scholar
(98) Jiang S, Yang FX, Zhang Y, Huang ZY. Study on properties of modified antibacterial polyvinyl alcohol packaging film (in Chinese). Food Mach. 2014;30(3):114–7 + 136. cnki:sun:spjx.0.2014-03-035.Search in Google Scholar
(99) Feng K, Wen P, Yang H, Li N, Lou WY, Zong MH, et al. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017;7(3):1572–80. 10.1039/c6ra25977d.Search in Google Scholar
(100) Meng LW, Wang HJ, Liu W. Preparation and properties of corn starch-polyvinyl alcohol antibacterial packaging films (in Chinese). Agric Product Process. 2018;19:20–2. cnki:sun:ncpj.0.2018-19-007.Search in Google Scholar
(101) Dey D, Dharini V, Periyar Selvam S, Rotimi Sadiku E, Mahesh Kumar M, Jayaramudu J, et al. Physical, antifungal, and biodegradable properties of cellulose nanocrystals and chitosan nanoparticles for food packaging application. Mater Today: Proc. 2021;38:860–9. 10.1016/j.matpr.2020.04.885.Search in Google Scholar
(102) Zou P. Preparation and biodegradable kinetic evaluation of poria-polyvinyl alcohol antibacterial film (in Chinese). Packag Eng. 2022;43(11):7.Search in Google Scholar
(103) Shojaee Kang Sofla M, Mortazavi S, Seyfi J. Preparation and characterization of polyvinyl alcohol/chitosan blends plasticized and compatibilized by glycerol/polyethylene glycol. Carbohydr Polym. 2020;232:115784. 10.1016/j.carbpol.2019.115784.Search in Google Scholar PubMed
(104) Wu JY, Ooi CW, Song CP, Wang CY, Liu BL, Lin GY, et al. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr Polym. 2021;262:117910. 10.1016/j.carbpol.2021.117910.Search in Google Scholar PubMed
(105) Lei Y, Mao L, Yao J, Zhu H. Improved mechanical, antibacterial and UV barrier properties of catechol-functionalized chitosan/polyvinyl alcohol biodegradable composites for active food packaging. Carbohydr Polym. 2021;264:117997. 10.1016/j.carbpol.2021.117997.Search in Google Scholar PubMed
(106) Wang Q, Wang H, Zhang T, Hu Z, Xia L, Li L, et al. Antibacterial Activity of Polyvinyl Alcohol/WO3 Films Assisted by Near-Infrared Light and Its Application in Freshness Monitoring. J Agric Food Chem. 2021;69(3):1068–78. 10.1021/acs.jafc.0c06961.Search in Google Scholar PubMed
(107) Yang W, Ding H, Qi G, et al. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. Reactive Funct Polym. 2021;162:104873. 10.1016/j.reactfunctpolym.2021.104873.Search in Google Scholar
(108) Zhang XT, Li MY, Huang XL. Molecular dynamic simulation of thymol release from PVA-based antibacterial film under different temperature. 2016 International Conference on Computer Engineering and Information Systems; 2016. 10.2991/ceis-16.2016.62.Search in Google Scholar
(109) Lee JS, Choi I, Han J. Mathematical modeling of cinnamon (Cinnamomum verum) bark oil release from agar/PVA biocomposite film for antimicrobial food packaging: The effects of temperature and relative humidity. Food Chem. 2021;363:130306. 10.1016/j.foodchem.2021.130306.Search in Google Scholar PubMed
(110) Monjazeb Marvdashti L, Yavarmanesh M, Koocheki A. In vitro release study of nisin from active polyvinyl alcohol-Alyssum homolocarpum seed gum films at different temperatures. Polym Test. 2019;79:106032. 10.1016/j.polymertesting.2019.106032.Search in Google Scholar
(111) Palmieri V, Niccolini B, Perini G, Augello A, De Maio F, Gervasoni J, et al. In situ N-acetylcysteine release from polyvinyl alcohol film for moisture-activated food packaging. Food Packag Shelf Life. 2021;29:100694. 10.1016/j.fpsl.2021.100694.Search in Google Scholar
(112) Crank J. The mathematics of diffusion. The Clarendon Press; 1977. 10.1007/978-1-4613-4145-1_3.Search in Google Scholar
(113) Ounkaew A, Janaum N, Kasemsiri P, Okhawilai M, Hiziroglu S, Chindaprasirt P. Synergistic effect of starch/polyvinyl alcohol/citric acid films decorated with in-situ green-synthesized nano silver on bioactive packaging films. J Environ Chem Eng. 2021;9(6):106793. 10.1016/j.jece.2021.106793.Search in Google Scholar
(114) Estevez-Areco S, Guz L, Candal R, Goyanes S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag Shelf Life. 2018;18:42–50. 10.1016/j.fpsl.2018.08.006.Search in Google Scholar
(115) Chen C, Li C, Yang S, Zhang Q, Yang F, Tang Z, et al. Development of new multilayer active packaging films with controlled release property based on polypropylene/poly(vinyl alcohol)/polypropylene incorporated with tea polyphenols. J Food Sci. 2019;84(7):1836–43. 10.1111/1750-3841.14681.Search in Google Scholar PubMed
(116) Ventura-Aguilar RI, Díaz-Galindo EP, Bautista-Baños S, Mendoza-Acevedo S, Munguía-Cervantes JE, Correa-Pacheco ZN, et al. Monitoring the infection process of Rhizopus stolonifer on strawberry fruit during storage using films based on chitosan/polyvinyl alcohol/polyvinylpyrrolidone and plant extracts. Int J Biol Macromol. 2021;182:583–94. 10.1016/j.ijbiomac.2021.03.187.Search in Google Scholar PubMed
(117) Shao P, Yan Z, Chen H, Xiao J. Electrospun poly(vinyl alcohol)/permutite fibrous film loaded with cinnamaldehyde for active food packaging. J Appl Polym Sci. 2018;135(16):46117. 10.1002/app.46117.Search in Google Scholar
(118) Kwon SJ, Chang Y, Han J. Oregano essential oil-based natural antimicrobial packaging film to inactivate Salmonella enterica and yeasts/molds in the atmosphere surrounding cherry tomatoes. Food Microbiol. 2017;65:114–21. 10.1016/j.fm.2017.02.004.Search in Google Scholar PubMed
(119) Pan J, Ai F, Shao P, Chen H, Gao H. Development of polyvinyl alcohol/beta-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019;300:125249. 10.1016/j.foodchem.2019.125249.Search in Google Scholar PubMed
(120) Muppalla SR, Kanatt SR, Chawla SP, Sharma A. Carboxymethyl cellulose–polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packag Shelf Life. 2014;2(2):51–8. 10.1016/j.fpsl.2014.07.002.Search in Google Scholar
(121) Hu Z, Wang H, Li L, Wang Q, Jiang S, Chen M, et al. pH-responsive antibacterial film based polyvinyl alcohol/poly (acrylic acid) incorporated with aminoethyl-phloretin and application to pork preservation. Food Res Int. 2021;147:110532. 10.1016/j.foodres.2021.110532.Search in Google Scholar PubMed
(122) Kanatt SR. Active/smart carboxymethyl cellulose‐polyvinyl alcohol composite films containing rose petal extract for fish packaging. Int J Food Sci Tech. 2021;56(11):5753–61. 10.1111/ijfs.15095.Search in Google Scholar
(123) Bi J, Tian C, Zhang GL, Hao H, Hou HM. Novel procyanidins-loaded chitosan-graft-polyvinyl alcohol film with sustained antibacterial activity for food packaging. Food Chem. 2021;365:130534. 10.1016/j.foodchem.2021.130534.Search in Google Scholar PubMed
(124) Kim S, Chang Y. Anti-Salmonella polyvinyl alcohol coating containing a virulent phage PBSE191 and its application on chicken eggshell. Food Res Int. 2022;162(Pt A):111971. 10.1016/j.foodres.2022.111971.Search in Google Scholar PubMed
(125) Motelica L, Ficai D, Ficai A, Oprea OC, Kaya DA, Andronescu E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods. 2020;9(10):1438. 10.3390/foods9101438.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings