Abstract
To enhance the mechanical properties of the Nitrate Ester Plasticized Polyether solid propellant matrix, the uniaxial tension of multi-component systems is simulated and the factors influencing the mechanical properties of the propellant matrix are investigated. First, mesoscale models of five types of systems include poly alpha olefin (PAO(3)), polyethylene glycol (PEG200, PEG400, PEG600), and 1,4-butanediol (BDO) are established, followed by uniaxial tensile simulations. The results show PEG600, PEG400, PEG200, BDO, and PAO(3) in order of enhancing the mechanical performance of the matrix. Second, the diffusion behavior of nitroglycerin (NG) and butanetriol trinitrate (BTTN) in various systems is investigated. The results show that NG exhibits higher diffusion capacity than BTTN, and the diffusion coefficient increases with an increment in the molecular weight of PEG. Additionally, the influence of different plasticizer ratios (2.8–3.0), curing parameters (1.58–1.62), and chain extension parameters (0.08–0.10) on the mechanical properties of the PEG600 system are investigated. The results demonstrate that as the plasticizer ratio increases, there is a gradual decrease in the modulus of the matrix. Additionally, an increase in the curing parameter leads to a substantial enhancement in the tensile strength of the matrix, while increasing the chain extension parameter significantly expands the maximum tensile length of the matrix. Finally, employing the Slip-Spring model, the effects of the physical and chemical cross-linked network of the propellant are simulated. The result shows that increasing the content of a chemical cross-linked network significantly improves the tensile strength of the matrix.
1 Introduction
Nitrate Ester Plasticized Polyether (NEPE) is a high-energy-density solid propellant composed of a mixture of materials, including metal particles, polymer binders, and combustion catalysts. This propellant has many applications in fields such as spacecraft launch, satellite orbiting, and deep space exploration (1,2). Compared with traditional solid propellants, NEPE has a higher combustion effectiveness and can considerably decrease fuel mass. Besides, it also has a simple manufacturing process that does not demand high temperature and high pressure (3). The mechanical performance of the propellant matrix plays a pivotal role in determining the overall performance of the propulsion system. However, the inherent characteristics of the matrix limit its application in high-load environments. Therefore, the auxiliary materials in the matrix are frequently necessary to enhance its mechanical properties. The crosslinker is capable of undergoing cross-linked reactions at high temperatures in a blend, which improves the mechanical properties and thermal stability of the mixture during combustion. It plays a crucial role in solid propellants (4). The chain extenders PEG and BDO can increase the molecular weight of propellants, enhance their viscosity, and strengthen the adhesion between particles, thereby improving the combustion and mechanical performance of propellants (5,6,7). In addition, different molecular weights of PEG have different effects on the performance of the propellant. For example, the smaller molecular mass of PEG200 can effectively reduce the surface tension of propellants and improve their flowability. The more significant molecular weight of PEG600 generates more vital activation energy to facilitate the combustion reaction and enhances the antioxidant properties of the liquid fuel in the propellant. It prevents the fuel from undergoing oxidative decomposition, thus improving the stability and reliability of the propellant.
Domestic and international scholars have made some progress in researching the performance of solid propellants. Dong et al. investigated the interface interaction between ammonium perchlorate (AP) and hydroxyl-terminated polybutadiene (HTPB) matrix in AP/HTPB propellant by molecular dynamics (MD) simulation. The results indicated that the cross-linked structure of the matrix, uniaxial strain rate, and contact area significantly influence the mechanical properties of composite solid propellants (8). Deng et al. used MD simulation to construct a molecular network structure of a polybutylene terephthalate propellant matrix. They studied the effects of different component contents on the mechanical properties of the propellant. The results showed that the variation in the content of trimethylphenol (TMP) and diethylene glycol has a negligible impact on the modulus of elasticity, and the tensile strength of the polymer matrix network positively correlates with the chain length and the number of cross-linked sites formed by TMP (9). Kitamura et al. conducted MD simulations on the uniaxial and biaxial extension and compression tests of polypropylene at different strain rates and temperatures using J-OCTA software. The results agreed with the Christensen failure criterion curve (10). The research mentioned methods above all utilize MD for simulation calculations. In addition, Lan et al. employed MD and dissipative particle dynamics (DPD) methods to investigate the influence of dimethylhydantoin on the mechanical properties of glycidyl azide polymer/cyclotrimethylene trinitramine (RDX) propellant (11). Fu et al. utilized MD to simulate the interactions between HTPB polymer and the NG/BTTN mixture, bis (2, 2-dintropropyl) acetal (BDNPA)/bis (2, 2-dintropropyl) formal (BDNPF) mixture, and high-energy plasticizers such as N-butyl-N-(2-nitroxy-ethyl) nitramine (Bu-NENA). The mechanical properties of HTPB polymer were calculated theoretically and studied experimentally (12). Kumar et al. investigated the migration of nitroglycerin (NG) and butanetriol trinitrate (BTTN) in NEPE propellant using high-performance liquid chromatography. They estimated that the diffusion coefficient of NG migration is higher than that of BTTN (13). Zhang used the MD method to simulate the interface interaction and mechanical properties of the neutral polymeric bonding agent (NPBA) on NEPE propellant. The results demonstrated that adding a small amount of NPBA can significantly enhance the mechanical properties of NEPE propellant, and the simulation results were in line with experimental results (14).
Most of the studies mentioned earlier mainly focus on investigating the compatibility, diffusion mechanisms, and interfacial interactions among the components of propellants. The impact of various elements and the cross-linked structures of physical or chemical on the mechanical properties of NEPE solid propellant at the mesoscale has not been documented. Given the complexity of the NEPE system and its high molecular weight, mesoscale simulation methods can effectively simulate the interactions and structural characteristics among molecules in propellants. It also can consider both computational efficiency and accuracy, thus revealing the changes in the performance of solid propellants under different conditions. In this study, based on the cross-scale simulation software J-OCTA (15), mesoscale modeling was performed on five cross-linked network systems of PAO(3), PEG200, PEG400, PEG600, and BDO. Based on uniaxial tension (16) simulations, the stress–strain curves (17) of different components are obtained. By comparing the simulation results with experimental data, the accuracy of the mesoscale model is verified. Furthermore, the diffusion behavior of NG and BTTN in various systems was investigated at the mesoscopic scale, and their diffusion coefficients were calculated. In addition, by modifying the plasticization ratio, curing parameters, and chain extension parameters of the PEG600 system, the effects of different factors on the mechanical properties of NEPE propellants were investigated. Finally, using the Slip-Spring (18) model, the number of sliding springs and frozen springs in the PEG600 system was varied to explore the effects of the physical and chemical cross-linked network (19) on the mechanical properties of the propellant (the simulation content is shown in Figure 1). The studies above provide a reference for developing high-performance solid propellant formulations.

The simulation content.
2 Materials and methods
The structures of the PAO(3), PEG200 (n = 4, n is the degree of polymerization), PEG400 (n = 9), PEG600 (n = 13), the PEG matrix (n = 250), BDO, NG, BTTN, and N-100 are depicted in Figure 2. In the Monomer module of J-OCTA software, the corresponding monomer models were created and subjected to geometric optimization (the monomer models for each component are shown in Figure 3). The following conditions were applied: the DREIDING force field, the built-in Smart minimizer optimization algorithm, atom-based van der Waals interactions, Ewald electrostatic interactions, and a spherical cut-off method for potential energy calculations with a cutoff of 9.5 Å.

Structures of various components in NEPE: (a) NG, (b) BTTN, (c) PAO(3), (d) N-100, (e) BDO, and (f) PEG.

The monomer models of various components in NEPE. The dots colored grey: C, red: O, blue: N, white: H. (a) NG, (b) BTTN, (c) PAO(3), (d) BDO, (e) N-100, and (f) PEG.
Taking the curing agent N-100 as an instance, according to the literature (20), its density is approximately 1.14 g·cm−3. In the COGNAC model of the J-OCTA software, a three-dimensional periodic boundary is constructed, and the MD calculations on the optimized amorphous system are carried out. The following calculation conditions were applied: the constant temperature and pressure ensemble (NPT) was used for density optimization at 298 K for 200 ps. Subsequently, the constant temperature and volume ensemble (NVT) was employed for MD calculations under vacuum conditions for 200 ps, and the entire trajectory file was recorded every 1 ps. During the simulation, the energy fluctuated continuously until dynamic stability was attained, with fluctuations within a range smaller than 5%, indicating that the system reaches an equilibrium state (Figure 4). Unless otherwise specified, the identical calculation conditions were applied for simulating and analyzing the other components.

The energy–time curve of the microscopic system.
To perform mesoscale simulations, the monomer models described above were coarse-grained using the DPD Model (21) to construct coarse-grained system models for each component. Based on the distribution of functional groups in the molecular structures of each component shown in Figure 2, a segmented treatment was conducted. The NG_1, NG_2, BTTN_1, BTTN_2, BTTN_3, PAO(3)_1, PAO(3)_2, BDO_1, BDO_2, N-100_1, N-100_2, N-100_3, PEG_1, and PEG_2 were considered as individual DPD beads (the coarse-grained bead model is illustrated in Figure 5). The Group Contribution Method (22) and the Fedors database provided by J-OCTA were used to calculate the segmental molar volume, cohesion energy, solubility parameters of each component, and the interaction parameters between the elements
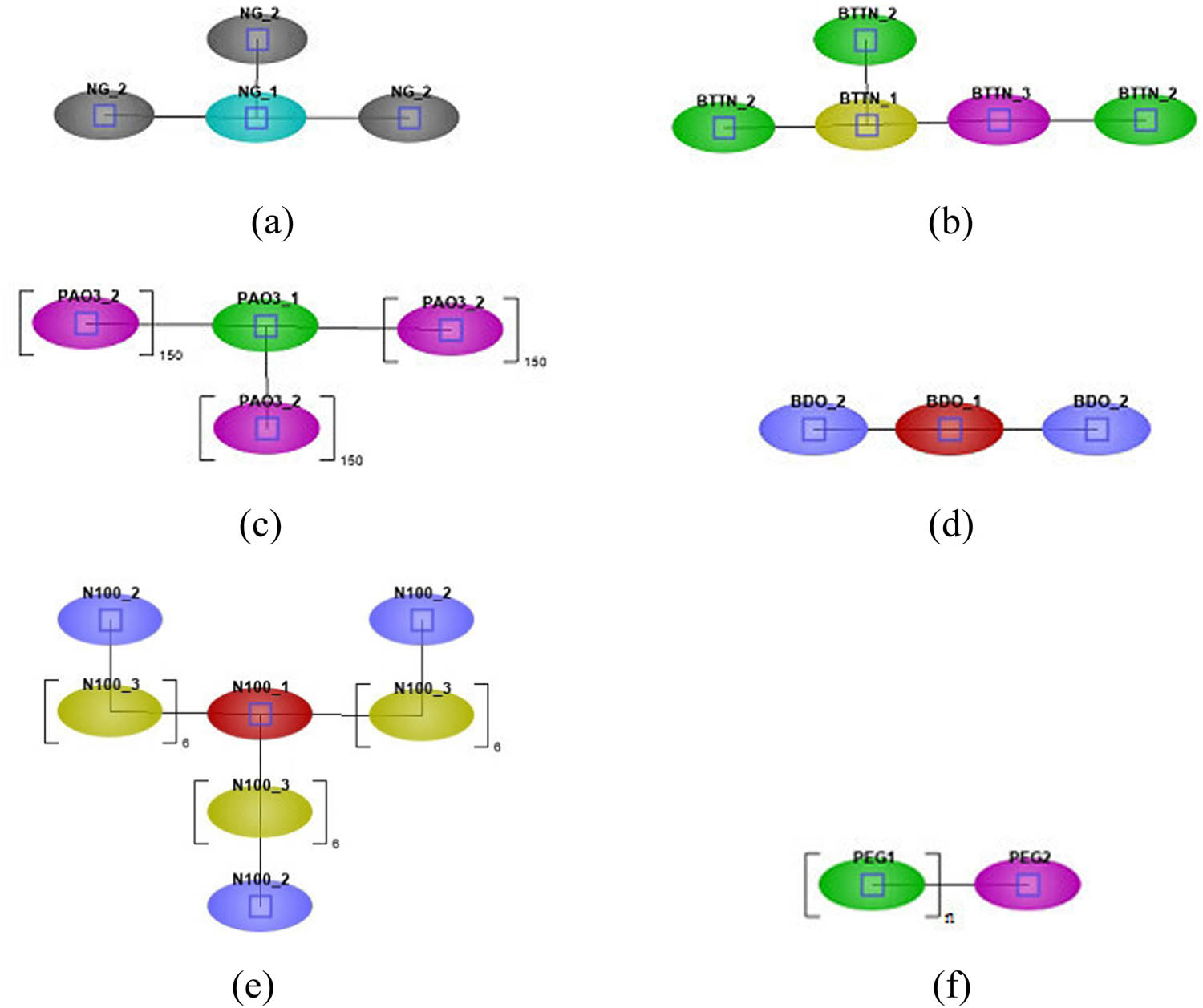
The coarse-grained bead model: (a) NG, (b) BTTN, (c) PAO(3), (d) BDO, (e) N-100, and (f) PEG.
The refinement parameters for each component are defined. The cure parameter represents the mole ratio of isocyanate groups to total hydroxyl groups in the cross-linked system. The chain extension parameter is the mole ratio of hydroxyl groups from the diol chain extender to the total hydroxyl groups. The cross-linked parameter is the mole ratio of hydroxyl groups from the triol cross-linker to the total hydroxyl groups (24). A coarse-grained system with a 1:1 formulation of NEPE was constructed, where the NG and BTTN were present in a mass ratio of 1:1, with a plasticizer ratio of 2.8. The cure parameter was set to 1.58. The chain extension parameter for the PEG200, PEG400, PEG600, and BDO systems was 0.10, and the cross-linked parameter for the PAO(3) system was 0.01.
The number of PEG matrix in each system was 2000, the number of NG was 135683, and the number of BTTN was 127801, while the quantities of the other components are shown in Table 1. Mesoscale models of the mixed systems of each element were established by the COGNAC_DPD module. The optimization was performed for 5,000 steps, with an entire trajectory file recorded every 100 steps. After the optimization, the last trajectory was extracted for DPD dynamic calculations. The relaxation model of the PAO(3) system is shown in Figure 6. The relaxation models of other systems are shown in Figure A1.
The number of molecular chains in each system
| System | N-100 | PAO(3) | PEG200 | PEG400 | PEG600 | BDO |
|---|---|---|---|---|---|---|
| PAO(3) system | 2,140 | 13 | — | — | — | — |
| PEG200 system | 2,330 | — | 198 | — | — | — |
| PEG400 system | 2,330 | — | — | 222 | — | — |
| PEG600 system | 2,340 | — | — | — | 222 | — |
| BDO system | 2,355 | — | — | — | — | 222 |

The relaxation model of PAO(3). The dots colored green: PEG matrix, red: NG, yellow: BTTN, blue: curing agent.
3 Results and discussion
3.1 Curing reactions and cross-linked network structures in multi-component systems
The cross-linked reaction of NEPE solid propellant primarily involves the interaction between the hydroxyl (−OH) groups and the isocyanate (−NCO) groups. Specifically, the three −OH groups at the end of PAO(3) molecular chain, the two −OH groups at the end of PEG200/PEG400/PEG600/BDO molecular chains, and the two −OH groups at the end of the PEG matrix molecular chains react with the three −NCO groups at the end of N-100 molecular chains, forming the aminoformate functional groups (25) (the curing reaction mechanism is illustrated in Figure 7). Taking the PAO(3) component as an instance, based on the curing reaction mechanism, the −OH groups of PAO(3) and the PEG matrix, as well as the −NCO groups of N-100, were set as reactive beads to simulate the cross-linked reaction. To visually depict the structure of the molecular chain after the reaction, only one reacted PEG backbone chain will be displayed while hiding the other components (the curing reaction model for the PAO(3) system is shown in Figure 8). The curing reaction models of other systems are shown in Figure A2.

Mechanism of curing reaction.

The curing reaction model for the PAO(3) system. The dots colored green: PEG matrix, red: NG, yellow: BTTN, blue: curing agent, and purple: reactive groups. (a) The curing reaction molecular chains and (b) the curing reaction model of the PAO(3) system.
The post-reaction system was subjected to uniaxial extension along the z-axis at a strain rate of 0.05 Å·ps−1, and snapshots of the stretching process for each system at the same time step are shown in Figure 9. The plasticizers (the “holes” in Figure 9) are dispersed within the matrix. For better visibility, all plasticizers in the trajectory were hidden.

The snapshots of the stretching process for each system. The dots colored green: PEG matrix, blue: curing agent, and purple: reactive groups: (a1) PEG600, (b1) PEG600, (c1) PEG600, (a2) PEG400, (b2) PEG400, (c2) PEG400, (a3) PEG200, (b3) PEG200, (c3) PEG200, (a4) BDO, (b4) BDO, (c4) BDO, (a5) PAO(3), (b5) PAO(3), and (c5) PAO(3).
From Figure 9, it can be observed that during the extending process, all systems exhibited similar geometric configurations. Before the rupture of the matrix, PEG molecules form a complex cross-linked network structure, forming a “fiber-like bundles” structure between each other (indicated by the tails of the arrows in Figure 9). As the extending process continues, the “fiber-like bundles” structure gradually becomes thinner, suggesting that a large number of PEG chains are pulled out from the “fiber-like bundles” structure, resulting in a sudden change in the load-bearing capacity of the matrix and ultimately leading to matrix fracture. Comparing the “fiber-like bundles” structures in c1 to c5 of Figure 9, it is evident that the tensile strength of the PEG600, PEG400, and PEG200 systems is greater than that of the BDO and PAO(3) systems.
The stress–strain curves for each component system are obtained. To verify the accuracy of the simulation results, experimental results for the same components are compared, as shown in Figure 10. From the stress–strain curves of both simulation and experiment, it can be observed that the trends of each component are approximately similar. However, the stress values in the simulation results are higher than those in the experimental results, which is attributed to the significantly higher strain rate used in the simulation compared to the actual experimental conditions. In addition, Guo et al. (26) proposed the influence of strain rate on the mechanical properties of NEPE propellant in their article. They predicted the power-law relationship between strain rate and the maximum tensile strength of NEPE at different temperatures (Table 2). By inputting the strain rate used in this study into the equation, the calculated maximum stress range matches the simulated stress–strain curves. Therefore, the simulation results can to some extent reflect the effects of each component on the mechanical properties of NEPE solid propellants.

The stress–strain curves obtained from the simulated and experimental single-axis tensile tests for each component system: (a) the simulated stress–strain curves and (b) the experimental stress–strain curves.
Relationship between maximum tensile strength and strain rate at different temperatures
| T (℃) | Equation | Correlation coefficient |
|---|---|---|
| 25 |
|
0.9987 |
| 50 |
|
0.9902 |
| 70 |
|
0.9947 |
From Figure 10, it can be observed that the stress of the NEPE solid propellant matrix increases approximately linearly with strain during extension. In the uniaxial tensile process, the cross-linked network structure of each system will change with the increase of strain. When the stress that the system can bear reaches the critical point, the cross-linked network structure will break, and the stress will drop rapidly on the stress–strain curve. In terms of improving the tensile strength of the solid propellant matrix, the order of effectiveness is PEG600 > PEG400 > PEG200 > BDO > PAO(3). The main reason is that the different components in each system form distinct cross-linked network structures during the curing reaction. The more complete the curing reaction of each component, the stronger the interaction between the cross-linked networks. Additionally, high levels of nitrate plasticizers can effectively swell the cross-linked network structure, thereby inhibiting the crystallization of polyethylene glycol and improving the tensile properties of the matrix.
3.2 Diffusion performance of plasticizers in multi-component systems.
After completing the DPD dynamic calculations, the entire trajectory files of each component system were analyzed to obtain the Mean Square Displacement (MSD) curves of NG and BTTN in PEG200/PEG400/PEG600/BDO/PAO3 systems (the MSD–t curves of plasticizers in each system are shown in Figure 11). Using the Einstein relationship Eq. 1, the diffusion coefficients D of plasticizers NG and BTTN in different systems can be determined (27).
where

MSD–t curves of plasticizers NG and BTTN in different systems: (a) MSD–t curves of the BDO system, (b) MSD–t curves of the PAO3 system, (c) MSD–t curves of the PEG200 system, (d) MSD–t curves of the PEG400 system, and (e) MSD–t curves of the PEG600 system.
The diffusion coefficient is an important parameter that characterizes the migration and diffusion capability of a substance, which is defined as the ratio between the diffusion flux through a unit area in unit time and the concentration gradient (28). By performing linear fitting using Origin software on the MSD–t curves of plasticizers in Figure 11, the slopes of the curves for each system were obtained. The diffusion coefficients of plasticizers NG and BTTN in different systems were calculated using Eq. 1 (Table 3).
The slopes of MSD–t curves and the diffusion coefficients D
| System | Slope of the curve ×
|
Diffusion coefficient D ×
|
|---|---|---|
| NG/BDO | 92 | 15.33 |
| BTTN/BDO | 59 | 9.83 |
| NG/PAO3 | 14 | 2.33 |
| BTTN/PAO3 | 11 | 1.83 |
| NG/PEG200 | 3 | 0.50 |
| BTTN/PEG200 | 2 | 0.33 |
| NG/PEG400 | 7 | 1.17 |
| BTTN/PEG400 | 3 | 0.50 |
| NG/PEG600 | 18 | 3.00 |
| BTTN/PEG600 | 8 | 1.33 |
According to reference (29), the diffusion coefficients obtained from MD simulations for similar systems are of the same order of magnitude as the calculated results in this study. Therefore, the computed results in Table 3 are accurate and reliable. From Figure 11 and Table 3, it can be observed that regardless of the system, the diffusion coefficient of NG is higher than that of BTTN. By examining the molecular structures in Figure 2, it can be inferred that NG, having one less functional group, exhibits stronger polarity than BTTN. Additionally, during the coarse-graining process, the number of beads of BTTN is greater than that of NG. Thus, the diffusion ability of NG is superior to that of BTTN. Furthermore, when the molecular weight of PEG is in the range of 200–600, the effect of PEG molecular weight on the diffusion ability of NG and BTTN was investigated, and the diffusion coefficient variability curves of plasticizers in different PEG molecular weight systems are shown in Figure 12. When the molecular weight of PEG is 200–600, it can be observed that the diffusion coefficients of NG and BTTN increase with increasing PEG molecular weight, with NG still exhibiting a higher diffusion coefficient than BTTN. It is mainly due to the decrease in the solubility parameter of PEG with increasing molecular weight, making migration more favorable, thereby leading to an increase in the diffusion coefficients of NG and BTTN (30).

The diffusion coefficient variability curves of plasticizers in different PEG molecular weight systems.
3.3 Effect of different parameters on the mechanical properties of the PEG600 system
NEPE solid propellant has a complex composition, and each component plays a different role. Therefore, it is necessary to simulate the effects of various parameters quantitatively. In this study, the PEG600 system with the highest tensile strength was chosen to explore the influences of varying plasticizer ratios, curing parameters, and chain expansion parameters on the mechanical properties of the propellant.
3.3.1 Effect of different plasticization ratios on the mechanical properties of the PEG600 system
Plasticizers, although not directly involved in the curing reaction of the system, can weaken the intermolecular interactions between molecular chains, thereby enhancing the mobility of the chains (31). Uniaxial tensile simulations were conducted on the propellant matrix with plasticizer ratios of 2.8, 2.9, and 3.0 in the PEG600 system. The simulations were performed under identical conditions for each plasticizer ratio. The stress–strain curves for NEPE propellant matrices with different plasticizer ratios are shown in Figure 13.

Stress–strain curves of NEPE propellant matrix with different plasticization ratios.
From Figure 13, it can be observed that the initial modulus of the matrix in various plasticizer ratio systems is not significantly different. However, with an increase in plasticizer ratio, the modulus and the tensile strength gradually weakened. The main reason is the increase in plasticizer content, which enhances the swelling effect between the cross-linked networks, thereby increasing the free movement space of the molecular chains. This weakens the entanglement between the cross-linked networks, resulting in a decrease in the tensile strength of the propellant matrix.
3.3.2 Effect of different curing parameters on the mechanical properties of the PEG600 system
The curing agent, as an essential component of the cross-linked reaction, directly affects the cross-linked network structure of the propellant matrix and changes the cross-linked density of the propellant matrix (32). In addition, the curing agent can generate a large amount of gas during the combustion process and enhance the thrust, thereby improving the performance of the solid propellant. Uniaxial tensile simulations were conducted on the propellant matrix with varying curing agent content in the PEG600 system. The curing parameters were set at 1.58, 1.60, and 1.62, and the simulations were performed under the same conditions. The stress–strain curves for NEPE propellant matrices with different curing parameters are shown in Figure 14.

Stress–strain curve of NEPE propellant matrix with different curing parameters.
From Figure 14, it can be observed that the curing parameter has little effect on the initial modulus of the matrix. However, as the tensile process progresses, increasing the curing parameter of the matrix leads to an increase in the modulus and a significant improvement in tensile strength. The main reason for this is that an increase in the content of the curing agent increases the probability of cross-linked reactions between components, thereby increasing the cross-linked density of the matrix and making the cross-linked network structure more robust, thus improving the mechanical properties of the matrix.
3.3.3 Effect of different chain extension parameters on the mechanical properties of the PEG600 system
The crosslinker molecule contains multiple reactive groups that can interact with the curing agent in the solid propellant, forming a strong solid network structure by chemical bonds. The network structure enhances the mechanical strength, hardness, and other mechanical properties of the propellant. The chain extender can change the physical properties of the solid propellant and improve the material’s toughness. Uniaxial tensile simulations were performed on the propellant matrix with varying chain extender content in the PEG600 system. The chain extension parameters were set at 0.08, 0.09, and 0.10, and the simulations were conducted under the same tensile conditions for each chain extension parameter. The stress–strain curves for NEPE propellant matrices with different chain extension parameters are shown in Figure 15.

Stress–strain curve of the NEPE propellant matrix with different chain extension parameters.
From Figure 15, it can be observed that increasing the chain extension parameter in the propellant system has little effect on the initial modulus of the matrix. As the tensile process progresses, the modulus of the matrix increases slightly, and the tensile strength rises to some extent. It is due to the increased plasticizer content, which increases the probability of curing reactions between hydroxyl (−OH) groups and isocyanate (−NCO) groups in the system, resulting in an increased content of the carbamate functional group in the matrix. Additionally, the change in the chain extension parameter significantly improves the maximum elongation of the matrix. This is mainly due to PEG600 being a binary hydroxyl compound as a large molecule plasticizer. With increased PEG600 content, the molar mass between cross-linked points in the system increases. During the process of stretching, the molecular chains can bear tremendous stress, resulting in a significant improvement in the maximum elongation of the matrix (33).
3.4 Effect of physical cross-linked and chemical cross-linked on the mechanical properties of propellants
Changing the content of components such as chain extender, curing agents, and plasticization ratio in the propellant can improve the mechanical properties of the propellant matrix by altering its cross-linked network structure. The essence of this process is to induce changes in the cross-linked network structure of the propellant matrix. In an ideal cross-linked network structure, both ends of each polymer chain are connected to cross-linked points, and when the cross-linked network undergoes deformation, all polymer chains contribute to the elastic force. However, the actual cross-linked network structure is much more complex and may have some defects. A polymer chain’s two ends may simultaneously react with a curing agent, forming a chain ring (Figure 16). In some cases, only one end of a polymer chain may connect to a cross-linked point, while the other end forms a suspension chain. Additionally, interpenetration between polymer chains or hydrogen bonding between the chains, known as physical cross-linked, may also occur. These complex phenomena contribute to the overall structure and properties of the cross-linked network (34).

Real cross-linked network structure.
In the J-OCTA software, the sliding spring can be used to characterize the physical sliding between intertwined molecular chains, while the frozen spring can be used to represent the interactions between molecular chains generated by chemical reactions. The Slip-Spring model is introduced to simulate the physical and chemical cross-linked network between DPD molecular chains by adjusting the numbers of sliding springs and frozen springs (35). When the Slip-Spring count in the model is set to 0, the stress–strain curve of the propellant matrix under uniaxial tensile simulation under the same stretching conditions is shown in Figure 17. With no physical or chemical cross-linked network in the matrix, there is minimal entanglement between the molecular chains during extension. As a result, the stress values fluctuate around 0 MPa.

The stress–strain curve of the substrate when Slip-Spring is 0.
The numbers of sliding springs and frozen springs were varied in the PEG600 system (Table 4). Uniaxial tensile simulations were performed on each system (the stress–strain curves are shown in Figure 18). From the graph, it can be observed that at the same strain level, as the number of frozen springs increases, indicating a higher quantity of chemical cross-linked network, the stress values sustained by the propellant matrix significantly increase. Therefore, it can be approximated that increasing the content of chemical cross-linking in the cross-linked network structure effectively enhances the mechanical properties of the matrix.
Number of Slip-Springs
| Number of Slip-Springs | Number of frozen springs | Number of sliding springs |
|---|---|---|
| 760 | 380 | 380 |
| 760 | 190 | 570 |
| 760 | 95 | 665 |
| 760 | 48 | 712 |
| 760 | 24 | 736 |

The Slip-Spring model stress–strain curve for the PEG600 system.
4 Conclusions
Based on the J-OCTA software, mesoscale simulations were performed on five cross-linked network systems: PAO(3), PEG200, PEG400, PEG600, and BDO. The main conclusions were as follows:
Uniaxial tension simulations were conducted to analyze the stress–strain curves of various components and validate the model results against experimental data. The results indicate that in terms of improving the mechanical properties of the matrix, the ranking is as follows: PEG600 > PEG400 > PEG200 > BDO > PAO(3).
The diffusion phenomena of NG and BTTN in various systems were investigated. The results showed that the diffusion capability of NG was greater than that of BTTN, and the diffusion coefficient increased with increasing PEG molecular weight.
The influence of different plasticizer ratios, curing agent parameters, and chain extender parameters on the mechanical properties of the PEG600 system was investigated. The results showed that within the range of plasticizer ratios from 2.8 to 3.0, the modulus of the propellant matrix gradually decreased with increasing plasticizer ratio. Within the range of curing agent parameters from 1.58 to 1.62, the tensile strength of the matrix significantly increased with increasing curing agent parameters. Within the range of chain extender parameters from 0.08 to 0.10, the maximum elongation of the matrix was significantly improved with increasing chain extender parameters.
The effects of physical and chemical cross-linked networks on the mechanical properties of the propellant were investigated using the Slip-Spring model. The results showed that under the same strain, an increased number of frozen springs, indicating a higher amount of chemical cross-linked network, significantly improved the stress that the propellant matrix could withstand.
The research findings provide valuable guidance for enhancing the mechanical performance of NEPE solid propellants and optimizing propellant formulations. The findings are of great significance for the ever-growing demands of space exploration and applications.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (Grant nos. 12172320).
-
Author contributions: Lipeng Zhang: writing – original draft, investigation, software, formal analysis; Chen Chen: investigation, software, and formal analysis; Xianqiong Tang: writing – review and editing, data curation, visualization, and funding acquisition; Xing Zhou: resources, supervision.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data have been given in the article.
Appendix

The relaxation models: (a) the relaxation model of PEG600, (b) the relaxation model of PEG400, (c) the relaxation model of PEG200, and (d) the relaxation model of BDO.

The curing reaction models: (a) the curing reaction model of PEG600, (b) the curing reaction model of PEG400, (c) the curing reaction model of PEG200, and (d) the curing reaction model of BDO.
References
(1) Hu QW, Fang QZ, Sha BL, Xu Q. Study on the viscoelastic damage properties of NEPE solid propellant. Propellants Explos Pyrotech. 2020;45(7):1076–88.10.1002/prep.201900385Search in Google Scholar
(2) Li H, Wang SX, Li M, Xu JS, Fan XG, Chen X. Experimental research on tensile mechanical properties of NEPE propellant under confining pressure. Propellants Explos Pyrotech. 2020;45(11):1769–79.10.1002/prep.201900412Search in Google Scholar
(3) Tao T, Sui X, Li SP, Wang NF. A study on consumption and characterization of stabilizer content of NEPE propellant via FTIR. Propellants Explos Pyrotech. 2019;44(7):889–95.10.1002/prep.201800340Search in Google Scholar
(4) Santosh MS, Sadavarte VS, Kumar A, Pande SM, Kulkarni PS. Examining the effect of trimethylol propane crosslinker on butanetriol trinitrate plasticized polycaprolactone polymer networks of propellant binder system. Propellants Explos Pyrotech. 2022;(6):47.10.1002/prep.202100377Search in Google Scholar
(5) Shen C, Yan S, Ou YP, Jiao QJ. Influence of fluorinated polyurethane binder on the agglomeration behaviors of aluminized propellants. Polymers. 2022;14(6):1124.10.3390/polym14061124Search in Google Scholar PubMed PubMed Central
(6) Chen KK, Wen XM, Li GP, Pang SP, Luo YJ. Improvement of mechanical properties ofin situ-prepared HTPE binder in propellants. RSC Adv. 2020;10(50):30150–61.10.1039/D0RA02613ASearch in Google Scholar
(7) Dou JK, Xu MG, Tan BJ, Lu XM, Mo HC, Wang BZ, et al. Research progress of nitrate ester binders. FirePhysChem. 2023;3(1):54–77.10.1016/j.fpc.2022.09.003Search in Google Scholar
(8) Dong G, Liu HZ, Deng L, Yu HY, Zhou X, Tang XQ, et al. Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation. e-Polymers. 2022;22(1):264–75.10.1515/epoly-2022-0016Search in Google Scholar
(9) Deng SW, Wang SX, Zhou HW, Miao CL, Wang JG. Molecular dynamics simulation of molecular network structure and mechanical properties of the polymer matrix in PBT propellant. Mater Today Commun. 2023;35.10.1016/j.mtcomm.2023.105723Search in Google Scholar
(10) Kitamura R, Kageyama T, Koyanagi J, Ogihara S. Estimation of biaxial tensile and compression behavior of polypropylene using molecular dynamics simulation. Adv Compos Mater. 2019;28(2):135–46.10.1080/09243046.2018.1469372Search in Google Scholar
(11) Lan YH, Zhai JX, Li DH, Yang RJ. Multiscale Simulation on the Influence of Dimethyl Hydantoin on Mechanical Properties of GAP/RDX Propellants. Propellants Explos Pyrotech. 2014;39(1):18–23.10.1002/prep.201200210Search in Google Scholar
(12) Fu XL, Fan XZ, Ju XH, Qi XF, Li JZ, Yu HJ. Molecular dynamic simulations on the interaction between an HTPE polymer and energetic plasticizers in a solid propellant. RSC Adv. 2015;5(65):52844–51.10.1039/C5RA05312ASearch in Google Scholar
(13) Kumar A, Chavan PV, Bhatkhanda DS, Sadavarte VS, Mada SS, Pande SM. Migration of energetic plasticizer in advanced energetic composite propellant grains. Propellants Explos Pyrotech. 2022;48(2).10.1002/prep.202200185Search in Google Scholar
(14) Zhang PA, Li TQ, Liu SM, Deng JR. Effects of NPBA on interface interaction and mechanical properties of NEPE propellant: Insight from molecular dynamics simulation. Comput Mater Sci. 2020;171(C):109135.10.1016/j.commatsci.2019.109135Search in Google Scholar
(15) Yoshida H, Nitta H, Chaki K, Ohata K, Ozawa T. Multiscale simulation technology and software for soft matter. NIHON GAZO GAKKAISHI (J Imaging Soc Jpn). 2015;54(6):578–86.Search in Google Scholar
(16) Pei SD, Qiang HF, Wang XR, Wang JX, Li SQ. Micromechanical behavior of HTPB propellant bonding interface under uniaxial tensile loading. Propellants Explos Pyrotech. 2022;47(10).10.1002/prep.202200106Search in Google Scholar
(17) Shekhar H. Studies on stress–strain curves of aged composite solid rocket propellants. Def Sci J. 2012;62(2):90–4.10.14429/dsj.62.773Search in Google Scholar
(18) Masubuchi Y, Langeloth M, Böhm MC, Inoue T, Müller-Plathe F. A multichain slip-spring dissipative particle dynamics simulation method for entangled polymer solutions. Macromolecules. 2016;49(23):9186–91.10.1021/acs.macromol.6b01971Search in Google Scholar
(19) Zhang W, Zhou X, Bao T, Deng L, Gao DL. Evaluation of mechanical properties of composite solid propellant by genetic engineering of materials: Part A. Propellants Explos Pyrotech. 2019;44(9):1167–74.10.1002/prep.201800385Search in Google Scholar
(20) Zhou SP, Tang G, Pang AM, Guo X, Wu F, Song HB, et al. Synthesis of an alkynyl neutral polymer-bonding agent and its enhancing effect on tensile strength of glycidyl azide polymer-based propellants. Iran Polym J. 2019;28(11):943–55.10.1007/s13726-019-00756-wSearch in Google Scholar
(21) Doi H, Okuwaki K, Mochizuki Y, Ozawa T, Yasuoka K. Dissipative particle dynamics (DPD) simulations with fragment molecular orbital (FMO) based effective parameters for 1-Palmitoyl-2-oleoyl phosphatidyl choline (POPC) membrane. Chem Phys Lett. 2017;684:427–32.10.1016/j.cplett.2017.07.032Search in Google Scholar
(22) Vicente MS, Gottifredi JC. Estimation of solvent activities in polymer solutions using a group-contribution method. Ind Eng Chem Process Des Dev. 1978;17(3):333–9.10.1021/i260067a021Search in Google Scholar
(23) Zenati A, Pokhrel A. Properties and molecular self-assembly of liquid crystalline hard–hard diblock and hard–soft–hard triblock copolymers influenced by Flory–Huggins interaction parameter and volume fraction of distinct segments. J Polym Sci. 2023;61(15):1596–611.10.1002/pol.20230075Search in Google Scholar
(24) Chen K, Ren QB, Li JJ, Chen DF, Li CX. A highly stretchable and self-healing hydroxy-terminated poly-butadiene elastomer. J Saudi Chem Soc. 2020;24(12):1034–41.10.1016/j.jscs.2020.11.002Search in Google Scholar
(25) Zhao X, Zhu WH. Optimization and design for the curing process of solid azide propellant: Influence of typical components on the curing reactions of PBT binders with TDI. J Chin Chem Soc. 2022;69(3):419–39.10.1002/jccs.202100510Search in Google Scholar
(26) Guo X, Zhang XP, Zhang W. Effect of tensile rate on mechanical properties of NEPE propellant. J Solid Rocket Technol. 2007;04:321–3 + 327.Search in Google Scholar
(27) Abou-Rachid H, Lussier LS, Ringuette S, Lafleur-Lambert X, Jaidann M, Brisson J. On the correlation between miscibility and solubility properties of energetic plasticizers/polymer blends: modeling and simulation studies. Propellants, Explosives, Pyrotech: An Int J Dealing Sci Technol Asp Energetic Mater. 2008;33(4):301–10.10.1002/prep.200700211Search in Google Scholar
(28) Gao HT, Chen FY, Cai RL, Ye SJ, Tan F, Xiong WJ, et al. The diffusion of components from propellant and liner at the interfaces of EPDM insulation. Propellants, Explosives, Pyrotechnics. 2021;46(3):460–7.10.1002/prep.202000270Search in Google Scholar
(29) Yu ZF, Wang WZ, Yao WS, Zhang W, Xie WX, Liu YF, et al. Simulation for the migration of nitrate ester plasticizers in different liners contacting with propellant by molecular dynamics. J Energetic Mater. 2021;39(1):74–84.10.1080/07370652.2020.1756987Search in Google Scholar
(30) Li SN, Liu Y, Tuo XL, Wang XG. Mesoscale dynamic simulation on phase sepa-ration between plasticizer and binder in NEPE propellants. Polymer. 2008;49(11):2775–80.10.1016/j.polymer.2008.04.020Search in Google Scholar
(31) Zou XB, Zhang WG, Zhang ZH, Gu YJ, Fu XC, Ge Z, et al. Study on properties of energetic plasticizer modified double‐base propellant. Propellants Explos Pyrotech. 2021;46(11):1662–71.10.1002/prep.202100085Search in Google Scholar
(32) Chen KK, Yuan S, Wen XM, Sang C, Luo YJ. Effect of mixed isocyanate curing agents on the performance of in situ‐prepared HTPE binder applied in propellant. Propellants Explos Pyrotech. 2021;46(3):428–39.10.1002/prep.202000190Search in Google Scholar
(33) Ross P, Sevilla G, Quagliano J. Effect of chain extender on the mechanical and thermal resistance properties of polyurethane liners for composite propellants. Polyurethanes. 2016;1(1):1–9.10.1515/polyur-2017-0001Search in Google Scholar
(34) Yuan S, Jiang SK, Luo YJ. Cross-linking network structures and mechanical properties of novel HTPE/PCL binder for solid propellant. Polym Bull. 2021;78:313–34.10.1007/s00289-020-03110-wSearch in Google Scholar
(35) Masubuchi Y. Multichain slip-spring simulations for branch polymers. Macromolecules. 2018;51(24):10184–93.10.1021/acs.macromol.8b01739Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

