Graphical abstract
Abstract
Water pollution remains a significant global challenge, with dye contamination from industrial activities being particularly problematic. Adsorption is widely recognized as an efficient, rapid, and cost-effective method for pollutant removal. To enhance adsorption efficiency, researchers have increasingly focused on the development of reusable adsorbent materials. Magnetic materials, in particular, have garnered attention due to their ability to be easily recovered through the application of an external magnetic field. In this study, ferrite spinel adsorbents were synthesized by substituting Co(ii) into the FeFe2O4 spinel structure using a coprecipitation method. The random distribution of a Co cation into host FeFe2O4 compounds, Fe1–x Co x Fe2O4 (x = 0–1), strongly influenced optical and magnetic properties. Nanoparticle sizes varying within 10–17 nm were obtained in Fe1−x Co x Fe2O4 compounds. X-ray diffraction and Fourier transform infrared spectroscopy analyses revealed a single-crystal phase that was well fabricated. The optical bandgap energy decreased from 2.27 to 1.89 eV, together with a reduction of magnetic moment from 93.5 to 43.1 emu/g. The Fe1−x Co x Fe2O4 materials demonstrated significant adsorption performance for Direct Red 79 (DR79), particularly at low pH levels, with optimal results observed at x = 0.5 (FC3). The adsorption behavior was effectively described by the Langmuir and Redlich–Peterson models. Kinetic analysis revealed that the pseudo-second-order and intragranular diffusion models provided the best fit. Thermodynamic analysis indicated an endothermic process (ΔH = 74.947 kJ/mol), suggesting that the adsorption mechanism is primarily physical. This study underscores the potential of Fe1−x Co x Fe2O4 materials for the effective removal of Direct Red 79 (DR79), offering valuable insights into their synthesis, characterization, and application for environmental remediation. After four cycles of recovery and reuse, the FC3 magnetic nanomaterial was able to remove 42% of the DR79 pollutant while maintaining good chemical stability. The adsorption efficiency of the material for the actual textile wastewater sample reached 57% for color removal and 54.5% for chemical oxygen demand reduction.
1 Introduction
The issue of water pollution remains a critical global concern, demanding innovative approaches for effective treatment. Various pollutants including dyes used in the textile and leather industries seriously contaminate water sources [1]. Dyes play a crucial role in many industries, particularly in the textile industry, where direct dyes are predominant. They are known environmental pollutants, with approximately 80% of direct dyes being azo compounds that need to be removed from water [2]. Direct Red 79 (DR79), classified as an azo dye, is a synthetic coloring agent extensively used across multiple industries, such as textile, paper, and leather processing. Despite its ubiquity and utility, the environmental ramifications of DR79 are noteworthy. Improper disposal practices can lead to the contamination of aquatic ecosystems, encompassing rivers, lakes, and streams. DR79, alongside other azo dyes, notably exhibits resistance to biodegradation mechanisms, thereby perpetuating the persistence of dye-induced water pollution over extended periods. Numerous methods have been studied for the removal of pollutants, such as photocatalytic degradation [3,4,5,6,7], membrane filtration [8], electrolysis [9], biodegradation [10], precipitation [11], and adsorption [12,13,14,15]. Among them, adsorption is highly feasible for practical applications owing to its simplicity, ease of operation, and wide variety of available adsorbent materials that are easy to fabricate [16].
Nanomaterials have large surface area-to-volume ratios and high surface energies, exhibiting strong adsorption capabilities. In particular, nanoparticles can treat pollutants at various depths within water bodies, a feature often overlooked by conventional technologies [14]. Some environment-friendly and cost-effective nanomaterials have been developed and play a crucial role in industrial-pollution remediation, surface-water treatment, groundwater treatment, and drinking water purification. Many materials based on metal oxides such as ZnO [12] and MnO2/Al2O3 [13], as well as magnetic materials like Fe3O4 [17,18], ferrites [15], etc., play significant roles in various types of nanoadsorbents. Ferrite nanoparticles used in water and wastewater treatment are highly stable and are easily regenerated without compromising their properties, thereby reducing treatment costs, particularly in terms of recovery [19,20,21]. Given their magnetic properties, they can also be separated from the environment using an external magnetic field, avoiding the need for centrifugation or complex filtration methods [22,23,24]. However, Fe3O4-based materials often lack stability and tend to undergo phase transition, affecting the magnetic properties of the particles and leading to changes in pollutant-removal efficiency. Hence, substituted-spinel ferrite Fe1−x M x Fe2O4 (M = divalent metal ion) is increasingly being used for contaminant removal [15]. Additionally, conventional oxides often exhibit poor stability in low pH environments, whereas their adsorption capacity for certain high-color pollutants decreases at low pH and gradually diminishes at high pH for metal-oxide-based adsorbents [25]. Conversely, spinel ferrites remain stable at low pH (pH 2–6) [15,26].
The synthesis of ferromagnetic ferrite materials has garnered significant attention due to their potential applications across various fields, including environmental remediation, catalysis, and energy storage. Among the numerous synthesis techniques – such as [27], sol–gel [28,29], hydrothermal [29,30], solvothermal [30,31] – the co-precipitation method stands out for its simplicity, cost-effectiveness, and ability to produce highly uniform nanoscale particles with tailored properties. This method, in particular, facilitates rapid nucleation at elevated temperatures, enabling the controlled formation of Fe1−x Co x Fe2O4 ferrites, which are of interest for their magnetic and adsorptive characteristics. For instance, Andersen et al. demonstrated that the rapid growth of MnFe₂O₄ and CoFe₂O₄ nanocrystals can achieve sizes as small as 10–20 nm [32].
In this study, we employed the co-precipitation method to synthesize Fe1−x Co x Fe2O4 ferrites, focusing on optimizing their structure for effective adsorption applications. The synthesized materials were tested for their ability to adsorb FDR79, a synthetic dye prevalent in industrial wastewater. The adsorption process was analyzed through various isothermal models, including Langmuir and Redlich–Peterson, to determine the adsorption mechanism and capacity. Additionally, kinetic studies utilizing pseudo-second-order and intragranular diffusion models provided insights into the adsorption dynamics. Our findings indicate that these materials have significant potential for practical application in environmental remediation, particularly in the treatment of dye-contaminated water.
2 Experiment
2.1 Chemicals and instruments
FeCl2·4H2O, FeCl3·6H2O, CoCl2⸱6H2O, and NaOH powdered chemicals were purchased from Merck. DR79 and acetone were purchased from China. Solutions of 2 M FeCl3, 2 M FeCl2, 2 M CoCl2, 2 M HCl, and 2 M NaOH were prepared from stock chemicals using twice-distilled water. A magnetic heating stirrer (C-MAG HS, IKA) was used for the synthesis of materials. IKA®KS 260 basic and control shakers were used to study adsorption. A pH meter was used to adjust the pH of the solution containing adsorbate. The concentration of adsorbate (DR79) was determined using a UV-1700 spectrophotometer (Shimadzu, Japan).
2.2 Fabrication of Fe1−x Co x Fe2O4
In the synthesis of Fe3O4 nanoparticles, a mixture comprising 2 mL of 2 M FeCl2 and 4 mL of 2 M FeCl3 (resulting in 8 mL of 1 M FeCl3) was combined with 10 mL of 2 M HCl. The resulting mixture was stirred for 1 h at room temperature. Subsequently, 60 mL of 2 M NaOH was brought to a boil at 100°C, and the iron salt solution was swiftly introduced into the boiling NaOH solution. A rapid pouring rate was ensured to secure uniform nucleation of iron salt with NaOH. The reaction was sustained for 1 h at 100°C. Following the reaction, the mixture was filtered, followed by two washes with distilled water until a pH of 7 was reached. The final step involved rinsing the product with acetone.
Fe1−x Co x Fe2O4 was fabricated similar to that of Fe3O4 nanoparticles. However, instead of using FeCl2, a portion of the mixed solution was replaced by CoCl2 in a certain ratio, as shown in Table 1.
Reagent composition for material preparation
| x | 2 M FeCl2 (mL) | 2 M FeCl3 (mL) | 2 M CoCl2 (mL) | 2 M HCl (mL) | 2 M NaOH (mL) | Denotation of product |
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 0 | 15 | 60 | F0 |
| 0.2 | 1.6 | 4 | 0.4 | 15 | 60 | FC1 |
| 0.4 | 1.2 | 4 | 0.8 | 15 | 60 | FC2 |
| 0.5 | 1.0 | 4 | 1.0 | 15 | 60 | FC3 |
| 0.6 | 0.8 | 4 | 1.2 | 15 | 60 | FC4 |
| 0.8 | 0.4 | 4 | 1.6 | 15 | 60 | FC5 |
| 1 | 0 | 4 | 2 | 15 | 60 | C0 |
2.3 Morphology and structures of materials
The crystal and phase structure, morphology, particle size, and chemical structure of the synthesized samples were characterized by field-emission scanning electron microscopy system (S-4800, Hitachi), X-ray diffraction (XRD) analysis (XRD-Bruker D8 Advance), Fourier transform infrared spectroscopy (FTIR; Nicolet Nexus 670), and ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis-DRS; Hitachi U-2900). Magnetic properties were observed on magnetization plots using a vibrating sample magnetometer (VSM) at room temperature. Specific surface area and pore size were examined through nitrogen adsorption at a low temperature (77.35 K) by using the Brunauer–Emmett–Teller (BET) equation (MicroActive for TriStar II Plus 2.03).
2.4 Adsorption properties
The pHpzc of absorbents was determined as follows. To adjust the pH of 0.1 M NaCl solutions to initial pH values (pHi) ranging from approximately 3 to 11, 0.1 M HNO3 and 0.1 M NaOH solutions were prepared. Nine Erlenmeyer flasks with a volume of 250 mL each were filled with 0.02 g of the adsorbent material. Subsequently, 100 mL of the prepared solutions with varying pHi values were sequentially added to each flask. The mixtures were allowed to stand for 48 h, after which the solutions were filtered, and their pH (pHf) was measured. The difference between the initial pH (pHi) and the equilibrium pH (pHf) was calculated as ∆pH = pHi – pHf. A graph illustrating the dependence of ∆pH on pHi was drawn. The intersection points of the curve with the x-axis (where ∆pH = 0) indicated the point of zero charge that needed to be determined.
This study involved the adsorption of DR79 (adsorbate) onto an adsorbent by shaking a conical flask containing the adsorbent in an aqueous solution of the absorbate at 250 rounds per min. The concentration of DR79 was determined using the spectrophotometric method (UV 1700) within the wavelength range of 300–700 nm.
The adsorption capacity was characterized by the adsorption efficiency or removal (%), calculated as the percentage of the adsorbate’s concentration at time t (C t , mg L–1) or equilibrium (C e, mg L–1) compared with the initial concentration (C 0, mg L–1) according to
The adsorption capacity, indicating the amount of adsorbate per unit adsorbent at time t (q t , mg g–1) or at equilibrium (q e, mg g–1) in a solution volume V (L) containing m (g) of adsorbent, was calculated by
In theory, depending on the adsorption system comprising adsorbent, adsorbate, and the adsorption environment, the dependence of adsorption capacity on concentration at equilibrium at a specific temperature follows different laws determined by the isothermal adsorption equation. The use of various isotherms such as Langmuir, Freundlich, Temkin, and Redlich–Peterson is common in analyzing adsorption phenomena.
The Langmuir single-layer homogeneous adsorption model in linear form, with a maximum adsorption capacity (q max) and Langmuir adsorption constant (K L), is represented by
In the Freundlich adsorption isotherm, an empirical adsorption model, the adsorbate forms a monomolecular layer on the surface of the adsorbent for a linear curve by
where K F is the Freundlich adsorption constant, and n is a constant (n > 1).
The Temkin isotherm model indirectly explores the influence of interactions between adsorbent and adsorbate under the assumption that the heat of adsorption for all molecules in a layer decreases linearly with increased surface coverage of the adsorbent. The Temkin isotherm model is mathematically expressed by
where K T (L mol−1) is the equilibrium binding constant, b is related to the adsorption heat, R is the universal gas constant (8.314 J K−1 mol−1), and T is the temperature (K).
The Redlich–Peterson isotherm is a combination of the Langmuir and Freundlich isotherms. It adopts the numerator from the Langmuir isotherm and offers the advantage of accessing the Henry region at infinite dilution. This isotherm model is an empirical three-parameter model, incorporating elements from the Langmuir and Freundlich equations; thus, the adsorption mechanism is a mixture and does not adhere to ideal single-layer adsorption. The linear form of the Redlich–Peterson equation is shown as follows:
where A is the Redlich–Peterson isotherm constant (L g–1), and β is an exponent between 0 and 1 [33].
3 Results and discussion
3.1 Morphology and structure of materials
The morphology and size of the Co-substituted Fe3O4 nanoparticles were studied through field emission scanning electron microscope (FESEM) and transmission electron microscopy (TEM) images, as shown in Figures 1 and 2. It can be seen that all samples exhibit a nearly cubic shape with a small size in the range of 7–25 nm, with the average size depending on the Co-substituted concentration. The ImageJ software was used to estimate the average size and the particle-size distribution of the obtained samples. The results reveal that the average size of the Co-substituted Fe3O4 nanoparticles increases with increasing the Co-substituted content, ranging from approximately 10 nm at F0 to 17 nm at C0 (Figure 3).

FESEM images of (a) F0, (b) FC1, (c) FC2, (d) FC3, (e) FC4, (f) FC5, (g) C0, and (h) FC2 recovered after adsorption.
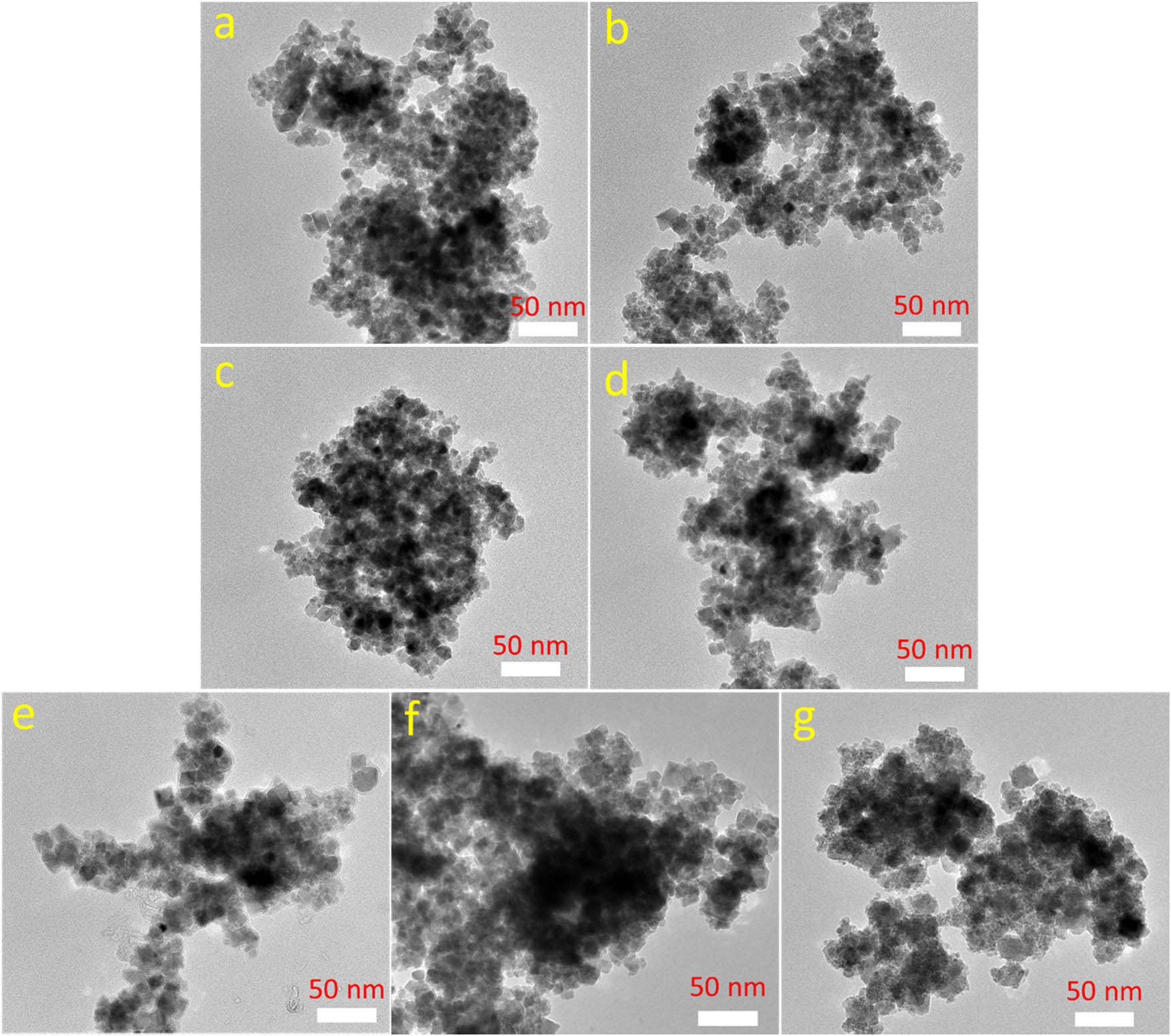
TEM images of (a) F0, (b) FC1, (c) FC2, (d) FC3, (e) FC4, (f) FC5, and (g) C0.
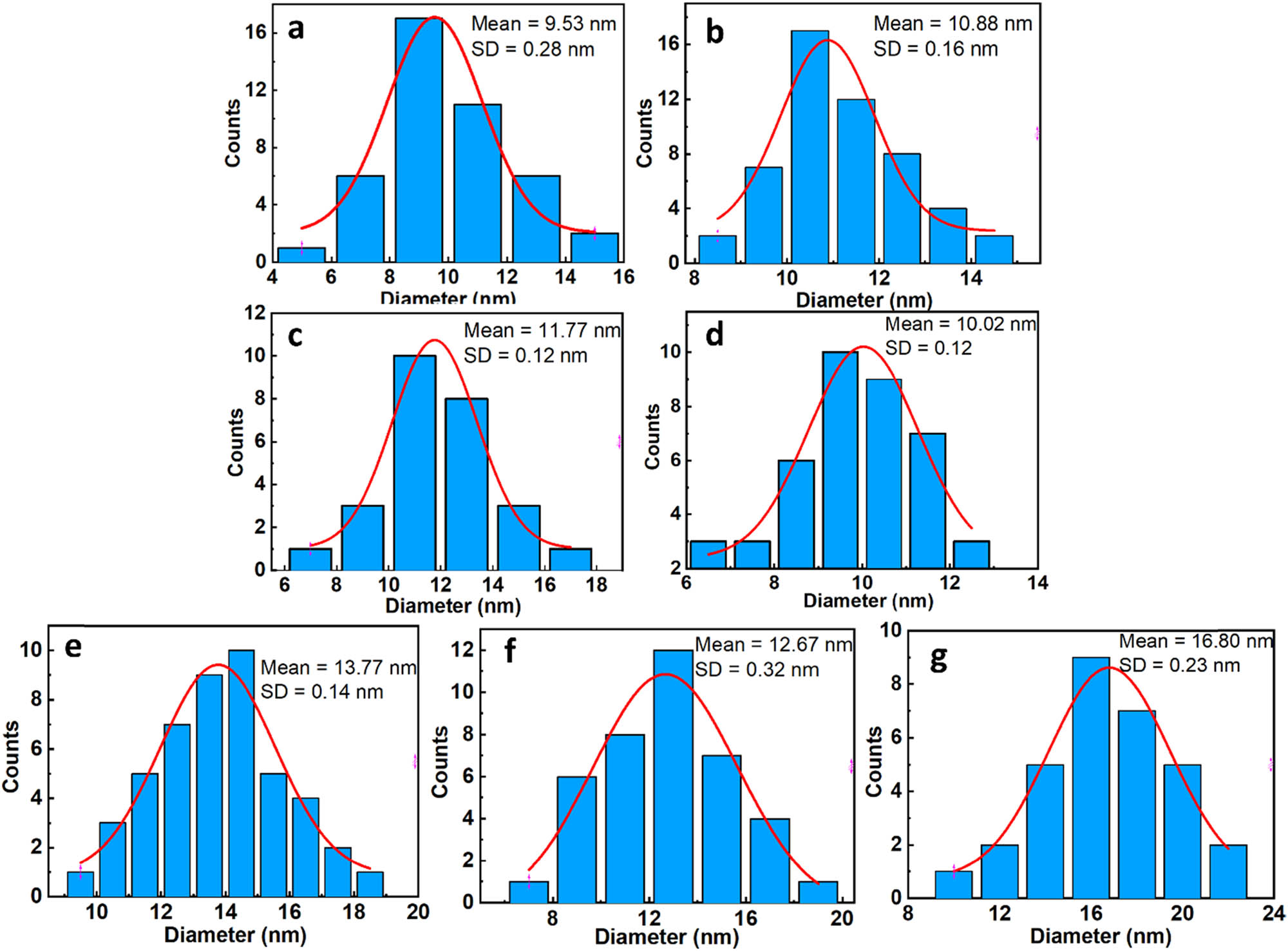
Size distribution of (a) F0, (b–f) FCn, and (g) C0.
The phase and crystalline structure of the as-prepared samples were analyzed by the XRD patterns, as presented in Figure 4(a). The XRD pattern of F0 shows the diffraction peaks at 2θ of 29.81°, 35.13°, 42.98°, 56.81°, and 62.52°, corresponding to the (220), (311), (400), (422), and (511) crystal planes belong to a cubic spinel structure of Fe3O4 (JCPDS # 01-086-1347c), respectively.
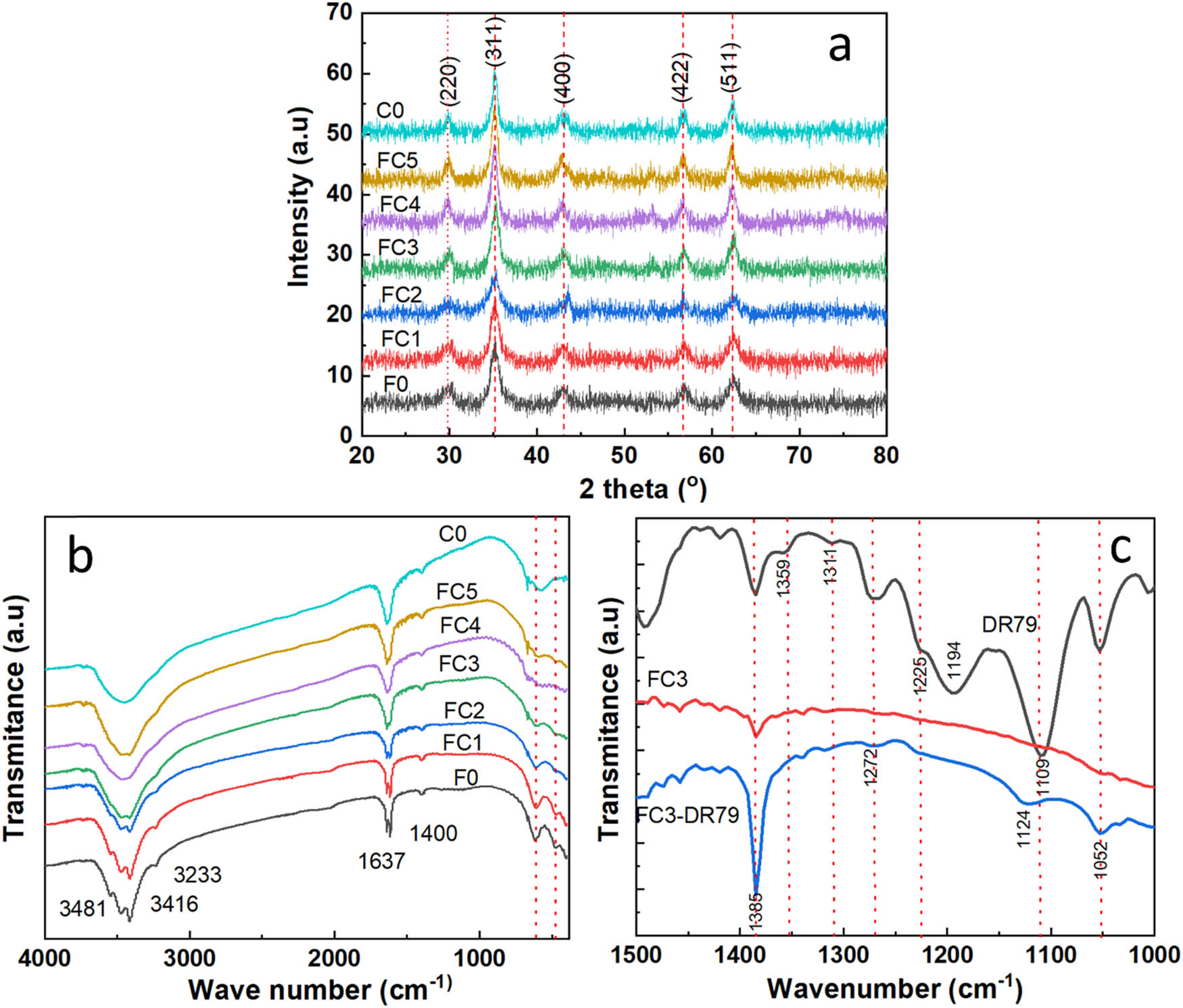
(a) XRD pattern of F0, FCn (n = 1–5), and C0. (b) FTIR spectrum of F0, FCn (n = 1–5), and C0. (c) FTIR spectrum of DR79, FC3 and DR79 adsorption.
The Scherrer equation was applied to determine the crystallite size based on the most intense diffraction peak (311), as shown in the following equation:
where k is a dimensionless shape factor (k = 0.9), λ is the X-ray wavelength (CuKa = 1.5405 Å), β is the full width at the half-maximum of the (311) diffraction peak, and θ is the angle of diffraction (2θ/2). The results are presented in Table 2. For the Co-substituted Fe3O4 nanoparticles, no new diffraction peaks were detected, and the existing diffraction peaks slightly left shifted, indicating Co(ii) ions effectively replaced the Fe(ii) ions in the Fe3O4 structure to form Co x Fe1−x Fe2O4 nanoparticles. Moreover, the obtained results from Table 2 also reveal that with increased Co content, the crystal size of the nanoparticles gradually increased initially (for x = 0, 0.2, 0.4, and 0.5), followed by a rapid escalation from x = 0.6 to 1. This trend was consistent with observations from the TEM image analysis presented earlier. The findings from TEM and XRD analyses may be due to the discrepancy in size between the trivalent(iii) and divalent(ii) oxidation states, specifically Co(ii) and Fe(iii) in the materials, compared with Fe(ii) and Fe(iii) in Fe3O4. The incorporation of Co(ii) into Fe(ii) sites induced lattice distortion, which expanded the crystal size.
Determination of crystal size using the Scherrer equation
| Samples | F0(x = 0) | FC1(x = 0.2) | FC2(x = 0.4) | FC3(x = 0.5) | FC4(x = 0.6) | FC5(x = 0.8) | C0(x = 1) |
|---|---|---|---|---|---|---|---|
| Particlesize (nm) | 6.61 | 6.58 | 6.60 | 6.96 | 8.44 | 8.96 | 9.93 |
The FTIR spectra of the as-prepared samples are shown in Figure 4(b). All samples exhibited similar spectral peaks around 3,200–3,400 cm–1, attributed to the presence of hydroxyl groups on the surface of the nanoparticles, likely influenced by moisture [34]. Two main stretching vibration bands corresponding to metal–oxygen bonds, ν 1 and ν 2, were characteristic of spinel materials. ν 1 corresponded with intrinsic stretching vibrations of the metal at the tetrahedral site, Mtetra ↔ O. ν 2 was assigned to octahedral-metal stretching Mocta ↔ O of Fe(iii) with O [35]. ν 1 shifted by about 610–569 nm from F0 to C0, indicating the replacement of Fe(ii) by Co(ii).
Figure 4(c) illustrates the FTIR spectra of DR79 and FC3 samples before and after DR79 adsorption. New peaks belonging to vibration bands of DR79 are observed in the FTIR spectrum of FC3 after DR79 adsorption compared to that of the FC3 sample. For instance, the band at 1,052 cm−1 reflects the C–O stretch vibration in DR79, and the peaks at 1,194 cm−1 (strong peak) and 1,225 cm−1 (shoulder peak) correspond to the symmetric and asymmetric vibrations of O═S═O in DR79. Additionally, the 1,109 cm−1 band observed in DR79 is attributed to the S–O stretching vibration, while in FC3-DR79, this vibration shifts to 1,124 cm−1, indicative of an interaction between −S−O and the metal ion present in FC3. Notably, the peak at 1,225 cm−1 in DR79 shifts to 1,229 cm−1 (weak peak) in FC3, potentially due to the linkage between O═S═O and the metal ion in FC3. Peaks at 1,272 cm−1, characteristic of C–N stretching in the structure of DR79, are also observed in FC3-DR79. These results confirm that DR79 was adsorbed onto FC3, primarily through interactions with the −SO3 − group on DR79 and the FC3 surface.
The impact of Co(ii) substitution on the energy-band structure, as assessed using UV–Vis-DRS measurements, is depicted in Figure 5(a). A red shift accompanied by multiple absorption peaks (e.g., ∼475 and 669 nm) was found in the absorption edge. This finding indicated a reduction in the bandgap width (E g) values and the presence of multiple absorptions owing to Co(ii) substitution at the Fe(ii) site. Recent studies have suggested that the energy-band structures of these materials undergo direct bandgap transitions. Utilizing the Wood–Tauc method to determine the bandgap width, the material was identified as a direct semiconductor, thereby establishing the dependence of αhυ 2 on hυ, as illustrated in Figure 5(b–h). In this equation, α, h, and υ represent the absorbance coefficient, Planck constant, and frequency, respectively. The bandgap energy was extrapolated from the linear portion intersecting the horizontal axis. These results demonstrated a reduction in bandgap energy with progressive substitution of Fe(ii) by Co(ii), decreasing rapidly from x = 0 to x = 0.5, followed by a slow or nearly negligible decrease from x = 0.6 to x = 1, as depicted in Figure 5(i). Hence, Co(ii) substitution at the Fe(ii) site decreased the bandgap width.
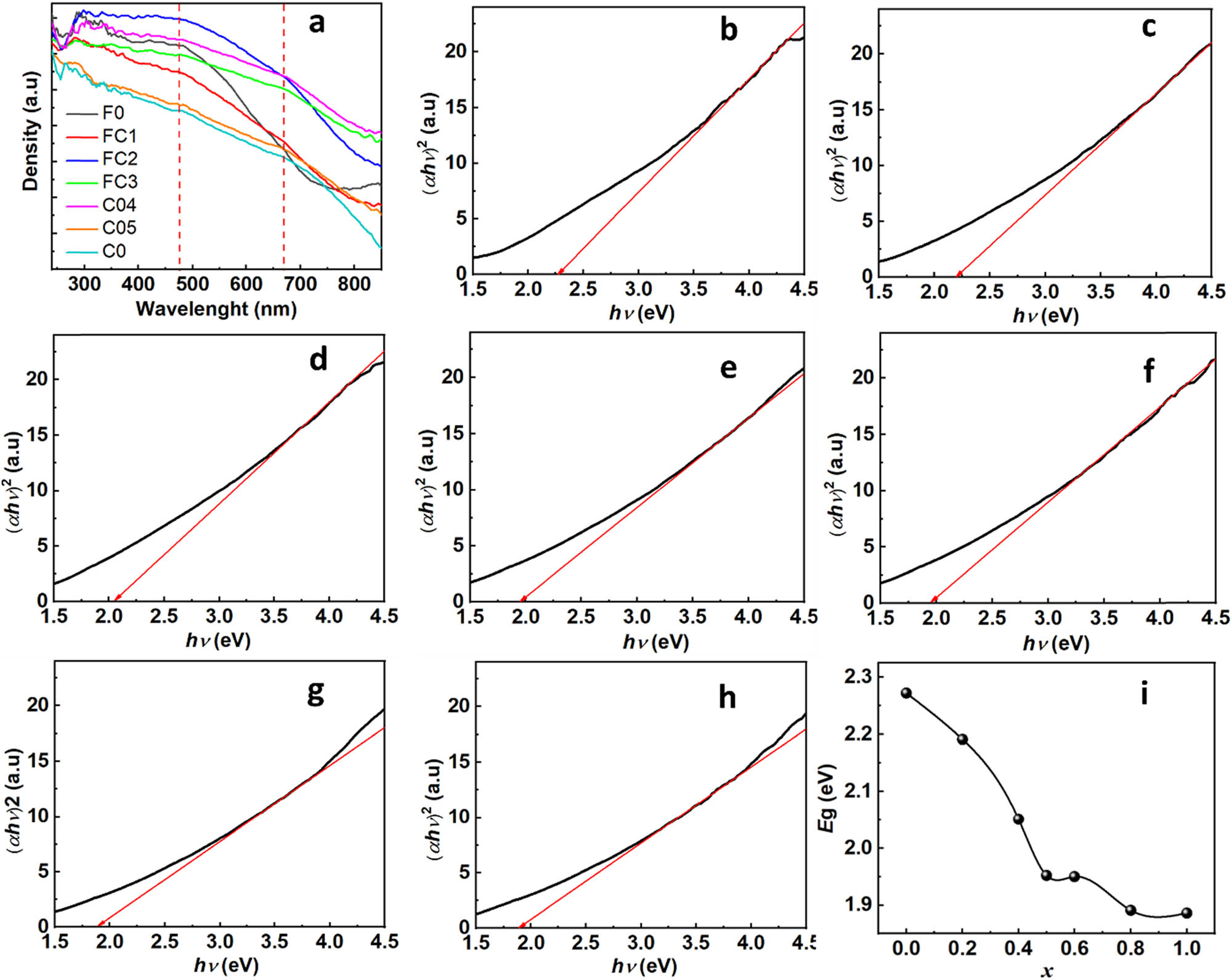
(a) UV–Vis-DRS spectra of F0, FCn, and C0. (b–h) Determination of optical band gap energy (E g) values of F0, FCn, and C0 using the Wood–Tauc method. (i) Plot of E g values of F0, FCn, and C0 vs x.
The field-dependent magnetization curves were obtained at room temperature of all samples shown in Figure 6(a), further confirming the ferromagnetic behavior of the nanoparticles at room temperature. The influence of Co content on the magnetic properties of Fe3O4 nanoparticles is shown in Figure 6(b). It can be seen that the saturation magnetization (M S) of the nanoparticles decreased when increasing the Co substituted content. Interestingly, for Co amounts below 50 mol%, the M S values of the nanoparticles decreased slowly and rapidly reduced for Co amounts over 50 mol%. The MS values obtained were around 93.5 emu/g for the Fe3O4 sample and decreased to 43.1 emu/g for the complete replacement of 1 Fe cation via 1 Co cation to form a CoFe2O4 composition. Our observation results were consistent with the recently reported magnetic properties of Co x Fe1−x Fe2O4 in nanoparticles. The magnetic moments depend on the amounts of Co substitution in Fe3O4 and are also strongly affected by crystal size [34,36,37,38]. Sangsuriyonk et al. [34] reported that the M S values of CoFe2O4 ranged within around 46.19–28.06 emu/g, which strongly depended on the surfactant templates, resulting in changed particle sizes from 42 to 16 nm. Similarly, the M S values of CoFe2O4 compounds are reportedly around 34.70 to 74.08 emu/g, which strongly depend on the nanograin size [36,37,38]. The nanograin size of Fe3O4 also affected the M S values, ranging from 63.36 to 100.41 emu/g [34,36,37,38]. Sangsuriyonk et al. suggested that the reduction of M S values of Co x Fe1−x Fe2O4 samples results from different magnetic moments of Co2+ (3.87 magnetons) and Fe2+ (4.09 magnetons) [34]. Sathya et al. [39] reported that particle size more strongly affects the magnetic moment than does the effect of cobalt content in Co x Fe3−x O4 compounds. Biswal et al. reported that the M S values of CoFe2O4 nanoparticles are affected by the molar ratios of Fe2+ to Co2+ ions, with M S values of 90 emu/g at 75:25 molar ratios of Fe2+ to Co2+ and a particle size of 13 nm [40]. Our TEM results exhibited that the samples’ nanograin size ranged between 10 and 17 nm. We suggested that the magnetic moment strongly depends on the nanograin size and the magnetic moment compensation between Co and Fe cations within the FeFe2O4 crystal structure during substitution to form as single phase.
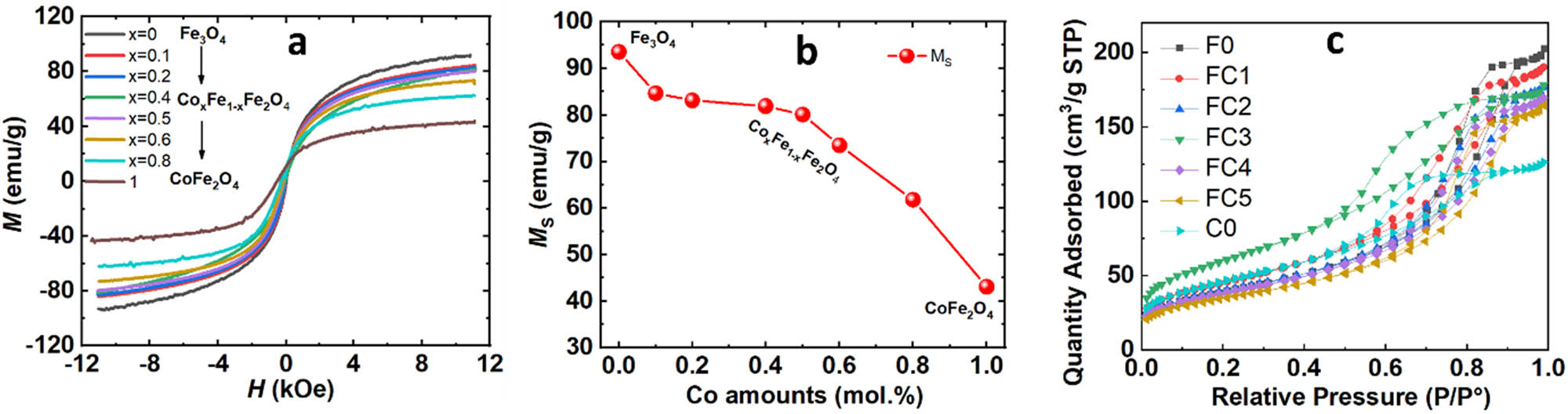
(a) Magnetic hysteresis loops of F0, FCn, and C0; (b) plot of M S vs F0, FCn, and C0; and (c) N2 – adsorption/desorption isotherms of F0, FCn, and C0.
The properties related to porosity and surface area of the materials were determined from the adsorption–desorption isotherms of nitrogen using the BET method. Figure 7 illustrates that the adsorption–desorption isotherms of F0, FCn, and C0 fell under type IV and H-type, which were characteristic of mesoporous materials.
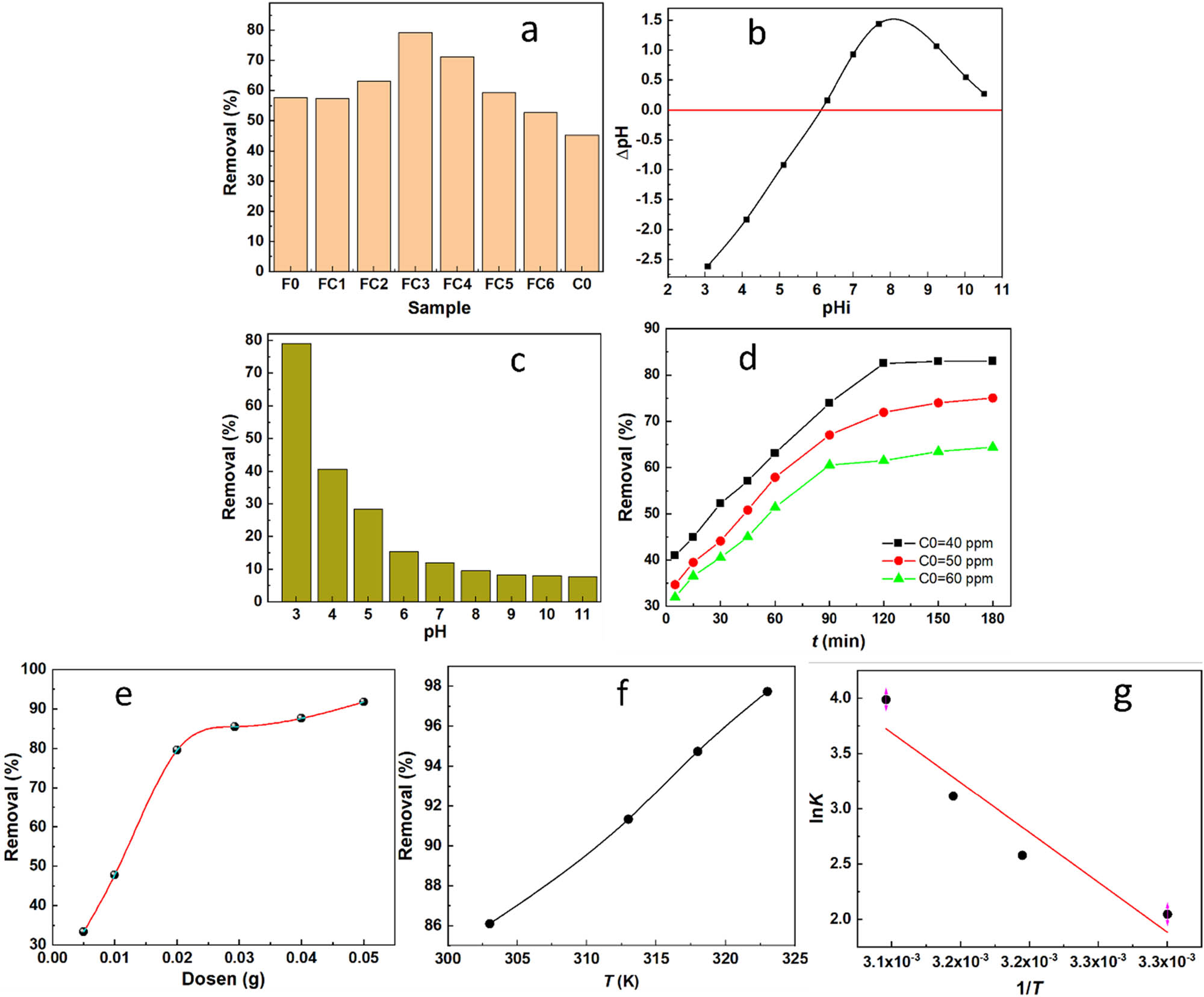
(a) Removal (%) of DR79 on different materials (F0, FCn, and C0); (b) determination of FC3’s isoelectric point; (c) adsorption efficiency of FC3 to DR79 at different pH values from 3 to 11; (d) effect of contact time on DR79 adsorption (FC3 mass of 0.02 g, pH 3, shake speed of 250 rpm, temperature of 25°C; (e) effect of adsorbent dose on the adsorption of DR79 (V = 25 mL, pH 3, 250 rpm, 25°C); (f) adsorption efficiency of FC3 to DR79 (C 0 = 50 mg/L, pH 3) at different temperatures; and (g) plot of lnK vs 1/T.
Surface area, pore volume, and pore diameter are listed in Table 3. Results indicated significantly large specific surface areas for the materials, with the highest observed in FC3. The pore size and volume suggested that the materials had average mesopore size, promising good adsorption capabilities.
Parameters in BET adsorption of materials
| Samples | BET area (m2/g) | Pore volume (cm3/g) | Pore size (nm) |
|---|---|---|---|
| F0 | 142.2478 | 0.309 | 8.9639 |
| FC1 | 165.5367 | 0.285394 | 7.3799 |
| FC2 | 143.6720 | 0.265636 | 7.8945 |
| FC3 | 217.9623 | 0.263953 | 5.0186 |
| FC4 | 139.3112 | 0.254646 | 7.8998 |
| FC5 | 126.1336 | 0.247761 | 8.5075 |
| C0 | 167.1040 | 0.181129 | 4.9022 |
3.2 Adsorption of Co x Fe1−x Fe2O4 onto DR79
3.2.1 Selection of Co x Fe1−x Fe2O4
A preliminary investigation was conducted to select the material with the appropriate value of x for studying the adsorption properties of the material. We determined the adsorption efficiency of 0.02 g of material for DR79 in a 25 mL solution with a concentration of 50 mg/L at pH 3 with a sample shaking rate of 250 rpm. As shown in Figure 7(a), FC3 (x = 0.5) exhibited the highest adsorption efficiency. Therefore, FC3 was chosen to investigate the adsorption properties for subsequent experiments.
3.2.2 Effect of initial pH
The determination of the isoelectric point of the ferrite material is depicted in Figure 7(b). The isoelectric point was defined as the intersection of the ΔpH – pHi curve with the pHi axis, yielding a value of 6.1. This finding indicated that below a pH of 6.1, the material surface was negatively charged, and above a pH of 6.1, the material surface was positively charged. At pH 6.1, the material surface was neutral.
The adsorption efficiency of DR79 dye within the pH range of 3–11, with an initial concentration of 50 mg/L in a volume of 25 mL, is depicted in Figure 7(c). The adsorption efficiency of DR79 dye onto ferrite highly depended on pH. Approximately 79.13% of the dye was adsorbed at pH 3, whereas the amount of dye adsorbed decreased with increased pH, reaching an adsorption efficiency of 7.5% at pH 11.
This finding can be explained as follows. With decreased pH, the concentration of H+ ions in the environment increased, facilitating electrostatic interaction with the negatively charged DR79 dye molecules, allowing them to adhere to the surface of ferrite and creating favorable conditions for adsorption. Conversely, with increased pH, the concentration of OH− ions increased, resulting in the surfaces of the adsorbent material becoming more negatively charged. In this scenario, repulsive forces dominated between the ferrite and DR79 dye molecules. Additionally, owing to the excess OH– ions in the solution, competitive adsorption occurred between the anionic DR79 dye molecules and OH– ions, reducing the adsorption efficiency. Therefore, pH 3 was selected for subsequent experiments.
3.2.3 Effect of contact time
Figure 7(d) illustrates the dependence of DR79 adsorption efficiency on contact time at various DR79 concentrations, and all materials had a consistent trend. Specifically, within the observation period ranging from 5 to 180 min, the adsorption efficiency increased relatively rapidly from 5 to 120 min and approached linearity. Subsequently, it stabilized gradually between 120 and 180 min. This finding can be explained as follows. Initially, adsorption occurred rapidly owing to the availability of vacant adsorption sites on the adsorbent surface. After 120 min, adsorption slowed down because the number of available adsorption sites decreased. In other words, adsorption had sufficient time to reach equilibrium. Therefore, an adsorption time of 120 min was used to study the processes related to adsorption equilibrium.
3.2.4 Effect of adsorbent dose
Figure 7(e) shows that the adsorption efficiency of DR79 increased with an increased dose of FC3. In the investigated FC3 mass range from 0.005 to 0.05 g, the adsorption efficiency of DR79 increased from 33.33 to 91.75%. This finding was due to the larger surface area, increased number of adsorption sites, and a larger number of surface functional groups. Adsorption efficiency increased linearly with increased FC3 mass from 0.005 to 0.02 g and then increased slowly. Therefore, we chose an FC3 mass of 0.02 g (or an adsorption dose of 0.8 g/L) for subsequent experiments.
3.2.5 Effect of adsorption temperature
Figure 7(f) shows that with increased temperature, the adsorption efficiency also increased. At various temperatures, the adsorption efficiency of DR79 on ferrite was found to be 86.09% at 303 K, 91.34% at 313 K, 94.74% at 318 K, and 97.73% at 323 K. The increased adsorption efficiency of DR79 on ferrite with increased temperature can be explained as follows. The adsorption of DR79 onto ferrite was endothermic. With increased temperature, the adsorption equilibrium shifted forward, reducing the adsorbate concentration in the solution. The outcome was enhanced adsorption capacity of the process.
3.2.6 Adsorption-isotherm models
All adsorption isotherms investigated are depicted in Figure 8, and the calculated constants and correlation coefficients derived from these plots are presented and compared in Table 4, which also illustrates the model parameters of the DR79 adsorption isotherm on FC3. Comparing the correlation coefficients, the Langmuir [Figure 8(a)] and Redlich–Peterson [Figure 8(d)] isotherm models were the most appropriate for the experimental data, with R 2 > 0.99. The maximum adsorption capacity determined by the Langmuir isotherm model was 44.07 mg g–1. The Freundlich [Figure 8(b)] and Temkin [Figure 8(c)] isotherm models exhibited considerably lower R 2 values (0.7831 and 0.7594, respectively), indicating poor fits of the experimental data to these two models. The adsorption capacity of ferrite and other adsorbents for various types of anionic dyes is compared in Table 6 [26,41,42,43,44,45,46,47,48,49].
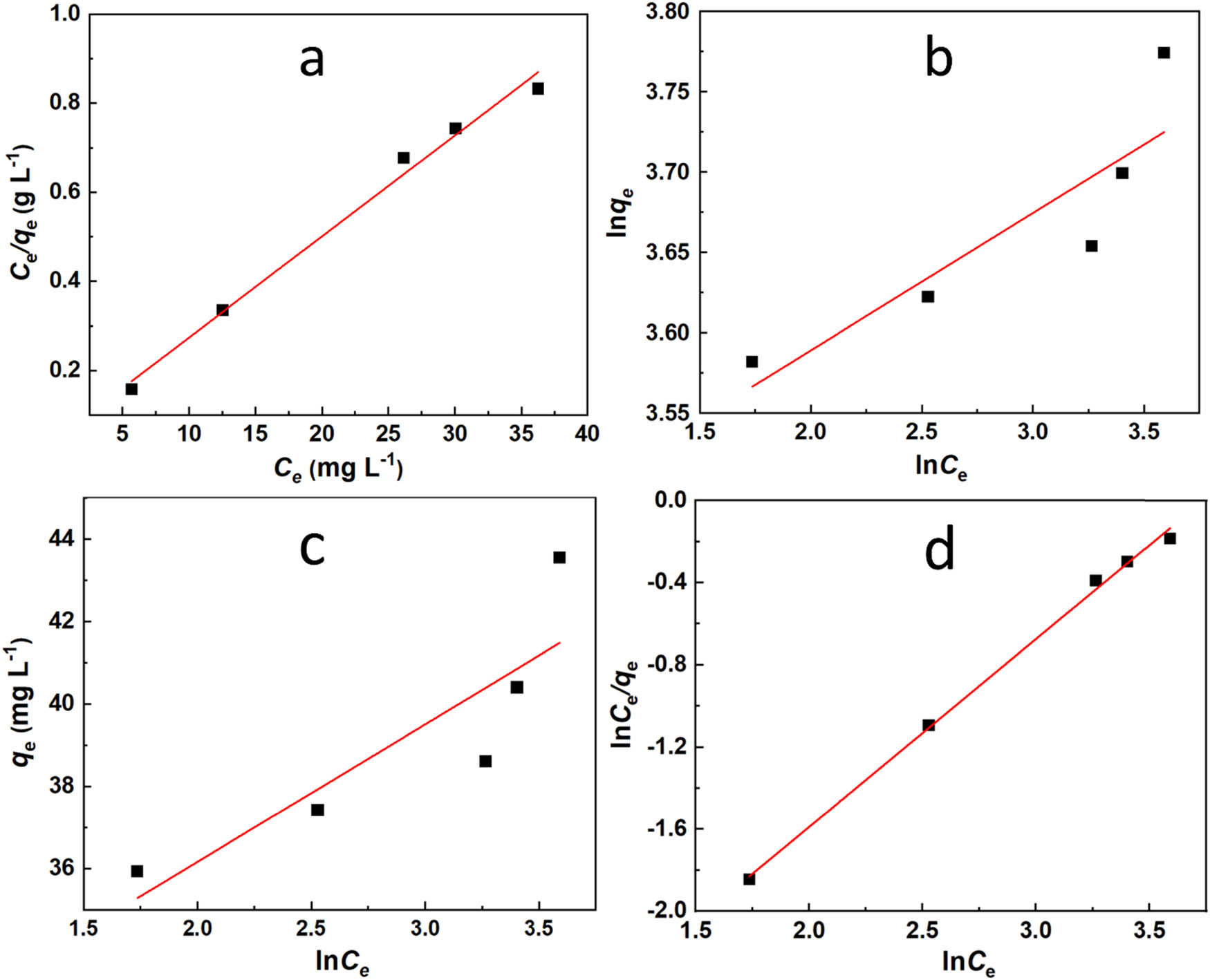
Representation of adsorption-isotherm models: (a) Langmuir, (b) Freundlich, (c) Temkin, and (d) Redlich–Peterson.
Experimental parameters from adsorption-isotherm models
| Langmuir | q max (mg g−1) | K L | R L | R 2 |
| 44.07 | 0.4819 | 0.0288 | 0.9901 | |
| Freundlich | K F | 1/n | R 2 | |
| 30.51 | 0.0854 | 0.7831 | ||
| Temkin | A T (L g−1) | B | b T (kJ mol−1 K−1) | R 2 |
| 6838.774 | 3.3395 | 741.889 | 0.7594 | |
| Redlich–Peterson | β | A (mg L−1) | R 2 | |
| 0.9146 | 30.5144 | 0.9976 |
3.2.7 Adsorption kinetics
The adsorption kinetics of the material were investigated by analyzing its adsorption capacity over time, as depicted in Figure 7(d). Various kinetic models, including pseudo-first-order, pseudo-second-order, intraparticle diffusion, and Elovich, were studied and are illustrated in Figure 9(a–d). The parameters determined from the pseudo-first-order, pseudo-second-order, intraparticle diffusion, and Elovich kinetic models are presented in Table 5. The values of k 1 and k 2 were calculated from the plots of log(q e – q t ) versus t and t/q t versus t, respectively, as depicted in Figure 9(a) and (b), respectively. Comparing the correlation coefficients (R 2) for all kinetic models in Table 5 revealed that the pseudo-second-order kinetic model was the most suitable. The correlation coefficients for the pseudo-second-order kinetic model (R 2 ≥ 0.983) were higher than those for the pseudo-first-order (R 2 ≤ 0.9814) and Elovich models (R 2 ≤ 0.9319), as well as the intraparticle diffusion model. The intragranular diffusion model exhibited a substantial R 2 of ≥0.9663, indicating that the adsorption kinetics of the system partially conformed to this model as well. The values of k id were obtained from the slope of the linear plot of q t versus t 0.5 from Figure 9(c). The intercept for this plot was not equal to 0, meaning that intraparticle diffusion was not solely rate-limiting.
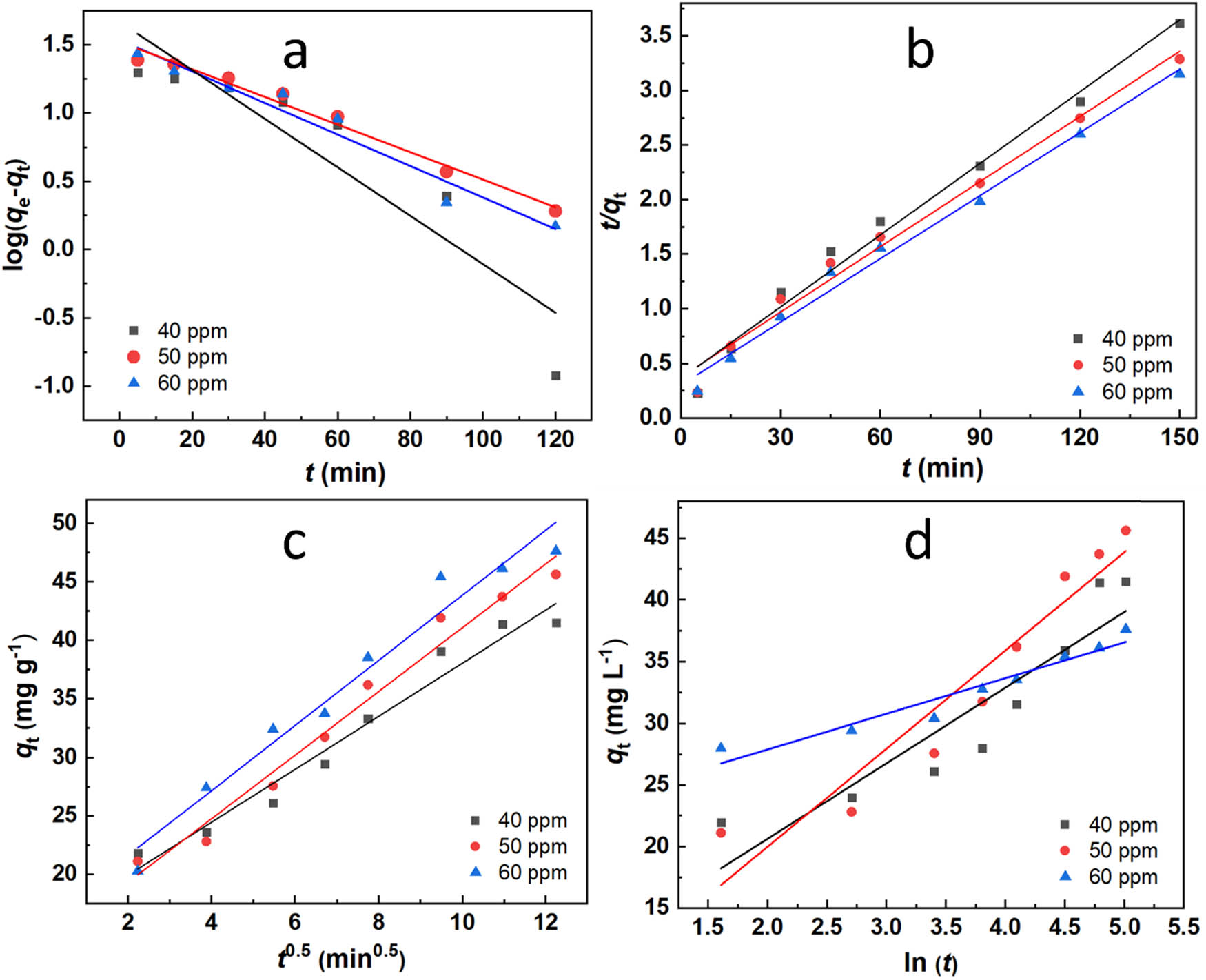
Plots of kinetic adsorption models: (a) pseudo-first-order, (b) pseudo-second-order, (c) intraparticle diffusion, and (d) Elovich.
Parameters and constants obtained from the kinetic models
| First-order kinetic | Second-order kinetic | |||||
|---|---|---|---|---|---|---|
| C o (mg L−1) | q e (mg g−1) | k 1 (min−1) | R 2 | q e (mg g−1) | k 2 (min−1 L mg−1) | R 2 |
| 40 | 46.6767 | 0.040993 | 0.8526 | 51.8135 | 0.000112268 | 0.9902 |
| 50 | 33.3811 | 0.023260 | 0.9814 | 50.2513 | 0.000147712 | 0.9859 |
| 60 | 34.4191 | 0.026715 | 0.9597 | 45.6621 | 0.000172708 | 0.9830 |
| Intragranular diffusion | Elovich | |||||
|---|---|---|---|---|---|---|
| k id | C | R 2 | α | β | R 2 | |
| 40 | 2.2645 | 15.39 | 0.9664 | 23.93317 | 0.162999 | 0.8493 |
| 50 | 2.7190 | 13.87 | 0.9773 | 13.23777 | 0.125560 | 0.9137 |
| 60 | 2.7778 | 16.05 | 0.9663 | 6110.2921 | 0.346332 | 0.9319 |
Comparison with some adsorption systems of the material for anionic dye reported in some literature demonstrated the excellent adsorption capacity of the material. The equilibrium adsorption model and the kinetic adsorption model were consistent with previous studies Table 6.
Comparison of some adsorption parameters of the material with those reported in previous studies
| Adsorbent | Adsorbant | q max (mg g−1) | Isothermal adsorption model/kinetic model | Ref. |
|---|---|---|---|---|
| Spent tea leaves (modified polyethyleneimine) | Reactive Black 5 | 71.9 | Temkin/pseudo-second-order | [41] |
| Nerium oleander flower (activated carbon) | Direct turquoise blues | 33.3 | Langmuir and Freundlich/pseudo-second-order | [42] |
| Cassava root husk | Direct Black ECO TFA | 46.1 | Langmuir/pseudo-second-order | [43] |
| Rice husk | Direct red 31 | 25.63 | Langmuir/pseudo-second-order | [44] |
| Direct Orang 26 | 19.96 | |||
| Clay | Direct Red 243 | 156.25 | Langmuir/pseudo-second-order | [26] |
| Canola hul | Reactive 198 | 2.8 | Temkin/pseudo-second-order | [45] |
| Reactive Blue 19 | 2.0 | |||
| Direct red 79 | 3.1 | |||
| Direct red 80 | 8.7 | |||
| Orange Pell | Direct red 79 | 151.5 | Langmuir and Freundlich/pseudo-second-order | [46] |
| Direct Yellow 27 | 153.85 | |||
| Powdered tourmaline | Direct red 23 | 153 | — | [47] |
| Fe x Co3−x O4 | Congo Red | 128.6 | Langmuir and Freundlich/pseudo-first-order | [48] |
| Commercial MCC | Reactive Blue 21 | 30 | Langmuir/pseudo-second-order | [49] |
| FC3 | Direct Red 79 | 44.07 | Langmuir, Redlich-Petorson/pseudo-second-order, intragranular diffusion | This work |
Thermodynamic parameters of adsorption
| T (K) | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (kJ/mol K) |
|---|---|---|---|
| 303 | −5.155 | 74.947 | 0.263 |
| 313 | −6.711 | ||
| 318 | −8.235 | ||
| 323 | −10.706′ |
3.2.8 Adsorption thermodynamic
Variations in the thermodynamic parameters, namely, free energy (∆G 0), enthalpy (∆H 0), and entropy (∆S°), of the adsorption process were calculated using the following equations:
where K D is the equilibrium constant, R is the gas constant, and T is the temperature in Kelvin scale.
The linear relationship between lnK D and 1/T Figure 7(g) allows for the determination of thermodynamic quantities, with the results presented in Table 7. The values of variation in free energy (∆G 0) determined ranged from −5.155 to −10.706 kJ/mol, and the entropy variation (∆S 0) was 0.263 kJ/mol. This finding indicated that the adsorption of DR79 onto ferrite was spontaneous. The calculated enthalpy variation (∆H 0) was 74.947 kJ/mol, demonstrating that the adsorption process was endothermic. In adsorption processes, when ∆H 0 <25 kJ/mol, Van Der Waals forces are the main factor for physical adsorption. If ∆H 0 = 40–200 kJ/mol, chemical bonding predominates, leading to chemical adsorption.
Comparison of FC3 with other adsorbents
| Adsorbent | Dye | Initial concentration of dye (ppm) | Dose of dye (g/L) | Adsroption time (min) | Optimal pH | H(%) – q max (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Powdered tourmaline | DR23 | 4.10−5 mol/L | 4.0 | 180 | 3 | 153 | [47] |
| Sepiolite | RB15 | 20 | 2.0 | 180 | 6 | 31.98 | [50] |
| Cassava root husk | Direct Black ECO TFA | 100 | 6.0 | 24 × 60 | 2 | 46.1 | [43] |
| Nano round polycrystalline | Congo Red (CR) | 200 | 1.6 | 100 | 2 | 77.4%; 98,216 | [51] |
| NBRC 1658 strain | RY18 | 300 | 10 | 24 × 60 | 2 | 88% – 90.17 | [52] |
| AR18 | 10 | 2 | 56% – 129.53 | ||||
| BB41 | 10 | 9 | 75% – 729.92 | ||||
| Clay | Astrazon Red | 100 | 2.0 | 60 | — | 95% – 67.11 | [53] |
| Astrazon Blue | 125 | 1.5 | 60 | — | 90% – 90.91 | ||
| Algerian kaolinite (DD3) | MB | 75 | 1.0 | 60 | Unadjus-ted pH (7) | 44.48 | [54] |
| EPGAC | DY12 | 125 | 3.0 | 120 | 2 | 42.01 | [55] |
| FC3 | DR79 | 50 | 0.8 | 120 | 3 | 79% – 44.07 | This work |
3.2.9 Comparison of the FC3 performance with other adsorbents and real wastewater
In Table 8, the organic matter removal efficiency of FC3 nanoparticles is comparable to other adsorbents [43,47,50,51,52,53,54,55], while the dosage used is very small and the treatment time is relatively short.
To evaluate the effectiveness of the real wastewater treatment process, we conducted UV–Vis spectroscopy scans and determined the chemical oxygen demand (COD) values of the wastewater samples before and after treatment. In this study, the textile dyeing wastewater samples were collected from a traditional dyeing village in Ha Dong district, Hanoi, Vietnam. The color of the textile dyeing wastewater could involve various dyes, so we performed UV–Vis scans on the red-colored wastewater samples before and after 120 min of adsorption by FC3, as shown in Figure 10(e). The results indicated that the actual red-colored wastewater sample exhibited a single maximum absorption wavelength at λ = 510 nm. The adsorption efficiency of the red-colored wastewater sample before and after 120 min of adsorption by FC3 was 57%.
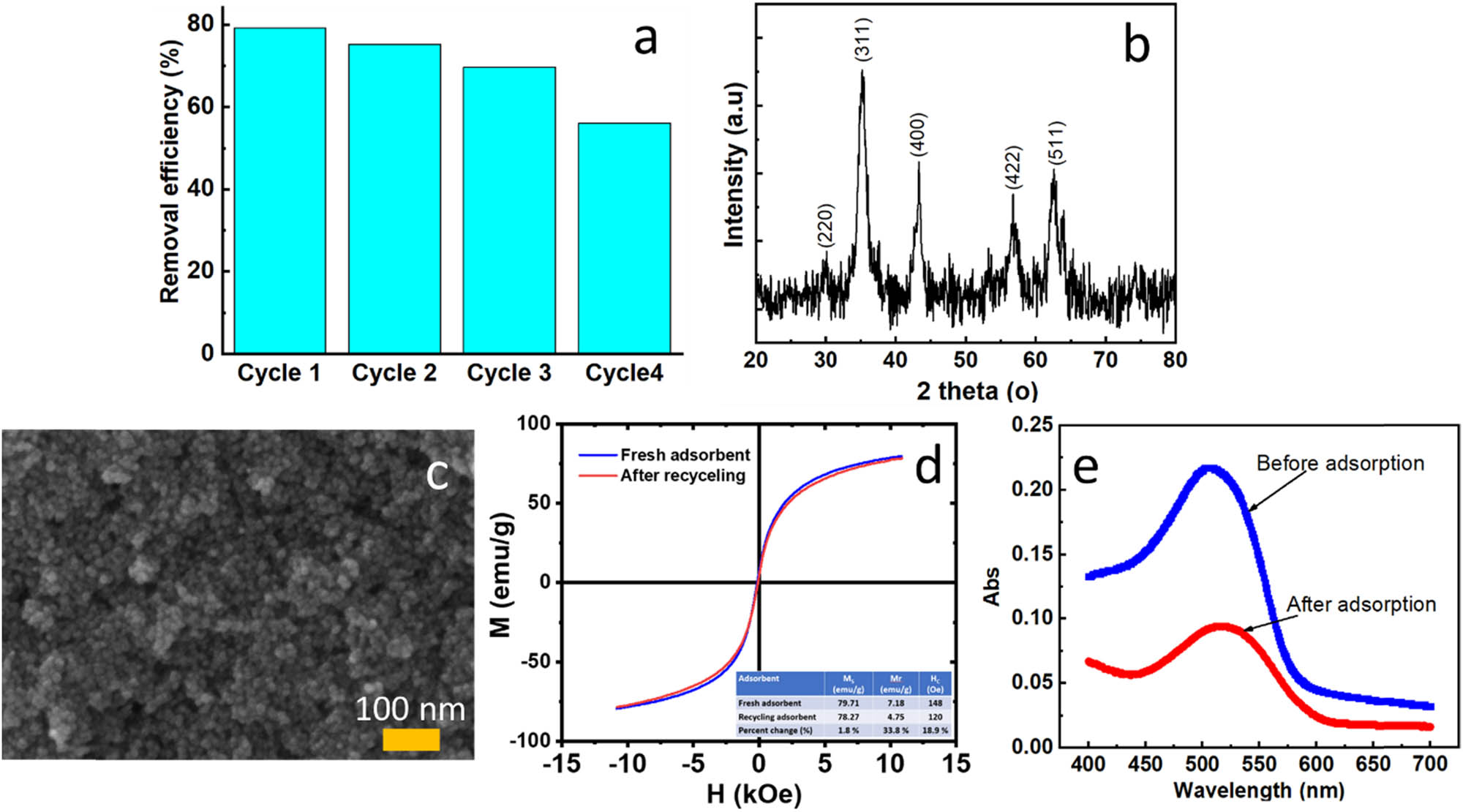
(a) Recycling of FC3 in the removal of DR79 (pH: 3, dose: 0.8 g/L, DR79 initial concentration: 50 mg/L), (b) XRD, (c) FESEM and (d) VSM analysis after four recovery steps, and (e) UV–Vis spectrum of the real wastewater sample before and after 120 min of adsorption (pH: 3, nano-adsorbent dosage: 0.8 g/L).
COD is an important method for measuring the concentration of organic substances in wastewater solutions, determining the total amount of oxygen required to oxidize the organic dyes into CO2 and water [56]. This implies that a low COD value indicates high organic matter treatment (adsorption) efficiency. Conversely, a high COD value reflects a significant oxygen demand to oxidize the remaining organic substances after the adsorption process.
After 120 min of adsorption, the COD of the wastewater decreased from 406.67 to 185 mg/L, achieving a COD removal efficiency of 54.5%. Notably, this COD value fell below the WHO’s permissible limit of 200 mg/L for wastewater, demonstrating the potential of FC3 in removing dyes through the adsorption process.
3.2.10 Chemical stability and reusability
From both economic and environmental perspectives, the recovery and reuse of adsorbent materials are crucial. Due to their strong magnetic properties, FC3 magnetic nanoparticles can be easily separated from the medium using a magnet. After the adsorbent was removed from the treatment medium, it was washed multiple times, first with double-distilled water and then with ethanol, until the washing solution was free of DR79 color [57]. The adsorbent was then dried in an oven at 80°C for 24 h before being subjected to reuse evaluation. Four regeneration cycles were conducted to assess the performance of the reused adsorbent. The DR79 removal efficiency of FC3 magnetic nanoparticles decreased from 79% in the first cycle to 67%. After four regeneration cycles, the efficiency dropped to 42% (DR79: 50 mg/L; 0.8 g/L; pH 3) under the same conditions Figure 10(a). This decline in efficiency may be attributed to the occupation of active sites on the adsorbent surface by DR79 molecules during previous adsorption cycles. According to Nasiri et al., the ZnCoFe2O4@Ch magnetic nano-adsorbent demonstrated an adsorption efficiency of 65% after five cycles of reuse for Tetracycline removal [58]. The structure of the material after the adsorption cycles was examined using XRD, FESEM, and VSM, as shown in Figure 10(b–d), indicating that the spinel ferrite structure of the FC3 material remained intact after four adsorption cycles. Additionally, the chemical stability of Co x Fe1−x Fe2O4 was evaluated by determining the concentrations of Co and Fe in the solution using atomic absorption spectroscopy with the spectrophotometer (ASS NOVAA 400p) at wavelengths of 241.7 and 243.3 nm, respectively. According to the measurements obtained from the equipment, the concentration of Co is 0.99 mg/L, which meets the permissible limit for Co (1.0 mg/L) in irrigation water [59]. Meanwhile, the concentration of Fe is 2.15 mg/L, which complies with the WHO standard for Fe content in industrial wastewater, where the permissible limit is 5 mg/L.
4 Conclusion
Ferromagnetic nanoparticles Co x Fe1−x Fe2O4 (x = 0–1) with spinel structural were synthesized via co-precipitation in which the formation of extremely small magnetic nanoparticles owing to sudden nucleation at elevated temperatures. These nanosized particles contributed to increased specific surface area of the material, thereby enhancing its adsorption capacity. Importantly, the ferromagnetic properties of Co x Fe1−x Fe2O4 exhibited superior environmental stability to Fe3O4 oxide. The adsorption performance of Co x Fe1−x Fe2O4 toward DR79, particularly noteworthy for its effectiveness at low pH, demonstrated its potential as a highly efficient adsorbent. Moreover, the magnetic properties of the material facilitated easy recovery post-adsorption. The optimal adsorption occurred at x = 0.5. Adsorption behavior was systematically analyzed using the Langmuir, Freundlich, Temkin, and Redlich–Peterson models. The Langmuir and Redlich–Peterson models proved to be the most suitable. Among the investigated kinetic models, pseudo-second-order kinetics was determined to be the most appropriate. This study provides valuable insights into the synthesis, characterization, and application of Co x Fe1−x Fe2O4 materials for the efficient removal of DR79. After four cycles of recovery and reuse, the material demonstrated a strong color removal efficiency while maintaining commendable chemical stability. Specifically, the material achieved an adsorption efficiency of 57% for color removal and 54.5% for COD reduction in an actual textile wastewater sample, promising its potential for future environmental remediation applications.
-
Funding information: This work was financially supported by Thai Nguyen University of Education under project number TNUE-2023-04.
-
Author contributions: Vu Thi Hau: writing – original draft; Pham Hoai Linh: formal analysis; Pham Thu Ha: data curation; Nguyen Thuy Chinh: methodology; Ngo Thi Mai Viet: methodology; Dang Duc Dung: formal analysis; Nguyen Quoc Dung: writing – review & editing; Thi Kim Ngan Tran: resources, validation.
-
Conflict of interest: The authors declare that they have no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Kandisa RV, Saibaba KN, Shaik KB, Gopinath R. Dye removal by adsorption: a review. J Biorem Biodegrad. 2016;7(6):371.10.4172/2155-6199.1000371Search in Google Scholar
[2] Ziane S, Bessaha F, Marouf-Khelifa K, Khelifa A. Single and binary adsorption of reactive black 5 and Congo red on modified dolomite: Performance and mechanism. J Mol Liq. 2018;249:1245–53.10.1016/j.molliq.2017.11.130Search in Google Scholar
[3] Fard MHA, Nasiri A, Daraei H. Green synthesis of AgCoFe. sub. 2O. sub. 4@ Ch/AC as a recyclable, magnetic nanohybrid heterogeneous catalyst in photodegradation of ceftriaxone from aqueous solutions with effluent bioassay. Appl Water Sci. 2023;13(11):NA-NA.10.1007/s13201-023-02026-wSearch in Google Scholar
[4] Gharaghani MA, Samaei M, Mahdizadeh H, Nasiri A, Keshtkar M, Mohammadpour A, et al. An effective magnetic nanobiocomposite: Preparation, characterization and its application for adsorption removal of P-nitroaniline from aquatic environments. Environ Res. 2024;246:118128.10.1016/j.envres.2024.118128Search in Google Scholar PubMed
[5] Rafiq A, Ikram M, Ali S, Niaz F, Khan M, Khan Q, et al. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J Ind Eng Chem. 2021;97:111–28.10.1016/j.jiec.2021.02.017Search in Google Scholar
[6] Sarkodie B, Amesimeku J, Frimpong C, Howard EK, Feng Q, Xu Z. Photocatalytic degradation of dyes by novel electrospun nanofibers: A review. Chemosphere. 2023;313:137654.10.1016/j.chemosphere.2022.137654Search in Google Scholar PubMed
[7] Arif N, Ma Y, Zafar MN, Humayun M, Bououdina M, Zhang SY, et al. Design and fabrication of biomass derived black carbon modified g‐C3N4/FeIn2S4 heterojunction as highly efficient photocatalyst for wastewater treatment. Small. 2024;20(20):2308908.10.1002/smll.202308908Search in Google Scholar PubMed
[8] Gan Y, Ding C, Xu B, Liu Z, Zhang S, Cui Y, et al. Antimony (Sb) pollution control by coagulation and membrane filtration in water/wastewater treatment: A comprehensive review. J Hazard Mater. 2023;442:130072.10.1016/j.jhazmat.2022.130072Search in Google Scholar PubMed
[9] Chen L, Xue Y, Luo T, Wu F, Alshawabkeh AN. Electrolysis-assisted UV/sulfite oxidation for water treatment with automatic adjustments of solution pH and dissolved oxygen. Chem Eng J. 2021;403:126278.10.1016/j.cej.2020.126278Search in Google Scholar PubMed PubMed Central
[10] Zhang H, Quan H, Yin S, Sun L, Lu H. Unraveling the toxicity associated with ciprofloxacin biodegradation in biological wastewater treatment. Environ Sci Technol. 2022;56(22):15941–52.10.1021/acs.est.2c04387Search in Google Scholar PubMed
[11] Pohl A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water, Air, Soil Pollut. 2020;231(10):503.10.1007/s11270-020-04863-wSearch in Google Scholar
[12] Li Z, Huang Y, Wang X, Wang D, Wang X, Han F. Three-dimensional hierarchical structures of ZnO nanorods as a structure adsorbent for water treatment. J Mater Sci Technol. 2017;33(8):864–8.10.1016/j.jmst.2016.11.022Search in Google Scholar
[13] Elbasuney S, Elsayed MA, Mostafa SF, Khalil WF. MnO2 nanoparticles supported on porous Al2O3 substrate for wastewater treatment: Synergy of adsorption, oxidation, and photocatalysis. J Inorg Organomet Polym Mater. 2019;29:827–40.10.1007/s10904-018-01057-0Search in Google Scholar
[14] Paswan SK, Kumar P, Singh RK, Shukla SK, Kumar L. Spinel ferrite magnetic nanoparticles: an alternative for wastewater treatment. In: Pollutants and water management: Resources, strategies and scarcity. John Wiley & Sons Ltd.; 2021. p. 273–305.10.1002/9781119693635.ch11Search in Google Scholar
[15] Reddy DHK, Yun YS. Spinel ferrite magnetic adsorbents: alternative future materials for water purification? Coord Chem Rev. 2016;315:90–111.10.1016/j.ccr.2016.01.012Search in Google Scholar
[16] Rashid R, Shafiq I, Akhter P, Iqbal MJ, Hussain M. A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res. 2021;28:9050–66.10.1007/s11356-021-12395-xSearch in Google Scholar PubMed
[17] Li X-M, Xu G, Liu Y, He T. Magnetic Fe3O4 nanoparticles: Synthesis and application in water treatment. Nanosci Nanotechnology-Asia. 2011;1(1):14–24.10.2174/2210682011101010014Search in Google Scholar
[18] Zeng H, Qiao T, Zhai L, Zhang J, Li D. Fe3O4@C particles synthesized with iron‐containing water treatment residuals and its potential for methylene blue removal. J Chem Technol Biotechnol. 2019;94(12):3970–80.10.1002/jctb.6202Search in Google Scholar
[19] Kefeni KK, Mamba BB, Msagati TA. Application of spinel ferrite nanoparticles in water and wastewater treatment: a review. Sep Purif Technol. 2017;188:399–422.10.1016/j.seppur.2017.07.015Search in Google Scholar
[20] Konicki W, Sibera D, Mijowska E, Lendzion-Bieluń Z, Narkiewicz U. Equilibrium and kinetic studies on acid dye Acid Red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J Colloid Interface Sci. 2013;398:152–60.10.1016/j.jcis.2013.02.021Search in Google Scholar PubMed
[21] Khosravi I, Eftekhar M. Characterization and evaluation catalytic efficiency of NiFe2O4 nano spinel in removal of reactive dye from aqueous solution. Powder Technol. 2013;250:147–53.10.1016/j.powtec.2013.10.021Search in Google Scholar
[22] Nithya R, Thirunavukkarasu A, Sathya AB, Sivashankar R. Magnetic materials and magnetic separation of dyes from aqueous solutions: a review. Environ Chem Lett. 2021;19:1275–94.10.1007/s10311-020-01149-9Search in Google Scholar
[23] Muliwa AM, Leswifi TY, Onyango MS, Maity A. Magnetic adsorption separation (MAS) process: An alternative method of extracting Cr (VI) from aqueous solution using polypyrrole coated Fe3O4 nanocomposites. Sep Purif Technol. 2016;158:250–8.10.1016/j.seppur.2015.12.021Search in Google Scholar
[24] Chi Z, Zhu Y, Liu W, Huang H, Li H. Selective removal of As(III) using magnetic graphene oxide ion-imprinted polymer in porous media: Potential effect of external magnetic field. J Environ Chem Eng. 2021;9(4):105671.10.1016/j.jece.2021.105671Search in Google Scholar
[25] Pormazar SM, Dalvand A. Adsorption of Direct Red 23 dye from aqueous solution by means of modified montmorillonite nanoclay as a superadsorbent: mechanism, kinetic and isotherm studies. Korean J Chem Eng. 2020;37:2192–201.10.1007/s11814-020-0629-8Search in Google Scholar
[26] Kavcı E. Adsorption of Direct Red 243 dye onto clay: Kinetic study and isotherm analysis. Desalin Water Treat. 2021;212:452–61.10.5004/dwt.2021.26861Search in Google Scholar
[27] Chavarriaga E, Lopera A, Franco V, Bergmann C, Alarcón J. Gel combustion synthesis and magnetic properties of CoFe2O4, ZnFe2O4, and MgFe2O4 using 6-aminohexanoic acid as a new fuel. J Magn Magn Mater. 2020;497:166054.10.1016/j.jmmm.2019.166054Search in Google Scholar
[28] Hakeem A, Alshahrani T, Muhammad G, Alhossainy M, Laref A, Khan AR, et al. Magnetic, dielectric and structural properties of spinel ferrites synthesized by sol-gel method. J Mater Res Technol. 2021;11:158–69.10.1016/j.jmrt.2020.12.064Search in Google Scholar
[29] Majid F, Rauf J, Ata S, Bibi I, Malik A, Ibrahim SM, et al. Synthesis and characterization of NiFe2O4 ferrite: Sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics. Mater Chem Phys. 2021;258:123888.10.1016/j.matchemphys.2020.123888Search in Google Scholar
[30] Kurian J, Lahiri B, Mathew MJ, Philip J. High magnetic fluid hyperthermia efficiency in copper ferrite nanoparticles prepared by solvothermal and hydrothermal methods. J Magn Magn Mater. 2021;538:168233.10.1016/j.jmmm.2021.168233Search in Google Scholar
[31] Zhang X, Chen Z, Wu C, Zhang J, Wang F. Solvothermal synthesis of spinel ZnFe2O4 nanoparticles with enhanced infrared radiation property. Chem Phys Lett. 2019;732:136647.10.1016/j.cplett.2019.136647Search in Google Scholar
[32] Andersen HL, Granados-Miralles C, Jensen KM, Saura-Múzquiz M, Christensen M. The chemistry of spinel ferrite nanoparticle nucleation, crystallization, and growth. ACS Nano. 2024;18(14):9852–70.10.1021/acsnano.3c08772Search in Google Scholar PubMed PubMed Central
[33] Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem. 2017;2017:3039817.10.1155/2017/3039817Search in Google Scholar
[34] Sangsuriyonk K, Paradee N, Rotjanasuworapong K, Sirivat A. Synthesis and characterization of CoxFe1−xFe2O4 nanoparticles by anionic, cationic, and non-ionic surfactant templates via co-precipitation. Sci Rep. 2022;12(1):4611.10.1038/s41598-022-08709-9Search in Google Scholar PubMed PubMed Central
[35] Köseoğlu Y, Bay M, Tan M, Baykal A, Sözeri H, Topkaya R, et al. Magnetic and dielectric properties of Mn0.2Ni0.8Fe2O4 nanoparticles synthesized by PEG-assisted hydrothermal method. J Nanopart Res. 2011;13:2235–44.10.1007/s11051-010-9982-6Search in Google Scholar
[36] Gonzalez-Sandoval M, Beesley A, Miki-Yoshida M, Fuentes-Cobas L, Matutes-Aquino J. Comparative study of the microstructural and magnetic properties of spinel ferrites obtained by co-precipitation. J Alloy Compd. 2004;369(1–2):190–4.10.1016/j.jallcom.2003.09.101Search in Google Scholar
[37] Sharifi I, Shokrollahi H, Doroodmand MM, Safi R. Magnetic and structural studies on CoFe2O4 nanoparticles synthesized by co-precipitation, normal micelles and reverse micelles methods. J Magn Magn Mater. 2012;324(10):1854–61.10.1016/j.jmmm.2012.01.015Search in Google Scholar
[38] Houshiar M, Zebhi F, Razi ZJ, Alidoust A, Askari Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J Magn Magn Mater. 2014;371:43–8.10.1016/j.jmmm.2014.06.059Search in Google Scholar
[39] Sathya A, Guardia P, Brescia R, Silvestri N, Pugliese G, Nitti S, et al. CoxFe3–xO4 nanocubes for theranostic applications: effect of cobalt content and particle size. Chem Mater. 2016;28(6):1769–80.10.1021/acs.chemmater.5b04780Search in Google Scholar
[40] Biswal D, Peeples BN, Peeples C, Pradhan AK. Tuning of magnetic properties in cobalt ferrite by varying Fe+2 and Co+2 molar ratios. J Magn Magn Mater. 2013;345:1–6.10.1016/j.jmmm.2013.05.052Search in Google Scholar
[41] Wong S, Tumari HH, Ngadi N, Mohamed NB, Hassan O, Mat R, et al. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J Clean Prod. 2019;206:394–406.10.1016/j.jclepro.2018.09.201Search in Google Scholar
[42] Durairaj K, Senthilkumar P, Velmurugan P, Divyabharathi S, Kavitha D. Development of activated carbon from Nerium oleander flower and their rapid adsorption of direct and reactive dyes. Int J Green Energy. 2019;16(7):573–82.10.1080/15435075.2019.1598419Search in Google Scholar
[43] Scheufele FB, Staudt J, Ueda MH, Ribeiro C, Steffen V, Borba CE, et al. Biosorption of direct black dye by cassava root husks: Kinetics, equilibrium, thermodynamics and mechanism assessment. J Environ Chem Eng. 2020;8(2):103533.10.1016/j.jece.2019.103533Search in Google Scholar
[44] Safa Y, Bhatti HN. Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination. 2011;272(1–3):313–22.10.1016/j.desal.2011.01.040Search in Google Scholar
[45] Mahmoodi NM, Arami M, Bahrami H, Khorramfar S. The effect of pH on the removal of anionic dyes from colored textile wastewater using a biosorbent. J Appl Polym Sci. 2011;120(5):2996–3003.10.1002/app.33406Search in Google Scholar
[46] AG ES, AM G, Mansour HF. Potential application of orange peel as an eco-friendly adsorbent for textile dyeing effluents. Res J Text Appar. 2013;17(4):31–9.10.1108/RJTA-17-04-2013-B004Search in Google Scholar
[47] Liu N, Wang H, Weng CH, Hwang CC. Adsorption characteristics of Direct Red 23 azo dye onto powdered tourmaline. Arab J Chem. 2018;11(8):1281–91.10.1016/j.arabjc.2016.04.010Search in Google Scholar
[48] Liu J, Wang N, Zhang H, Baeyens J. Adsorption of Congo red dye on FexCo3-xO4 nanoparticles. J Environ Manag. 2019;238:473–83.10.1016/j.jenvman.2019.03.009Search in Google Scholar PubMed
[49] Kale RD, Potdar T, Gorade V. Treatment of CI Reactive Blue-21 effluent by microcrystalline cellulose grafted with APTES: kinetics, isotherm and thermodynamic study. Sustain Environ Res. 2019;29:1–12.10.1186/s42834-019-0007-6Search in Google Scholar
[50] Tabak A, Eren E, Afsin B, Caglar B. Determination of adsorptive properties of a Turkish Sepiolite for removal of Reactive Blue 15 anionic dye from aqueous solutions. J Hazard Mater. 2009;161(2–3):1087–94.10.1016/j.jhazmat.2008.04.062Search in Google Scholar PubMed
[51] Ofudje EA, Al-Ahmary KM, Alshdoukhi IF, Alrahili MR, Kavil YN, Alelyani SS, et al. Nano round polycrystalline adsorbent of chicken bones origin for Congo red dye adsorption. Sci Rep. 2024;14(1):7809.10.1038/s41598-024-57412-4Search in Google Scholar PubMed PubMed Central
[52] Hassan Ibrahim AH, Cihangir N, Idil N, Aracagök YD. Adsorption of azo dye by biomass and immobilized Yarrowia lipolytica; equilibrium, kinetic and thermodynamic studies. World J Microbiol Biotechnol. 2024;40(5):140.10.1007/s11274-024-03949-5Search in Google Scholar PubMed PubMed Central
[53] Açıkyıldız M, Gürses A, Güneş K, Şahin E. Adsorption of textile dyes from aqueous solutions onto clay: Kinetic modelling and equilibrium isotherm analysis. Front Chem. 2023;11:1156457.10.3389/fchem.2023.1156457Search in Google Scholar PubMed PubMed Central
[54] Hamri N, Imessaoudene A, Hadadi A, Cheikh S, Boukerroui A, Bollinger J-C, et al. Enhanced adsorption capacity of methylene blue dye onto kaolin through acid treatment: batch adsorption and machine learning studies. Water. 2024;16(2):243.10.3390/w16020243Search in Google Scholar
[55] Reddy YS, Jose TJ, Dinesh B, Kumar RN, Kumar PS, Kaviyarasu K. Equilibrium, kinetic, and thermodynamic study of Direct Yellow 12 dye adsorption by biomass-derived porous graphitic activated carbon. Biomass Convers Biorefin. 2024;1–17.10.1007/s13399-024-05464-xSearch in Google Scholar
[56] Mehrabanpour N, Nezamzadeh-Ejhieh A, Ghattavi S. A comparative photocatalytic activity between PbS NPs and PbS-clinoptilolite towards Cefotaxime. Solid State Sci. 2022;131:106953.10.1016/j.solidstatesciences.2022.106953Search in Google Scholar
[57] Rajabi S, Derakhshan Z, Nasiri A, Feilizadeh M, Mohammadpour A, Salmani M, et al. Synergistic degradation of metronidazole and penicillin G in aqueous solutions using AgZnFe2O4@ chitosan nano-photocatalyst under UV/persulfate activation. Environ Technol Innov. 2024;35:103724.10.1016/j.eti.2024.103724Search in Google Scholar
[58] Nasiri A, Golestani N, Rajabi S, Hashemi M. Facile and green synthesis of recyclable, environmentally friendly, chemically stable, and cost-effective magnetic nanohybrid adsorbent for tetracycline adsorption. Heliyon. 2024;10(2):1–21.10.1016/j.heliyon.2024.e24179Search in Google Scholar PubMed PubMed Central
[59] Mahmud HN, Huq AO, binti Yahya R. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. Rsc Adv. 2016;6(18):14778–91.10.1039/C5RA24358KSearch in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

