Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
-
Dheenadhayalan Sivakumar
Abstract
Biocorrosion, or microbiologically influenced corrosion, is a phenomenon where microorganisms deteriorate the metals. While corrosion is generally considered undesirable due to its negative impact on the integrity and lifespan of materials, the significance of biocorrosion is a major problem because it can cause material deterioration, financial losses, and environmental issues. Conventional corrosion protection techniques frequently use chemicals, which come with risks to human health and the environment. Biosurfactants are surface tension-reducing agents with a low molecular weight that attract many researchers and industrialists due to their excellent chemical properties and stability at extreme temperatures, pH, and under alkaline conditions. These compounds reduce the surface tension of liquids, leading to improved wetting and spreading on metal surfaces. This can help to create a more uniform and protective layer, preventing the accumulation of corrosive agents. This review explores different types of biosurfactants, which include lipopeptides, glycolipids, phospholipids, etc., and how they work to prevent corrosion. The investigation of biosurfactants in corrosion protection not only addresses environmental concerns but also holds promise for innovation in the development of efficient and long-lasting corrosion mitigation strategies for a variety of metal substrates, given the growing demand for green and sustainable technolo gies.
Graphical abstract

1 Introduction
Surfactants are amphiphilic compounds that contain both hydrophobic and hydrophilic ends and reduce interfacial tension between any two phases. These are surface-active agents, and when added in small quantities to the liquid, they improve the ability to spread and moisten the surfaces [1]. These surface-active compounds are capable of acting as emulsifiers, moistening agents, dispersants, cleaners, foaming agents, anti-adhesives, anticorrosive, anti-bacterial, and antistatic agents [2,3,4,5,6,7]. According to the hydrophobic and hydrophilic ends, surfactants are classified as anionic, cationic, and amphoteric [8].
Recently, the international market has driven researchers in the synthesis of both chemical and green surfactants as they possess a vital role in the field of Science, Engineering, and Technology [9]. The main active commercially produced surfactants belong to the various classes of groups like alkylbenzene sulphonates, alcohol ethoxylates, sulphates, and ethersulphates [10]. These groups of surfactants are used in the field of detergent, households, and personal care products [11], and they are economically viable. Even though they are not expensive, chemical surfactants possess several disadvantages. The added surfactants may have a chance of being absorbed by wildlife plants and deposited in water bodies, thus causing high toxicity to aquatic life, reducing dissolved oxygen, and reducing the photosynthesis process in plants [12].
In the past few years, scientists have focused on the synthesis of bio-based surfactants to compensate for the performance of chemical surfactants and diversify the range of applications in various fields. Biosurfactants, also known as bio-based surfactants or green surfactants, are synthesized by yeast, fungi, and certain bacteria [13,14]. They exhibit a remarkable diversity of chemical structures, encompassing lipopeptides, glycolipids, phospholipids, and proteins, and are useful in many different industrial applications because of their structural variability, which contributes to a wide range of functional properties. Biosurfactants are used in various fields, such as food, agriculture, petroleum, and cosmetics, as they reduce surface and internal surface tension and possess emulsifying, stabilizing, wetting, spreading, foaming, and cleaning properties similar to chemical surfactants [15,16,17]. More recently, biosurfactants have found various applications in the fields of corrosion, healthcare, textiles, and bioremediation [18]. The importance of these naturally occurring compounds keeps growing as researchers continue to explore new microbial sources, optimize production processes, and learn more about the field of biosurfactants. Because of their adaptability and sustainability, biosurfactants are positioned to play a significant role in the development of green technologies and ecologically friendly solutions for a variety of industries (Figure 1).

Properties of biosurfactants.
Biosurfactants play a significant role in the corrosion inhibition of metals due to their unique properties and environmentally friendly nature. Corrosion poses a serious hazard and pulls down the economy, industry, and country. Bio-corrosion caused by aerobic and anaerobic bacteria causes severe damage to different industries and services, and it is recognized as an important category of corrosion [19,20]. To control and prevent such hazardous bio-corrosion, bio-based biosurfactants can be used. The present review focuses on the types of biosurfactants and their roles in the prevention and control of corrosion. We report a few real-world examples from the literature that use biosurfactants as intelligent and environmentally friendly biocides. This review gives some novel insights and ideas for researchers on emerging trends and future directions in using biosurfactants as corrosion inhibitors for metals. With increasing concerns about environmental pollution and sustainability, there is a growing interest in developing green corrosion inhibitors. Biosurfactants offer a sustainable alternative to traditional chemical inhibitors since they are produced from renewable resources and are biodegradable. Future research may focus on optimizing biosurfactant production processes to enhance their environmental friendliness. A better understanding of these mechanisms will facilitate the design of more effective biosurfactant-based corrosion inhibitors. As research into biosurfactant-based corrosion inhibitors progresses, there is a need for scalable production methods and commercialization strategies. Future efforts may focus on scaling up biosurfactant production processes, conducting large-scale corrosion testing, and collaborating with industry partners to bring these innovative inhibitors to market.
2 Types of biosurfactants
Due to their high efficiency and biodegradability, biosurfactants have been used as effective corrosion inhibitors for metals. According to the hydrophilic and hydrophobic ends, surfactants are classified into anionic, cationic, and amphoteric surfactants [21]. Biosurfactants are usually classified by their microbial origin, polarity, and chemical composition, as represented in Figure 2. The microbial origin of biosurfactants can be glycolipids, lipoproteins and lipopeptides, surfactants, lichensysin, fatty acids, and phospholipids [22]. Biosurfactants contain hydrophobic and hydrophilic parts. The hydrophobic part is composed of a functional group of carbohydrates and amino acids, while the hydrophilic part typically contains long-chain fatty acids. The biosurfactants obtained from various microorganisms are listed in Table 1.
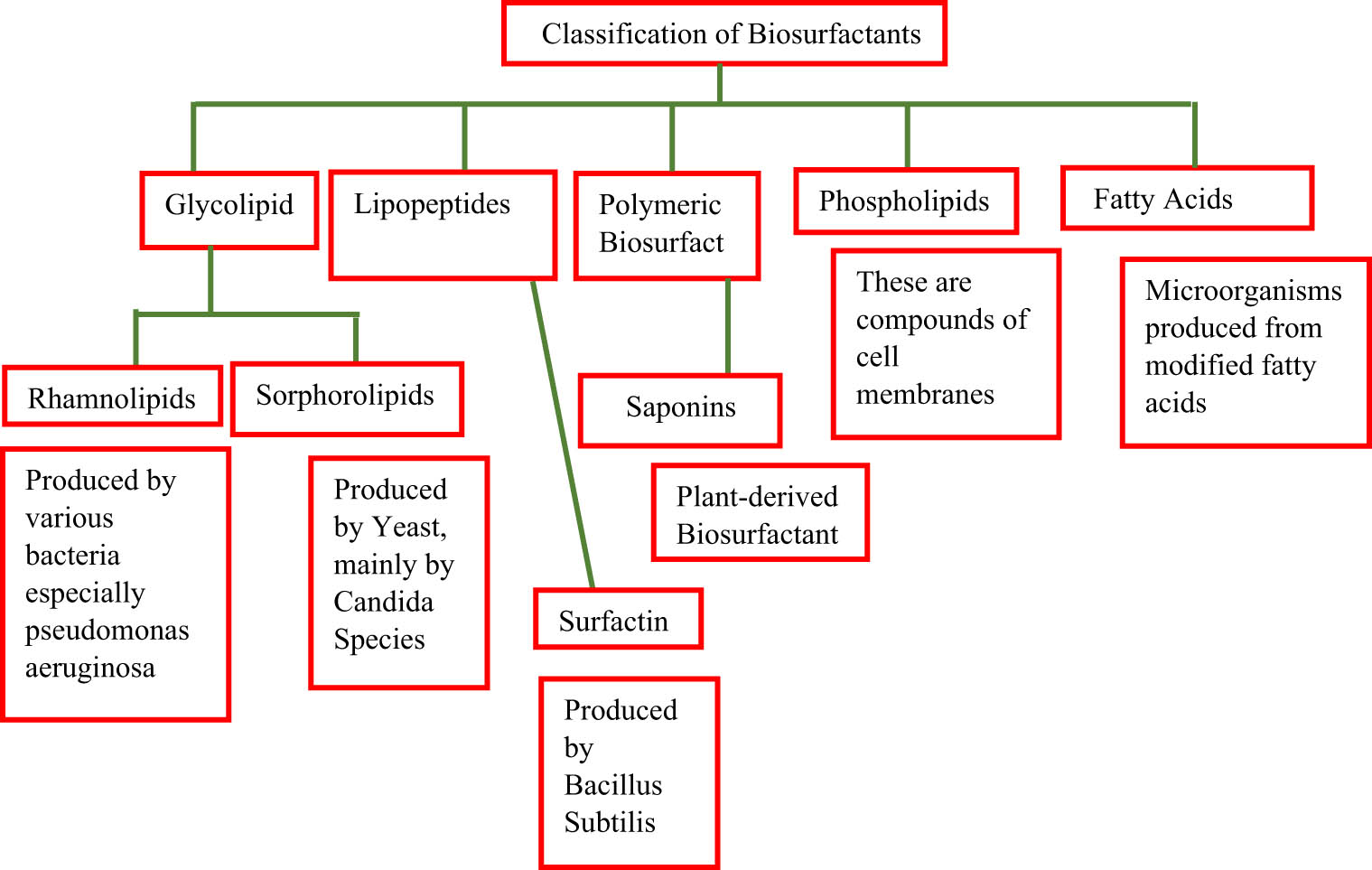
Classification of biosurfactants.
Biosurfactants derived from microorganisms
| Nature of biosurfactants | Class | Microorganism |
|---|---|---|
| Rhamnolipids, sophorolipids, trehalolipids | Glycolipid | Pseudomonas aeruginosa, other species of Pseudomonas [98], Rhodococcus sp., Candida bombicola |
| Surfactin | Lipopeptide | Bacillus subtilis |
| Viscosin | Lipoproteins | Pseudomonas fluorescens |
| Emulsan, lipomannan | Polymer | Candida tropicalis, Acinetobacter radioresistens |
| Arthrofactin | Polypeptide | Arthrobacter spp. |
2.1 Glycolipids
Glycolipids are compounds that contain fatty acids and carbohydrate moieties. They correspond to the group of compounds based on the nature of lipids and carbohydrate components [23]. According to the nature of lipids, they are categorized as rhamnolipids (RH), trehalose lipids, and sophorose lipids. They contain a hydrophobic lipid tail and one or more hydrophilic sugar groups linked by a glycosidic bond. The main structure of the glycolipids consists of a mono- or oligosaccharide group attached to a sphingolipid or glycerol group with one or two fatty acids. They are commonly found in Gram-positive microorganisms. Glycolipids are compounds containing fatty acids and carbohydrate moieties. They correspond to the group of compounds based on the nature of lipids and carbohydrate moieties [23]. Based on the nature of lipids, they are categorized as RHs, trehalose lipids, and sophorose lipids.
The other groups of compounds, like RHs, are derived from Pseudomonas aeruginosa and trehalose lipids and are found in various Gram-positive bacteria belonging to the mycolates group [24]. The sophorose lipids are derived from non-pathogenic yeast [25].
2.2 Lipopeptides and lipoproteins
Lipopeptides contain linear or cyclic oligopeptides acylated with fatty acids. They are commonly synthesized from Bacillus and Pseudomonas [26]. They are fatty acids linked to a peptide chain with a hydrophilic head and are linked to the lactone ring in the case of cyclic peptides. As lipopeptides are highly stable, biodegradable, and non-toxic, they play a vital role in various fields like cosmetics, healthcare, biomedical, pharmaceutical, and food industries [27]. Lipoproteins are surface-active biosurfactants produced from Bacillus subtilis. They are complex particles that have a hydrophobic core at the centre of non-polar lipids. They are surrounded by a hydrophilic membrane surrounded by an amphipathic coat of protein, phospholipid, and cholesterol [28].
2.3 Surfactants
Surfactants are cyclic lipopeptides produced mainly by various strains of Gram-positive bacteria, specifically from the genus B. subtilis, and they are the most efficient biosurfactants used in various industries. The composition consists of hydroxy fatty acid and seven amino acid peptide rings [29]. These seven amino acids contain l-asparagine, glutamic acid, l-leucine, l-violin, and two d-leucines with a long hydrophobic fatty acid chain. Surfactants are found to possess many biomedical properties, including antiviral, antifungal, anti-mycoplasma, and anti-bacterial activities [30,31,32].
2.4 Lichensysin
Lichensysin is a highly effective anionic cyclic lipoheptapeptide that is produced by Bacillus licheniformis using glucose as its main source. It produces several biosurfactants that act efficiently in the presence of some other biosurfactants, and they are very stable at higher temperatures and pH and under alkaline environments [33].
2.5 Fatty acids and phospholipids
Phospholipids contain hydrophobic fatty acid chains with hydrophilic moieties. In nature, phospholipids occur as several classes with different polar moieties. Because of their amphiphilic nature, they can be used as emulsifiers. The growth of n-alkanes results in the production of large quantities of phospholipid fatty acid surfactants by several bacteria and yeast. The amphiphilic nature of these lipids depends on the length of the hydrocarbon chain in their structure [34].
3 Microbially induced corrosion or biocorrosion
Microbiologically influenced corrosion (MIC), or biocorrosion, is the term used to describe the degradation of materials, particularly metals, brought on by the actions of microorganisms [35]. Algae, bacteria, fungi, and other microorganisms all contribute to the acceleration of the corrosion process in biocorrosion. These microbes can cause corrosive byproducts or foster environments that are more conducive to corrosion [36]. These byproducts are frequently formed as a consequence of the microorganisms’ metabolic processes. These microorganisms form a thin film on the surface of the metal, called biofilms, and these biofilms adhere to the metal surface, trapping the corrosive agents and leading to localized corrosion. Before looking into the process or mechanism of inhibition of corrosion by biosurfactants, we must identify how the corrosive byproducts are formed on the metal surface by microorganisms. The ways in which corrosive byproducts can be formed are explained as follows.
During the process of biocorrosion, a very low level of oxygen is present in the corrosive environment. At that time, microorganisms engage in anaerobic respiration, which in turn produces corrosive byproducts like organic acids and hydrogen sulphide. The main bacteria involved in the formation of biocorrosion are sulphate-reducing bacteria [37]. These sulphate-reducing bacteria metabolize sulphate present in the environment and produce hydrogen sulphide.
On surfaces, microorganisms have the ability to form biofilms, which produce an EPS-protective matrix. Corrosive agents can be trapped by this biofilm, which concentrates them against the material surface and encourages localized corrosion.
Some microorganisms produce organic acids as metabolic byproducts. By fostering an acidic environment, organic acids like formic or acetic acid can hasten the corrosion of metals.
On the metal surface, microorganisms might speed up electrochemical reactions. For instance, corrosive agents may be produced as a result of the microorganism and transfer electrons between microorganism and the metal surface [38]. Depending on the type of microorganisms and environmental conditions, the formation of corrosive byproducts may vary.
4 Corrosion inhibition mechanism of biosurfactants
Bio-corrosion caused by aerobic and anaerobic bacteria causes severe damage to different industries and services, and it is recognized as an important category of corrosion [39,40,41]. It is a complex interaction between metal surfaces and microorganisms that can induce corrosion. The degradation of metals can be accelerated by the corrosive byproducts that are produced by the metabolic activities of the microorganisms in the biofilm, such as hydrogen sulphide and organic acids [42]. Such microbially induced corrosion by microbes’ forms biofilms that can be prevented by biosurfactants that are produced from some other microbes. Biosurfactants interact with biofilms and are greatly influenced by their structure and chemical properties; consequently, they disrupt the formation of biofilms via various mechanisms. These characteristics allow biosurfactants to function as agents that inhibit biofilm formation, adhesion, fouling, and corrosion.
These biosurfactants destroy the biofilms by disrupting their plasma membrane or cell wall. When the cell wall is disturbed, it is very difficult for the microorganism to combat the added biosurfactant [43,44]. Hence, the added biosurfactants attack the cell walls of corrosive bacterial cells and thus retard the movement of ions inside and outside of the bacteria, which leads to bacterial death. Thus, the lipid compounds are reported to effectively dissolve the biofilm and protect the metal surface from corrosion. Specific ways of disrupting biofilms by a few biosurfactants are shown in Figure 3.

Action of lipopeptides and glycolipids on biofilms.
Corrosion inhibition of metal surfaces involves complex mechanisms, and the extent of inhibition depends on the type of protective layers formed on the metal surface. The protective layers may be mono- or multidimensional. At the same time, the inhibitive nature may be attributed to the following factors: (i) interaction of inhibitor molecules on the metal surface, (ii) adsorption of the inhibitor molecules on the metal surface, (iii) chemical reactions, (iv) concentration of the inhibitors, (v) temperature, etc. Before discussing the mechanism of inhibition of metal surfaces by biosurfactants, we must perceive the complete structure of biosurfactants. As discussed in this review, the chemical constituents of biosurfactants can vary widely depending on the producing organism, which mainly constitutes glycolipids, lipopeptides, phospholipids, proteins, peptides, fatty acids, etc.
When these biosurfactant-containing functional groups like >C═O, –OH, –NH, COOH, and –
When the concentration of biosurfactant increases, it tends to form a multilayer of protective film on the metal surface. In addition, the biosurfactant molecules accumulate or aggregate in a head-to-tail pattern and form a micelle. Metal surfaces are hydrophilic in nature, and consequently, the surfactant molecules are attracted to their surfaces. Thus, in turn, biosurfactants permeate and adsorb onto the interface, reducing the cohesion and thereby either preventing or promoting the attachment of biofilm-forming organisms. By creating a stable emulsion or protective layer on hydrophobic surfaces, biosurfactants can disrupt the biofilm due to their emulsifying and micelle-forming properties [46]. On the metal surface, they promote the development of a passivation layer that serves as a barrier between the metal and the corrosive environment, known as the passivation layer, and helps stop additional corrosion from occurring. Thus, it modifies the electrochemical reactions occurring on the metal surface and alters the kinetics of corrosion reactions by suppressing both anodic and cathodic reactions.
The hydrophilic and hydrophobic nature of biosurfactants enables them to interact with interfaces between the aqueous and non-aqueous phases of the corrosive medium. A simple mechanism of corrosion inhibition of metals by biosurfactants involves the following reactions, and the mechanism is shown in Figure 4.

Mechanism of corrosion inhibition by biosurfactants.
An anodic reaction by which the metal is corroded is given by equation (1):
A cathodic reaction takes place as per equation (2):
The anions present in the corrosive media adsorb onto the metal surface, causing the metal to act as negatively charged. Biosurfactants have the ability to form micelle and, in turn, emulsify the hydrophobic compounds. This property can be utilized to disperse or solubilize corrosion-inducing substances, preventing them from coming into contact with the metal surface.
Also, the heteroatoms present in the biosurfactants are assumed to protonate and produce cations. Now, the protonated biosurfactants were adsorbed on the negatively charged metal surfaces and formed a film, which acts as a protective layer.
The adsorption of biosurfactants in anodic locations occurs through heteroatoms, leading to delayed metal corrosion. Also, adsorption on the cathodic locations reduces the evolution of oxygen. Furthermore, ligand formation also occurs between heteroatoms of the biosurfactants and unoccupied d-orbital on the metal involved in the adsorption process and inhibits metal corrosion.
5 Corrosion protection of metals using various biosurfactants
Corrosion protection of metals depends on the specific type of biosurfactant, the metal being protected, and certain environmental factors. Ongoing research in this field continues to explore new ways to harness the potential of biosurfactants for corrosion control in various industrial applications.
5.1 Coco monoethanolamide (CMEA)
CMEA was investigated as a corrosion inhibitor for the protection of mild steel using 1 M HCl [47]. The inhibition efficiency of CMEA at 0.6163 mM and 60°C was found to be 99.01%. It performs as a mixed-type inhibitor, and its adsorption at the metal–acid interface follows the Langmuir isotherm. Theoretical findings from density functional theory, Monte Carlo, and molecular dynamics simulations were used to demonstrate their experimental results, and the potentiodynamic polarization studies revealed that the Tafel slopes at different inhibitor concentrations follow the same trend with reduced corrosion current density (j corr). The inhibition was mainly cathodic and inhibited the hydrogen evolution reaction, as evidenced by the shift towards the cathodic direction. This shows that solution resistance was greater for the solution containing inhibitor, and corrosion resistance was also increased when the inhibitor dosage increased, which shows that the inhibitors form a protective layer on the surface of the metal. Further, they suggest that CMEA develops a defensive layer on the surface of the metal through a physiochemisorption mode. The functional groups like >C═O, –OH, and >NH present in the CMEA get protonated in an acidic solution and form cationic molecules in the medium, as well as the counter ions (anions) induce the metallic surface to behave as negative. Thus, the cations formed by CMEA and anions on the metal surface form a defending layer on the metal surface. They reported that the CMEA bio-surfactant can act as a potent inhibitor for mild steel.
5.2 Asparagine and arginine
The two biosurfactants, viz. sodium N-dodecyl asparagine, and sodium N-dodecyl arginine, were employed as green corrosion inhibitors on the dissolution of mild steel (MS37-2) in aqueous sodium chloride solutions [48]. Electrochemical impedance measurements showed that sodium N-dodecyl asparagine and sodium N-dodecyl arginine surfactants were effective in inhibiting MS37-2 corrosion in NaCl solution. The polarization measurements revealed that the biosurfactants added to the sodium chloride solution altered both the anodic and cathodic branches of the polarization curves towards lower current densities. The anodic and cathodic reactions were slowed down, preventing corrosion on MS-37-2.
Electrochemical impedance studies showed that the increase in corrosion resistance and decrease in the double-layer capacitance was due to the continuous replacement of the water molecule by the adsorption of the biosurfactants, which reduces the interfacial tension and forms a protective film on the surface of MS-37-2. The study concluded that the inhibition efficiency depends on the concentration, nature, and type of the surfactant. With increasing the concentration of biosurfactants, the interfacial tension on the metal surface is minimized, thus protecting it from corrosion. The microbial-influenced corrosion by the Pseudomonas species on carbon steel ST37 was investigated using biosurfactants derived from the Bacillus species [49]. They determined the least inhibitory concentration and minimum biofilm inhibitory concentration using the broth microdilution technique, and the corrosion of carbon steel was determined by weight loss measurements. The corrosion rates were determined by antibiofilm assays and they revealed that the biofilm on the metal surface formed by aerobic microorganism Pseudomonas species induces the corrosion by utilizing the oxygen present in the biofilm and results in the formation of anodic sites on the surface. A thin layer of biofilm was found on carbon steel treated with a biosurfactant, and a few bacteria cells were attached to the surface of the layer. Thus, the added biosurfactant eradicated the biofilm and reduced the microbial corrosion of carbon steel.
5.3 RH (Pseudomonas species)
The corrosion inhibition of aluminium alloy in acidic rainwater by RH biosurfactant was investigated [50]. They synthesized RH biosurfactants from the strains of Pseudomonas species and carried out the corrosion test in Aluminium alloy D16T A by electrochemical studies at room temperature. They showed that the aluminium alloy in acidic rainwater was protected when it was immersed in the RH biosurfactant. After 3 h of exposure to acid rain and the addition of biosurfactants at concentrations of 0.1, 0.25, and 0.5 g L−1, the impedance magnitude increases by 0.1 Hz. A similar effect was also observed after 24 h exposure to the metal alloy with a biosurfactant in rainwater. The increase in impedance magnitude represents the formation of a barrier film on the aluminium surface by the biosurfactants. As RHs contain hydroxyl, carboxyl, and carbonyl functional groups, they exist as carboxylate anions in the solution. These anions are bonded to the outer regions of the oxide film through the physical mode of adsorption. In addition, the added RHs form a low soluble complex with the aluminium alloys and are deposited at the anodic sites. They concluded that environmentally safe corrosion inhibitors could be synthesized for aluminium alloy corrosion by using sustainable surfactants.
5.4 Glycolipid (Pseudomonas mosselii F01)
Parthipan et al. investigated the microbial corrosion inhibition using the glycolipid biosurfactant against bacterial strains of Streptomyces parvus B7, B. subtilis A1, Acinetobacter baumannii MN3, and Pseudomonas stutzeri NA3 on carbon steel. The glycolipid-based biosurfactant was synthesized from Pseudomonas mosselii F01 [51]. The rate of corrosion was monitored using weight loss and electrochemical studies. They reported 87% corrosion inhibition using weight loss, decreased corrosion current and potential, and high resistance capacity. The highest level of corrosion inhibition was due to the high energy interaction between the added glycolipid and carbon steel. They proved that glycolipid-based biosurfactants can act both as corrosion inhibitors and biocide. The reason for inhibition was explained by the mechanism of inhibition, as biosurfactants possess anti-bacterial activity: the active microbes present in the biosurfactant absorb onto the corrosive bacterial cell and restrict the biological reaction to take place. It was also attributed to the fact that biosurfactant molecules’ adsorption on the metal surface is that their interaction energy with the metal surface is higher than that of water molecules with the metal surface. Metals in different corrosive environments protected by several microorganisms are provided in Table 2.
List of metals protected by various biosurfactant
| Microorganism/biosurfactant | Metal | Medium |
|---|---|---|
| CMEA biosurfactant [41] | Mild steel | 1 M HCl |
| N-Dodecyl arginine, asparagine [48] | Mild steel (MS 37-2) | Aqueous NaCl solution |
| Biosurfactant from Bacillus sp. [49] | Carbon steel ST-37 | Oil |
| Glycolipid from, Streptomyces parvus B7, Bacillus subtilis A1, Pseudomonas stutzeri NA3, and Acinetobacter baumannii MN3 [70] | Carbon steel | Acid medium |
| Marine bacteria from Marinobacter salsuginis [47] | X80 pipeline steel | Alkaline medium |
| Bacillus subtilis and Bacillus licheniformis | Aluminium | Neutral |
| RH produced by Pseudomonas aeruginosa [53] | X70 carbon steel | Sea water |
| Rhamnolipid [54] | X65 carbon steel | CO2-saturated oilfield |
| Phospholipids [55] | Carbon steel | 15% hydrochloric acid |
5.5 Marino bacter salsuginis
The Bacillus marine bacteria, Marino bacter salsuginis, was employed to study the corrosion behaviour of X80 pipeline steel in marine environments [52]. Electrochemical impedance spectroscopy and polarization studies were carried out for the corrosion monitoring process. Due to the formation of the biofilm, the pipeline steel has higher polarization resistance, charge transfer resistance, and reduced corrosion current density. Bacillus subtilis and Bacillus licheniformis were employed as pitting corrosion inhibitors for aluminium 2024 by electrochemical impedance spectroscopy [53]. They reported that the secretion of 20 amino acids reduced the corrosion rate of the aluminium alloy at pH 6.5. They found that many uniform pits were found on the surface of the alloy in the uninhibited medium, whereas reduced pits in the presence of microorganisms due to the formation of biofilm and the capacitive nature of the impedance spectra were observed using electron microscopes. The presence of carboxylic acid groups decreases the corrosion rates due to the production of polyaspartate and γ-polyglutamate by B. licheniformis.
5.6 RH (P. aeruginosa)
The mechanism of inhibition was explained by the chelation of aluminium ions and carboxylic acid through Coulombic interaction, hydrogen bonds, and dipole–dipole interaction. RH produced by P. aeruginosa was investigated as an effective corrosion inhibitor for X70 carbon steel in seawater [54]. According to the weight loss measurements, they reported that the corrosion rate of carbon steel decreased from 18 to 0.05 mm/year with the addition of 0.1% (m/V). The increase in inhibition rate was attributed to the interaction of hydroxide molecules of RH on the metal surface.
5.7 RH
The corrosion protection efficiency of RH was studied on X65 carbon steel in CO2-Saturated Oilfield-Produced Water [55] using the gravimetric method and electrochemical measurements. They found an inhibition effect of 90% on increasing the concentration of biosurfactant. From the polarization bipolar branch curves, the cathodic and anodic branches represent only one reaction process but predominate along the anodic side. They also reported that the addition of RHs decreased the corrosion current density, and they act as a mixed type of inhibitor by forming a thick protective layer on the surface of the metal. The interaction of the RHs and metal surface occurs between the rhamnopyranose ring and C═O groups and the vacant d orbitals of carbon steel.
RHs extracted from P. aeruginosa were used against corrosive strains of B. subtilis A1, S. parvus B7, P. stutzeri NA3, and A. baumannii MN3 on concrete rebars in 0.5 N NaCl using electrochemical studies [51]. According to their report, the biosurfactant system included in mixed consortia demonstrated an inhibition efficiency of approximately 87% in the presence of naturally occurring corrosive bacterial consortia. Also, the XRD and experiments on weight loss and electrochemical analysis validate the function of biosurfactants in the corrosion inhibition process and the biosurfactant’s efficacy as a microbial corrosion inhibitor. The electrochemical studies reported that a good corrosion inhibitory effect was observed for carbon steel, which contains RHs and increased corrosion current and charge transfer resistance. Further, the interaction of RHs onto the metal surface was confirmed by the formation of a protective layer with the help of microscopic studies.
Zin et al. [56] investigated the corrosion inhibition efficiency of aluminium alloy by RH bio-complex synthesized from Pseudomonas sp. PS-17 strain using Tafel plots. It was found that the aluminium alloy in the presence of RH showed a pattern of frequency dependence phase angle, which indicates the possible formation of a barrier film on the surface of the metals. Finally, they concluded that an increase in the rate of re-passivation of the aluminium alloy on newly formed surfaces indicates that the RH biocomplex can act as an effective corrosion inhibitor.
5.8 Phospholipids
Phospholipids extracted from vegetable oil waste were used to prevent corrosion on the carbon steel surface in 15% hydrochloric acid. Various chemical surfactants like acetone C.70 (C12-C14 alkyltrimethylammonium chloride), cationic SAA, catapav (C16-C18 alkyldimethylbenzylammonium chloride, cationic surfactant), oxypav (tertiary amine oxides, nonionic surfactant), CP-20 (condensation products of mono- and dialkylphenols with ethylene oxide, nonionic surfactant), and oleylamidopropyldimethylamine (a mixture of zwitterionic surfactant, oleylamidopropyldimethylamine, and sodium chloride) were added as additives with the phospholipids [57]. The corrosion rate was determined using a weight loss method, and its electrochemical corrosion rate was estimated based on the corrosion current density. They reported that phospholipids were found to possess 86.5% inhibition efficiency. The combined use of surfactants and phospholipids tends to show a synergistic effect on the corrosion inhibition efficiency. Phospholipids, when combined with surfactants, tend to show a high level of corrosion protection, up to 95%.
5.9 Penicillium citrinum and Trichoderma
The biosurfactant produced from P. citrinum and Trichoderma, which were isolated from municipal dumpsite, was used as a corrosion inhibitor for mild steel bar in NaCl solution [58]. Corrosion inhibition efficiency was tested using weight loss measurements at different concentrations of the fungi. Mild steel bars were immersed in different concentrations (v/v) of 5, 7.5, and 10% of inhibitors for immersion times of 14, 28, and 100 days. To compare its efficiency, parallelly, Tween 80, a non-organic surfactant, was also used to study the corrosion inhibition property of the mild steel bar in NaCl solution. It was found that the biosurfactant with 10% concentration showed the highest inhibition efficiency of 58.01%, and also, the biosurfactant of Penicillium citrinum was found to be more effective in corrosion protection than the Trichoderma and non-organic surfactant. In addition, they reported that when mild steel bars were immersed in the corrosive media with and without inhibitors, the colour of the solution changed from clear to brownish, and some sludge formation was observed. However, the solution containing biosurfactants does not produce sludge and does not contain any colour. According to the study, biosurfactants not only prevent corrosion but are also used for the bioremediation process [59].
5.10 Pseudomonas fluorescens
The corrosion behaviour of AISI 304 stainless steel was investigated using Gram-negative bacteria Pseudomonas fluorescens in NaCl medium by electrochemical studies [60]. Hiemenz and Rajagopalan determined the surface activity and critical micelle dilution by surface tension measurements using the Wilhelmy plate method [61]. According to them, when the samples are immersed in biosurfactants, the formed oxide layer on the steel surface is more compact, which decreases the density of pits to a higher anodic/cathodic surface area. When the corrosive medium is too aggressive, the corrosion will increase at a faster rate; the formed oxide layer does not nucleate before the biosurfactant gets adsorbed on the metal surface. Hence, they concluded that for better protection efficiency, the surface of the metal must be pre-passivated before the biosurfactant addition.
5.11 Bacillus sp.
Biosurfactant produced by indigenous oil reservoir bacteria Bacillus sp. was aimed to determine the corrosion caused by Pseudomonas sp. 1 and Pseudomonas sp. 2 on Carbon Steel ST37 using the weight loss method [62]. The authors used 15% of glutaraldehyde as a positive control. They revealed that biosurfactant decreased the corrosion rates due to the formation of biofilms, which reduces the microbial-induced corrosion, and the added Bacillus Sp. as an inhibitor able to inhibit the film formation of Pseudomonas sp. 1 and Pseudomonas sp. 2. The intervention effects given by their studies are summarized as follows:
Minimum inhibitory concentration of the biosurfactant: 62.5 μg ml−1
Minimum biofilm inhibitory concentration (MBIC) of the biosurfactant: 31.25 μg ml−1
Minimum biofilm eradication concentration for 50% eradication (MBEC50) of the biosurfactant: 500 μg ml−1
Biosurfactant is able to inhibit the attachment of Pseudomonas sp. 1 and Pseudomonas sp. 2 to Carbon Steel ST37 surface
Biosurfactant is able to eradicate the preformed biofilm on the steel surface
Reduction of corrosion rate in Carbon Steel ST37: from 4.56 × 10−4 to 3.31 × 10−4 mm year−1 based on the MBIC value and from 5.18 × 10−5 mm year−1 to 2.7 × 10−5 after biofilm eradication at the MBEC value.
The mechanism of inhibition was explained as follows: the biosurfactant prevented certain bacteria from adhering to the steel surface and removed the existing biofilm, which lowered the rate at which Carbon Steel ST37 corroded. This type of biosurfactant can be used as a good anti-corrosion agent in the oil industry.
5.12 Bio-MOF-1
Feng et al. [63] encapsulated benzimidazole in the micropores of bio-MOF-1 and investigated its anti-corrosion durability on mild steel using electrochemical studies. As benzimidazole has hetero atoms with lone pair of electrons, both benzimidazole and bio-MOF-1 showed synergistic corrosion protection. They reported that the impedance arc value decreased when the immersion time increased and showed the highest inhibition efficiency of 85.36% recorded for the encapsulated inhibitor.
5.13 Surfactins and quorum quenching lactonase
MIC on steel coupons was investigated by Huang et al. using some biologically active compounds, such as surfactin, capsaicin, gramicidin, and an enzyme quorum quenching lactonase [64]. They evaluated the effectiveness of different coating additives in reducing the formation of corrosion tubercles on steel surfaces. They found that the coating additives, surfactin and the quorum quenching lactonase, significantly reduced the formation of corrosion tubercles by 31, 36, and 50%, respectively. They demonstrated the potential of highly stable quorum quenching lactonases to provide a reliable, cost-effective method to treat steel structures and prevent biocorrosion. The main intervention effects given by them are summarized as follows: surfactin: reduced the number and coverage of tubercles on steel coupon surfaces by 31 and 37%, respectively. Quorum quenching lactonase reduced the formation of corrosion tubercles by 50%. Bacterial community composition: significant differences were observed in the bacterial communities on experimental and control coupons for all treatments (p < 0.05). The mechanism of inhibition was attributed to the use of a silica gel matrix to encapsulate anti-corrosion biochemicals and enzymes, allowing their activity to last for several months, even after all cells were dead. The enzymatic activity of the silica gel coating, including the lactonase enzyme, was greatly reduced after 42 days of exposure to water. The decrease in the observed corrosion compared to the silica gel coating control was statistically significant for surfactin and the lactonase enzyme. These findings suggest that the encapsulated additives, particularly the lactonase enzyme, play a role in inhibiting corrosion on steel surfaces.
5.14 Pseudomonas fragi
The corrosion inhibition effect of some aerobic bacterium Pseudomonas fragi, facultative anaerobe Escherichia coli DH5α on SAE 1018 carbon steel was investigated by Jayaraman et al. [65]. They reported that the presence of P. fragi or E. coli DH5α resulted in 2- to 10-fold lower mass loss compared to sterile controls. Increasing the temperature from 23 to 30°C resulted in a 2- to 5-fold decrease in corrosion inhibition with P. fragi, whereas the same shift in temperature resulted in a 2-fold increase in corrosion inhibition with E. coli DH5α. Also, in a few steel coupons, corrosion observed with non-biofilm-forming S. lividans TK24 was observed. The mechanism of inhibition was explained as follows: the live cells produce a protective biofilm that inhibits corrosion, resulting in a significant reduction in mass loss when compared to sterile controls. The biofilm, which is made up of both living and dead cells, is embedded in a sparse glycocalyx matrix and is essential for inhibiting corrosion.
6 Other applications of biosurfactants in various industries
6.1 Bioremediation
Bioremediation is a process that uses living organisms to remove or neutralize pollutants from a contaminated environment. Biosurfactants, which are surface-active compounds produced by microorganisms, play a crucial role in enhancing bioremediation and biodeterioration efforts [66,67,68]. Pollutants such as air, soil, and water are universally present in the environment. Many researchers reported the carcinogenic effects of organic pollutants on human health [69,70]. The microbial species take up an efficient role in the eradication of pollution and are involved in the formation of intermediate compounds like glycolipids. These glycolipids play an active role in the bioremediation of pollutants [71,72,73].
It was reported that biosurfactants play a crucial role in dispersing hydrocarbons in the medium [74]. Pseudomonas species produce RH with very low surface tension and alter their cell wall. Man-made activities like manufacturing, mining, and usage of electronic devices have led to the liberation of metals like Ba, Cd, Hg, Ni, Pb, and Sr in the environment [75]. These toxic metals become a major contaminant when it is mixed with water and soil. The contaminated soil sites may be converted into landfill sites in the future. In such cases, this may cause a severe impact on the environment and affect the food chain.
Recently, biosurfactants were employed to remove these pollutants from the environment in order to resolve this issue [77]. When the biosurfactants are added to the environment, they interact with the pollutants through any one of the possible reactions like electrostatic interaction, ion exchange, counter-ion binding, or precipitation dissolution [78]. The added biosurfactants chelate with the metal ion surface and form a micelle [79]. This micelle helps to remove the metals from the contaminated environment [80].
Cadmium metal was removed from the environment by an RH-based biosurfactant [81], and it was found that it was very effective in the removal of cadmium by forming a complex between cadmium(ii) and the added RH, thus reducing its toxicity. Similarly, strontium was removed by adding very low concentration (80 ppm) of RH [82]. It was reported that the biosurfactant lipopeptide was synthesized from C. lipolytica and was employed to remove Cu and Zn. The study found that the added lipopeptide exhibited 96% efficiency in the removal of metals [83]. Singh et al. also found that lipopeptides were also effective in reducing heavy metals like CD, Fe, and Pb [84].
6.2 Pharmaceutical industries
The various functional groups of biosurfactants confer them to possess many potential applications in various fields. The hydrophilic and hydrophobic nature of biosurfactants is supposed to act like detergents on the cell walls of many bacteria and fungi and tend to possess antimicrobial activity [85]. Several biosurfactants have strong anti-bacterial, antifungal, and antiviral activity.
Das et al. reported that the biosurfactants extracted from marine Bacillus circulans exhibit excellent antimicrobial activity against Gram-positive and Gram-negative pathogens [86]. Fernandes et al. [87] investigated the antimicrobial properties of Bacillus subtilis R14 against various bacterial strains. They reported that lipopeptides were very effective against the bacterial strains. Also, it was reported that the biosurfactants were found to control the adhesion of microorganisms on the infected sites of the wound [88]. Thus, the biosurfactants were employed on the wounded surface before the application of medicine in order to prevent the colonization or grouping of microorganisms (Rivardo et al., [89]).
6.3 Nanoparticle synthesis
Nanotechnology is a wide area and attracts the attention of many researchers as it converts matter into the near-atomic scale to produce new structures. It provides many scientific advancements in various fields, such as energy, materials, electronics, coatings, cosmetics, manufacturing, and many other daily activities. Nanomaterials synthesized through nanotechnology are unique due to their physical, chemical, and mechanical properties. Many researchers are focusing on the synthesis of the nanomaterial through various methods, like chemical reduction, co-precipitation, hydrothermal, sol–gel, and micro-emulsion. These methods involve high temperature, pressure, and energy during the synthesis, and they also generate many biologically toxic byproducts as they pollute the environment [90]. Moreover, it faces a main disadvantage of the use of chemicals and self-agglomeration in aqueous solutions [91]. Hence, the bio-fabrication of nanomaterial is a more sustainable, cheap, and eco-friendly method. Biosynthesis of nanoparticles involves the usage of natural plant extracts, polysaccharides, gum exudates, algae, fungi, yeast, etc. [92,93,94]. More recently, researchers have focused on the synthesis of nanomaterials using biosurfactants as they reduce environmental impacts, reduce waste, and improve the stabilization of nanomaterials using sustainable substrates [95]. Biosurfactants are surface-active compounds that possess both hydrophilic and hydrophobic properties. They are also called surface-active biomolecules, which are mainly synthesized from microorganisms, and they tend to show exceptional properties like low toxicity, specificity, and relative ease of synthesis [96]. Also, when compared with chemical surfactants, biosurfactants have several advantages, such as environmental cleaning by biodegradation and depollution of industrial effluents [97,98,99].
Silver nanoparticles were synthesized using a biosurfactant produced by Bacillus cereus UCP [100]. The synthesized nanoparticles were characterized using UV-VIs spectroscopy, scanning electron microscopy, and zeta potential methods. The study reported that the average size of their obtained nanoparticles was 20 nm. And it was found that the synthesized nanoparticles were found to possess 85% inhibition against P. fellutanum and A. niger.
Nickel oxide nanorods were synthesized using commercial RH as a stabilizer. The synthesized nanorods were found to possess a size of 22 nm in diameter [101]. Silver nanoparticles were characterized by UV-vis absorption spectroscopy. The morphology was found to be spherical by TEM and SEM.
Water-soluble RHs from P. aeruginosa were employed to synthesize ZnS spherical nanoparticles [102]. They obtained the nanoparticle in the size of 4.5 nm.
Polyaniline nanofibres and nanotubes were synthesized using RHs [103]. They reported that when comparing the synthesis of PAN with other additives, the PAN synthesized using RH showed uniform morphology and size.
7 Conclusions
For the past few years, biosurfactants have attracted many researchers as it possesses many versatile amphiphilic properties. These amphiphilic compounds are good anti-bacterial and anti-biofilm agents against various microbial organisms, which induce biocorrosion in different industries. Even though various biosurfactants were studied for their anti-corrosion properties, the most studied are RHs and lipopeptides, as they possess well-characterized structures. Their distinct qualities – such as their surface activity, biodegradability, and variety of molecular structures – are desirable options for applications involving corrosion prevention. Because of their biodegradability and eco-friendly nature, biosurfactants are a greener option for corrosion inhibitors than conventional ones. This quality is particularly important in industries where corrosion and environmental considerations are among the paramount problems, aligning with the growing emphasis on sustainable practices. Much interest in employing these eco-friendly sustainable compounds in the prevention of the formation of biofilms or fouls on the metal surface and corrosion protection on metals is less studied, and very few works have been reported. Even though there is a good sign in the progress of understanding the mechanism of corrosion by biosurfactants, their production costs, scalability, and optimization for specific applications remain challenging and are left for future research purposes. Ongoing research is essential to address these challenges, explore new biosurfactant formulations, and optimize their performance under various conditions. Hence, biosurfactants are emerging as important materials that have the potential to completely change the corrosion landscape as industries look for long-term, cost-effective solutions for corrosion control.
-
Funding information: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
-
Author contributions: Dr. T. Brindha – original draft preparation, writing, reviewing, editing. Dr. S.Dheenadhayalan – investigation and formal analysis. Dr. R. Rathinam, Natrayan L, Abhinav Kumar – investigation, validation, and formal analysis. Dr. R.T. Yamuna - writing, reviewing, and validation, Dr. K. Umapathi – supervision and editing and reviewing. Mohd. Irfanul Haque Siddiqui – investigation and formal analysis. Mohd. Asif Shah – reviewing, Natrayan L – reviewing, Abhinav Kumar – reviewing.
-
Conflict of interest: The authors declare no competing interests.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Verma C, Hussain CM, Quraishi MA, Alfantazi A. Green surfactants for corrosion control: Design, performance and applications. Adv Colloid Interface Sci. 2023 Jan;311:102822.10.1016/j.cis.2022.102822Search in Google Scholar PubMed
[2] Jan CJB, Gucciardi E, Cavallaro S. Biolubricants. ScienceDirect. (2024). https://www.sciencedirect.com/book/9780857092632/biolubricantsSearch in Google Scholar
[3] Krishnan R, Pandiaraj S, Muthusamy S, Panchal H, Alsoufi MS, Ibrahim AMM, et al. Biodegradable magnesium metal matrix composites for biomedical implants: synthesis, mechanical performance, and corrosion behavior – a review. J Mater Res Technol. 2022;20:650–70. 10.1016/j.jmrt.2022.06.178.Search in Google Scholar
[4] Tadros TF. An introduction to surfactants. Berlin, Boston: De Gruyter; 2014.10.1515/9783110312133Search in Google Scholar
[5] Mehta B, Subhedar D, Panchal H, Sadasivuni KK. Stability and thermophysical properties enhancement of Al2O3-water nanofluid using cationic CTAB surfactant. Int J Thermofluids. 2023;20:100410. 10.1016/j.ijft.2023.100410.Search in Google Scholar
[6] Vieira DB. Cationic lipids and surfactants as antifungal agents: mode of action. J Antimicro Chemother. 2006 Jul;58(4):760–7.10.1093/jac/dkl312Search in Google Scholar PubMed
[7] Yalçın G, Huminic G, Huminic A, Panchal H, Dalkılıç AS. Investigation on the effect of surfactants on the viscosity of graphite-water-based nanofluids. J Mol Liq. 2024;398:124197. 10.1016/j.molliq.2024.124197.Search in Google Scholar
[8] Sumeyra Gurkok A, Ozdal M. Microbial biosurfactants: properties, types, and production. Anatol J Biol. 2021 Dec;42:2687–444.Search in Google Scholar
[9] Ly M, Mekonnen TH. Cationic surfactant modified cellulose nanocrystals for corrosion protective nanocomposite surface coatings. J Ind Eng Chem. 2020 Mar;83:409–20.10.1016/j.jiec.2019.12.014Search in Google Scholar
[10] Sujit K, Ajaya B, Sujeet C. Applications of surfactants in modern science and technology. Modern trends in Science and Technology, Nepal Biological Society, Nepal Physical Society (Eastern Chapter) and Research council of Science and Technology; 2013. p. 147–58.Search in Google Scholar
[11] Rosen MJ, Kunjappu JT. Surfactants and Interfacial Phenomena. New York: John Wiley & Sons; 2012.10.1002/9781118228920Search in Google Scholar
[12] Fernandes N, de AT, Simões LA, Dias DR. Biosurfactants produced by yeasts: fermentation, screening, recovery, purification, characterization, and applications. Fermentation. 2023 Feb;9(3):207.10.3390/fermentation9030207Search in Google Scholar
[13] Velioglu Z, Ozturk Urek R. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turkish J Biol. 2015;39:160–6.10.3906/biy-1406-44Search in Google Scholar
[14] Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, et al. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol. 2010 Apr;87(2):427–44.Search in Google Scholar
[15] Campos JM, Montenegro Stamford TL, Sarubbo LA, de Luna JM, Rufino RD, Banat IM. Microbial biosurfactants as additives for food industries. Biotechnol Prog. 2013 Sep;29(5):1097–108.10.1002/btpr.1796Search in Google Scholar PubMed
[16] Mnif I, Ghribi D. Glycolipid biosurfactants: main properties and potential applications in agriculture and food industry. J Sci Food Agric. 2016 Jun;96(13):4310–20.10.1002/jsfa.7759Search in Google Scholar PubMed
[17] Bouassida M, Ghazala I, Ellouze-Chaabouni S, Ghribi D. Improved biosurfactant production by bacillus subtilis SPB1 mutant obtained by random mutagenesis and its application in enhanced oil recovery in a sand system. J Microbiol Biotechnol. 2018 Jan;28(1):95–104.10.4014/jmb.1701.01033Search in Google Scholar PubMed
[18] Souza EC, Vessoni-Penna TC, de Souza Oliveira RP. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int Biodeterior Biodegrad. 2014 Apr;89:88–94.10.1016/j.ibiod.2014.01.007Search in Google Scholar
[19] Kadukova J, Pristas P. Biocorrosion – Microbial Action. Elsevier eBooks; 2018. p. 20–7. 10.1016/b978-0-12-409547-2.13500-0.Search in Google Scholar
[20] Cernousek T, Sevcu A, Shrestha R, Steinova J, Kokinda J, Vizelkova K. Microbially influenced corrosion of container material. Elsevier eBooks; 2021. p. 119–36. 10.1016/b978-0-12-818695-4.00006-x.Search in Google Scholar
[21] Sumeyra Gurkok A, Ozdal M. Microbial biosurfactants: properties, types, and production. Anatol J Biol. 2021 Dec;42:2687–444.Search in Google Scholar
[22] Ines M, Dhouha G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr Res. 2015 Oct;416:59–69.10.1016/j.carres.2015.07.016Search in Google Scholar PubMed
[23] Gensler WJ, Alam I, Prasad RS, Radhakrishna AI, Chaudhuri AP. 3-Hydroxy-2-alkyl carboxylic acids related to mycolic acid. Tetrahedron. 1979 Jan;35(21):2595–600.10.1016/0040-4020(79)88026-6Search in Google Scholar
[24] Reshmy R, Philip E, Madhavan A, Binod P, Awasthi MK, Pandey A, et al. Applications of biosurfactant as an antioxidant in foods. Elsevier EBooks; 2023. p. 391–401. 10.1016/b978-0-12-824283-4.00008-3.Search in Google Scholar
[25] Carolin CF, Kumar PS, Ngueagni PT. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J Hazard Mater. 2021 Apr [cited 2021 Dec 29]; 407:124827.10.1016/j.jhazmat.2020.124827Search in Google Scholar PubMed
[26] Zabetakis I, Lordan R, Tsoupras A. The impact of nutrition and statins on cardiovascular diseases. London, United Kingdom: Elsevier/Academic Press; 2019.Search in Google Scholar
[27] Inamuddin R, BoddulaAsiri, AM. Green sustainable process for chemical and environmental engineering and science. Analytical Techniques for Environmental and Industrial Analysis. Amsterdam: Elsevier; 2020.Search in Google Scholar
[28] Huang X, Lu Z, Bie X, Lü F, Zhao H, Yang S. Optimization of inactivation of endospores of Bacillus cereus by antimicrobial lipopeptides from Bacillus subtilis fmbj strains using a response surface method. Appl Microbiol Biotechnol. 2007 Feb;74(2):454–61.10.1007/s00253-006-0674-1Search in Google Scholar PubMed
[29] Singh P, Cameotra SS. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004 Mar;22(3):142–6.10.1016/j.tibtech.2004.01.010Search in Google Scholar PubMed
[30] Nir-Paz R, Prévost M-C, Nicolas P, Blanchard A, Wróblewski H. Susceptibilities of Mycoplasma fermentans and Mycoplasma hyorhinis to membrane-active peptides and enrofloxacin in human tissue cell cultures. Antimicrobial Agents Chemotherapy. 2002 May;46(5):1218–25.10.1128/AAC.46.5.1218-1225.2002Search in Google Scholar PubMed PubMed Central
[31] Tendulkar SR, Saikumari YK, Patel V, Raghotama S, Munshi TK, Balaram P, et al. Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J Appl Microbiology. 2007 Aug;103(6):2331–9.10.1111/j.1365-2672.2007.03501.xSearch in Google Scholar PubMed
[32] Dufour S, Deleu M, Nott K, Wathelet B, Thonart P, Paquot M. Hemolytic activity of new linear surfactin analogs in relation to their physico-chemical properties. Biochimica et Biophysica Acta (BBA) – Gen Subj. 2005 Oct;1726(1):87–95.10.1016/j.bbagen.2005.06.015Search in Google Scholar PubMed
[33] Rismani E, Fooladi J, Ebrahimi Por GH. Biosurfactant Production in batch culture by a bacillus licheniformis isolated from the persian Gulf. Pak J Biol Sci. 2006 Jun;9(13):2498–502.10.3923/pjbs.2006.2498.2502Search in Google Scholar
[34] Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: multifunctional biomolecules of the 21st Century. Int J Mol Sci. 2016 Mar;17(3).10.3390/ijms17030401Search in Google Scholar PubMed PubMed Central
[35] Makhlouf ASH, Botello MA. Failure of the metallic structures due to microbiologically induced corrosion and the techniques for protection. In: Handbook of materials failure analysis. Elsevier EBooks. 2018; p. 1–18. 10.1016/b978-0-08-101928-3.00001-x.Search in Google Scholar
[36] Maji K, Lavanya M. Microbiologically influenced corrosion in stainless steel by pseudomonas aeruginosa: an overview. J Bio- Tribo-Corrosion. 2024 Jan;10(1).10.1007/s40735-024-00820-wSearch in Google Scholar
[37] Gu JD, Mitchell R. Biodeterioration. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Springer, Berlin, Heidelberg; 2013; 309–41. 10.1007/978-3-642-31331-8_31.Search in Google Scholar
[38] Lekbach Y, Liu T, Li Y, Moradi M, Dou W, Xu D, et al. Microbial corrosion of metals: The corrosion microbiome. In: Advances in microbial physiology; 2021. p. 317–90. 10.1016/bs.ampbs.2021.01.002.Search in Google Scholar PubMed
[39] Janek T, Rodrigues LR, Gudiña EJ, Czyżnikowska Ż. Metal-biosurfactant complexes characterization: binding, self-assembly and interaction with bovine serum albumin. Int J Mol Sci. 2019 Jun;20(12):2864.10.3390/ijms20122864Search in Google Scholar PubMed PubMed Central
[40] Puentes-Cala E, Tapia-Perdomo V, Espinosa-Valbuena D, Reyes-Reyes M, Quintero-Santander D, Vasquez-Dallos S, et al. Microbiologically influenced corrosion: the gap in the field. Front Environ Sci. 2022;10:924842.10.3389/fenvs.2022.924842Search in Google Scholar
[41] Ganjoo R, Sharma S, Sharma PK, Dagdag O, Berisha A, Ebenso EE, et al. Coco monoethanolamide surfactant as a sustainable corrosion inhibitor for mild steel: theoretical and experimental investigations. Molecules. 2023 Feb;28(4):1581.Search in Google Scholar
[42] Rajitha K, Nancharaiah YV, Venugopalan VP. Inhibition of biofilm formation and settlement of barnacle larvae by a biosurfactant produced from a marine biofilm-forming Exiguobacterium sp. R58. Int Biodeterior Biodegrad. 2024 Feb;187:105724.10.1016/j.ibiod.2023.105724Search in Google Scholar
[43] Płaza G, Chojniak J, Banat I. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int J Mol Sci. 2014 Aug;15(8):13720–37.Search in Google Scholar
[44] Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, et al. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol. 2010 Apr;87(2):427–44.10.1007/s00253-010-2589-0Search in Google Scholar PubMed
[45] Jimoh AA, Booysen E, Van Zyl L, Trindade M. Do biosurfactants as anti-biofilm agents have a future in industrial water systems? Front Bioeng Biotechnol. 2023 Sep;11.10.3389/fbioe.2023.1244595Search in Google Scholar PubMed PubMed Central
[46] Zhu Y, Free ML, Woollam R, Durnie W. A review of surfactants as corrosion inhibitors and associated modeling. Prog Mater Sci. 2017 Oct;90:159–223.10.1016/j.pmatsci.2017.07.006Search in Google Scholar
[47] Ganjoo R, Sharma S, Sharma PK, Dagdag O, Berisha A, Ebenso EE, et al. Coco monoethanolamide surfactant as a sustainable corrosion inhibitor for mild steel: theoretical and experimental investigations. Molecules. 2023 Feb;28(4):1581.10.3390/molecules28041581Search in Google Scholar PubMed PubMed Central
[48] Fawzy A, Abdallah M, Alfakeer M, Altass HM, Althagafi II, El-Ossaily YA. Performance of unprecedented synthesized biosurfactants as green inhibitors for the corrosion of mild steel-37-2 in neutral solutions: a mechanistic approach. Green Chem Lett Rev. 2021 Jun;14(3):488–99.10.1080/17518253.2021.1943543Search in Google Scholar
[49] Little BJ, Blackwood DJ, Hinks J, Lauro FM, Marsili E, Okamoto A, et al. Microbially influenced corrosion – Any progress? Corros Sci. 2020 Jul;170:108641.10.1016/j.corsci.2020.108641Search in Google Scholar
[50] Zin IM, Pokhmurskii VI, Korniy SA, Karpenko OV, Lyon SB, Khlopyk OP, et al. Corrosion inhibition of aluminium alloy by RH biosurfactant derived from pseudomonas sp. PS-17. Anti-Corros Methods Mater. 2018 Oct;65(6):517–27.10.1108/ACMM-03-2017-1775Search in Google Scholar
[51] Parthipan P, Sabarinathan D, Angaiah S, Rajasekar A. Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J Mol Liq. 2018 Jul [cited 2023 Mar 23]:261:473–9.10.1016/j.molliq.2018.04.045Search in Google Scholar
[52] Saleem Khan M, Yang C, Zhao Y, Pan H, Zhao J, Babar Shahzad M, et al. An induced corrosion inhibition of X80 steel by using marine bacterium Marinobacter salsuginis. Colloids Surf B: Biointerfaces. 2020 May;189:110858.10.1016/j.colsurfb.2020.110858Search in Google Scholar PubMed
[53] Ornek A, Jayaraman B, Syr D. Pitting corrosion inhibition of aluminum 2024 by Bacillus biofilms secreting polyaspartate or γ-polyglutamate. Appl Microbiol Biotechnol. 2002 Apr;58(5):651–7.10.1007/s00253-002-0942-7Search in Google Scholar PubMed
[54] Li Z, Yuan X, Sun M, Li Z, Fan Y, Lei Y, et al. Rhamnolipid as an eco-friendly corrosion inhibitor for microbiologically influenced corrosion. Soc Sci Res Netw. 2021;23–8.10.2139/ssrn.3980353Search in Google Scholar
[55] Zhang J, Wang M, Liu C, Fang Z. The corrosion inhibition performance and mechanism of rhamnolipid for X65 Steel in CO2 ‐saturated oilfield‐produced water. J Surfactants Deterg. 2021 Apr;24(5):809–19.10.1002/jsde.12478Search in Google Scholar
[56] Zin ІM, Pokhmurs’kyi VІ, Khlopyk OP, Karpenko OV, Pokyn’broda TY, Kornii SA, et al. Inhibition of the corrosion of aluminum alloy in aqueous solution of ethylene glycol by the rhamnolipid biocomplex. Mater Sci. 2020 Mar;55(5):633–9.10.1007/s11003-020-00353-wSearch in Google Scholar
[57] Lesik EI, Buryukin FA, Vaganov RA. Phospholipids from plant materials as a corrosion inhibitor in oil production. J Physics: Conf Ser. 2021 Nov;2094(5):052044.10.1088/1742-6596/2094/5/052044Search in Google Scholar
[58] Olivia R, Ang CH, Clotilda P, Caroline M, Rudy T, Joe N. Corrosion inhibition of mild steel bars by biosurfactant produced by Penicillium citrinum. IOP Conf Ser. 2023 Jan;1135(1):012057.10.1088/1755-1315/1135/1/012057Search in Google Scholar
[59] Gu JD. On environmental biotechnology of bioremediation. Appl Environ Biotechnol. 2021;5(2):3–8.10.26789/AEB.2020.02.002Search in Google Scholar
[60] Dagbert C, Meylheuc T, Bellon-Fontaine MN. Corrosion behaviour of AISI 304 stainless steel in presence of a biosurfactant produced by Pseudomonas fluorescens. Electrochim Acta. 2006 Jul;51(24):5221–7.10.1016/j.electacta.2006.03.063Search in Google Scholar
[61] Hiemenz PC, Rajagopalan R. Principles of colloid and surface chemistry Dekker M. New York; 1997.Search in Google Scholar
[62] Purwasena IA, Astuti DI, Ardini Fauziyyah N, Putri DAS, Sugai Y. Inhibition of microbial influenced corrosion on carbon steel ST37 using biosurfactant produced by Bacillus sp. Mater Res Express. 2019 Oct;6(11):115405.10.1088/2053-1591/ab4948Search in Google Scholar
[63] Feng J, Chen J, Wang S, Jia M, Zhang Z, Yu T, et al. Rational design of inhibitor-encapsulated Bio-MOF-1 for dual corrosion protection. Inorg Chem. 2022 Oct;61(45):18285–92.10.1021/acs.inorgchem.2c03151Search in Google Scholar PubMed
[64] Huang S, Bergonzi C, Schwab M, Elias M, Hicks RE. Correction: Evaluation of biological and enzymatic quorum quencher coating additives to reduce biocorrosion of steel. PLOS One. 2021 Jun;16(6):e0253354.10.1371/journal.pone.0253354Search in Google Scholar PubMed PubMed Central
[65] Jayaraman A, Earthman JC, Wood TK. Corrosion inhibition by aerobic biofilms on SAE 1018 steel. Appl Microbiol Biotechnol. 1997 Jan;47(1):62–8.10.1007/s002530050889Search in Google Scholar
[66] Xu X, He Z, Ji F, Zhang M, Bai J, Wang B. Insight into the interactions between surfactants and microorganisms for the biodegradation of polycyclic aromatic hydrocarbons: Enhancing efficiency, cellular response, and elucidating mechanisms. Int Biodeterior Biodegrad. 2024 Feb;187:105710–0.10.1016/j.ibiod.2023.105710Search in Google Scholar
[67] Liu L, Si L, Yang J, Peng L, Qiao S, Sun Y, et al. Biodegradation and process optimization of phenol and formaldehyde by Aspergillus nomius SGFA1. Int Biodeterior Biodegrad. 2023 Aug;182:105630.10.1016/j.ibiod.2023.105630Search in Google Scholar
[68] Komar M, Nowicka-Krawczyk P, Ruman T, Nizioł J, Dudek M, Gutarowska B. Biodeterioration potential of algae on building materials - Model study. Int Biodeterior Biodegrad. 2023 May [cited 2023 Nov]; 180:105593.10.1016/j.ibiod.2023.105593Search in Google Scholar
[69] Yoshikawa M, Zhang M, Toyota K. Biodegradation of volatile organic compounds and their effects on biodegradability under co-existing conditions. Microbes Environ. 2017;32(3):188–200.10.1264/jsme2.ME16188Search in Google Scholar PubMed PubMed Central
[70] Gao L, Gu JD. A new unified conceptual framework involving maintenance energy, metabolism and toxicity for research on degradation of organic pollutants. Int Biodeterior Biodegrad. 2021 Aug;162:105253.10.1016/j.ibiod.2021.105253Search in Google Scholar
[71] Liston SD, Mann E, Whitfield C. Glycolipid substrates for ABC transporters required for the assembly of bacterial cell-envelope and cell-surface glycoconjugates. Biochimica et Biophysica Acta (BBA) – Mol Cell Biol Lipids. 2017 Nov;1862(11):1394–403.10.1016/j.bbalip.2016.10.008Search in Google Scholar PubMed
[72] Patowary R, Patowary K, Kalita MC, Deka S. Application of biosurfactant for enhancement of bioremediation process of crude oil contaminated soil. Int Biodeterior Biodegrad. 2018 Apr;129:50–60.10.1016/j.ibiod.2018.01.004Search in Google Scholar
[73] Cameotra SS, Singh P. Bioremediation of oil sludge using crude biosurfactants. Int Biodeterior Biodegrad. 2008 Oct;62(3):274–80.10.1016/j.ibiod.2007.11.009Search in Google Scholar
[74] Ayangbenro A, Babalola O. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health. 2017 Jan;14(1):94.10.3390/ijerph14010094Search in Google Scholar PubMed PubMed Central
[75] Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;2011(402647):1–20.10.5402/2011/402647Search in Google Scholar
[76] Joginder S, Simranjeet S. Environmental exposure and health risks of the insecticide monocrotophos -a review. J biodiverse Environ Sci. 2014;5:2220–6663.Search in Google Scholar
[77] Kumar V, Singh S, Singh J, Upadhyay N. Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol. 2015 Mar;94(6):807–14.10.1007/s00128-015-1523-7Search in Google Scholar PubMed
[78] Kosaric N, Sukan FV. (Editors). Biosurfactants: Production and utilization-processes, technologies, and economics (1st ed.). CRC Press; 2014. 10.1201/b17599.Search in Google Scholar
[79] Sarubbo LA, Rocha RB, Luna JM, Rufino RD, Santos VA, Banat IM. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem Ecol. 2015 Nov;31(8):707–23.10.1080/02757540.2015.1095293Search in Google Scholar
[80] Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B. Remediation technologies for heavy metal contaminated groundwater. J Environ Manag. 2011 Oct;92(10):2355–88.10.1016/j.jenvman.2011.06.009Search in Google Scholar PubMed
[81] Sandrin TR, Chech AM, Maier RM. A Rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation. Appl Environ Microbiol. 2000 Oct;66(10):4585–8.10.1128/AEM.66.10.4585-4588.2000Search in Google Scholar PubMed PubMed Central
[82] Elouzi AA, Akasha AA, Elgerbi AM, El Gammudi BA. Using of microbial bio-surfactants (rhamnolipid) in heavy metals removal from contaminated water. J Sebha Univ Pure Appl Sci. 2012;11:85–95.Search in Google Scholar
[83] Rufino R, Luna J, Campos-Takaki G, Ferreira SRM, Sarubbo L. Application of the biosurfactant produced by candida lipolytica in the remediation of heavy metals. Chem Eng Trans. 2012 Jun;27:61–6.Search in Google Scholar
[84] Singh AK, Cameotra SS. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ Sci Pollut Res. 2013 May;20(10):7367–76.10.1007/s11356-013-1752-4Search in Google Scholar PubMed
[85] Rahman MS, Ano T. Production characteristics of lipopeptide antibiotics in biofilm fermentation of Bacillus subtilis. J Environ Sci. 2009 Jan;21:S36–9.10.1016/S1001-0742(09)60032-2Search in Google Scholar PubMed
[86] Das P, Mukherjee S, Sen R. Antiadhesive action of a marine microbial surfactant. Colloids Surf B: Biointerfaces. 2009 Jul;71(2):183–6.Search in Google Scholar
[87] Fernandes PAV, Arruda IR, deSantos AFAB, Araújo AA, deMaior, Ximenes AMS, et al. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz J Microbiol. 2007 Dec;38:704–9.10.1590/S1517-83822007000400022Search in Google Scholar
[88] Das P, Mukherjee AK, Sen R. Antiadhesive action of a marine microbial surfactant. Colloids Surf B: Biointerfaces. 2009;71:183–6. 10.1016/j.colsurfb.2009.02.004.Search in Google Scholar PubMed
[89] Rivardo F, Turner RJ, Allegrone G, Ceri H, Martinotti MG. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl Microbiol Biotechnol. 2009 Jun;83(3):541–53.10.1007/s00253-009-1987-7Search in Google Scholar PubMed
[90] Płaza G, Chojniak J, Banat I. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int J Mol Sci. 2014 Aug;15(8):13720–37.Search in Google Scholar
[91] Dixit M, Shukla P. Microbial nanotechnology for bioremediation of industrial wastewater. Front Microbiol. 2020 Nov;11.10.3389/fmicb.2020.590631Search in Google Scholar PubMed PubMed Central
[92] Boroumand Moghaddam A, Namvar F, Moniri M, Tahir P, Azizi S, Mohamad R. Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules. 2015 Sep;20(9):16540–65.10.3390/molecules200916540Search in Google Scholar PubMed PubMed Central
[93] Srivastava S, Usmani Z, Atanasov AG, Singh VK, Singh NP, Abdel-Azeem AM, et al. Biological nanofactories: using living forms for metal nanoparticle synthesis. Mini-Rev Med Chem. 2021 Feb;21(2):245–65.10.2174/1389557520999201116163012Search in Google Scholar PubMed
[94] Vuong LD, Quang DA, Chuc NH, Van L, Quoc Bao VV. Natural gums as a sustainable source for synthesizing copper nanoparticles. Elsevier eBooks; 2022 Jan. p. 81–98. 10.1016/b978-0-12-823833-2.00022-2.Search in Google Scholar
[95] Vecino X, Rodríguez-López L, Rincón-Fontán M, Cruz JM, Moldes AB. Nanomaterials synthesized by biosurfactants. In: Comprehensive analytical chemistry. Elsevier; 2021. p. 267–301.10.1016/bs.coac.2020.12.008Search in Google Scholar
[96] Viajayakumar S, Saravanan V. Biosurfactants-Types, Sources and Res J Microbiol. 2015;10(5):181–92.10.3923/jm.2015.181.192Search in Google Scholar
[97] Silva R, Almeida D, Rufino R, Luna J, Santos V, Sarubbo L. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci. 2014 Jul;15(7):12523–42.10.3390/ijms150712523Search in Google Scholar PubMed PubMed Central
[98] Diaz De Rienzo MA, Stevenson PS, Marchant R, Banat IM. Pseudomonas aeruginosabiofilm disruption using microbial surfactants. J Appl Microbiol. 2016 Feb;120(4):868–76.10.1111/jam.13049Search in Google Scholar PubMed
[99] Gu JD. Biodegradation testing: so many tests but very little new innovation. Appl Environ Biotechnol. 2016 Apr;1(1):92.10.18063/AEB.2016.01.007Search in Google Scholar
[100] Batista J, Morais Meira H, Oliveira B, Diniz Rufino R, Converti A, Sarubbo LA. Green synthesis of silver nanoparticles using a biosurfactant from Bacillus cereus UCP 1615 as stabilizing agent and its application as an antifungal agent. Fermentation. 2021 Oct;4:233–37.10.3390/fermentation7040233Search in Google Scholar
[101] Płaza G, Chojniak J, Banat I. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int J Mol Sci. 2014 Aug;15(8):13720–37.10.3390/ijms150813720Search in Google Scholar PubMed PubMed Central
[102] Narayanan J, Ramji R, Sahu H, Gautam P. Synthesis, stabilisation and characterisation of rhamnolipid-capped ZnS nanoparticles in aqueous medium. IET Nanobiotechnol. 2010;4(2):29.10.1049/iet-nbt.2009.0010Search in Google Scholar PubMed
[103] Worakitsiri P, Pornsunthorntawee O, Thanpitcha T, Chavadej S, Weder C, Rujiravanit R. Synthesis of polyaniline nanofibers and nanotubes via rhamnolipid biosurfactant templating. Synth Met. 2011 Feb;161(3–4):298–306.10.1016/j.synthmet.2010.11.039Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

