Abstract
Here we have shown the novel biosynthesis of silver nanoparticles (Ag NPs) encapsulated by chitosan polymers in the presence of Achillea millefolium aqueous extract (Ag NPs@CHI). The Ag ions were first embedded over the chitosan surface enriched with polar organofunctions like amines (NH2) and hydroxyls, and subsequently the ions were reduced green-metrically by the electron rich phytochemicals of the plant extract. After the synthesis numerous techniques, including the UV-vis spectrum, transmission electron microscopy, FE-SEM, EDS-elemental mapping, and ICP-AES, were used to study the physicochemical characteristics of the nanocomposite biomaterial. Next, we explored the material biologically in the anti-cutaneous squamous cell carcinoma effects against the corresponding cell lines like PM1, MET1, MET 4, SCC T9, SCC IC1MET, SCC IC19, SCC T8, and SCC T11. The related IC50 values of the nanocomposite against them were 182, 158, 177, 178, 177, 99, 62, and 183 µg/mL, respectively. The cytotoxicity in terms of percentage cell viability of cancer cells were decreased with the increase in the nanocomposite doses.
1 Introduction
Now a days medical researchers are inclined to employ plant extracts to cure cancer in addition to chemical medications which have severe side effects, following the human awareness of the efficacy of medicinal plants in treating deadly diseases like cancer [1–4]. Herbal medicines, either in combination with anticancer drugs or alone, have demonstrated positive therapeutic outcomes in certain resistant cases. In contrast, modern medicine alone has proven ineffective in treating these cases [3–6]. Medicinal plants are a viable option for mitigating the disease efficacies. The medicinal plants’ affordable price, lower toxicity, minimal side effects, and accessibility make them valuable alternatives to chemical drugs [6–9]. Achillea millefolium has been utilized for various purposes such as treating gall bladder insufficiency, reducing blood pressure, preventing kidney stones, stopping bleeding, regulating women’s menstrual cycles, addressing children’s enuresis, inducing relaxation, repelling Ascaris parasites, alleviating cold symptoms, treating skin acne, and relieving muscle pain [10–12]. The antidiabetic properties have been scientifically substantiated. Studies have demonstrated its efficacy in decreasing blood pressure, lowering blood cholesterol and fat levels, exhibiting antimicrobial effects, and mitigating the seizures severity [13–15].
In addition to the wide array of chemical drugs employed in carcinoma treatment, the medicinal plants integration with diminished negative impacts can function as a supplementary approach and potentially a substitute for chemical drugs. There has been a collaborative effort between the traditional and modern scientific communities to develop advanced medications for combating this fatal illness [3,6–9]. With the emergence of nanotechnology, researchers have focused on creating nanomedicines that combine traditional medicine and plant-based remedies, resulting in a diverse range of efficient compounds for carcinoma management [16].
The nanoparticle’s sustainable production has garnered notable attention owing to its several advantages as compared with the conventional methods. Furthermore, the artificial procedure is capable of being carried out under normal pressure conditions, resulting in substantial energy savings [17–21]. Various plant components, including root extract, seeds, leaves, stems, and fruit extracts, are utilized in the process as reducing agents and stabilizers [20–22]. Despite the existence of various other approaches in the NPs biosynthesis, such as the bio-macromolecules and microorganisms, as well as plant extracts, the latter option proves to be more advantageous due to its cost-effectiveness, abundance, and scalability [23,24].
Among the assortment of NPs, silver nanoparticles (Ag NPs) have indicated many therapeutic effects. This is primarily attributed to their remarkable efficacy in combating microbes, bacteria, and cancer cells, in addition to their exceptional chemical stability [25–28]. The Ag NPs use in various industries such as electronics, textiles, appliances, food and beverage, cosmetics, and healthcare has caused a notable enhance in their global consumption [29]. In addition, the green synthesis utilization for these NPs holds notable efficacy in the realm of applied pharmaceutics. Medicinal herbs have been utilized to mediate Ag NPs with green methods, which have revealed promise in curing several cancer forms [25–27]. According to recent findings, it has been discovered that the Ag NPs effectively eliminate carcinoma cells by increasing the reactive oxygen species levels within the cells [26–28]. Hence, separate research has indicated that the Ag NPs play a crucial role in permeating the membrane of carcinoma cells, ultimately leading to their demise [27,28]. The goal of this particular study is to introduce a novel method for the synthetic design and development of chitosan-capped Ag NPs, green synthesized and promoted by plant extract (Figure 1), a bioinspired procedure without using any harmful and toxic agents followed by their biological evaluation in the treatment of cancer cells. Chitosan is a unique naturally occurring carbohydrate polymer having plenteous hydroxyl groups as well as high density of amino functions, both of which provides electron rich environment that is necessary for anchoring the incoming metal ions as well as their green reduction to generate the corresponding nanoparticles. In addition, the electron rich environment also enables the NPs to stabilize by encapsulation. Also, the positively charged chitosan helps in improved ionic gelation through self-association or by different physicochemical interactions like Van der Waals, H-bonds, hydrophobic or ionic interactions, etc., which is important in determining the size of the NPs.

A. millefolium’s image.
The green reduction is further promoted by the plenteous oxygenated phytochemicals of A. millefolium extract. Our devised green Ag NPs were analyzed and their cytotoxicity was clarified on a number of cutaneous squamous carcinoma cell lines like PM1, MET1, MET 4, SCC T9, SCC IC1MET, SCC IC19, SCC T8, and SCC T11 with some excellent outcomes. In literature there has not been much report on the green-mediated Ag NPs in the treatment of cutaneous squamous cell carcinoma and therefore this method itself proves its novelty.
2 Experimental
2.1 Materials and methods
The catalyst preparation involved the use of chemicals such as AgNO3, chitosan, [3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide] (MTT), and various solvents, all of which were of analytical grade with a purity of over 99.5%. These chemicals were obtained from Sigma Aldrich, USA. Biochemicals like streptomycin, penicillin, glutamine, and fetal bovine serum were sourced from Fluka. The cells were purchased from Fieser, USA. The trypsin-EDTA was procured from Gibco BRL, Scotland. No further purification was required for their use. For the analytical research of the catalyst, a double beam UV–vis instrument (PG, T80+) was utilized, along with quartz cuvettes measuring 10 mm. The energy dispersive X-ray spectroscopy (EDX) studies and scanning electron microscopy (SEM) analysis were conducted using the FESEM-TESCAN MIRA3 microscope. Transmission electron microscopy (TEM) method was performed using a Zeiss microscope, operating at 300 kV.
2.2 Preparation of A. millefolium extract
The A. millefolium (1.0 g) plant (Figure 1) being washed and dried was added to 20 mL of DI water, and warmed for 0.5 h at 80°C. The plant suspension was then filtered over Whatman-1 paper and applied as intended.
2.3 Synthesis of Ag NPs
A solution of 0.1 g chitosan in 10 mL A. millefolium plant extract was prepared by sonication and then the Ag precursor, AgNO3 aqueous solution (50 mL, 1 mM) was added to it. The mixture was again sonicated at 40°C for 0.5 h when the solution color became reddish brown, validating the generation of Ag NPs (Figure 2, inset). The prepared Ag NPs@CHI nanocomposite was collected and centrifuged for 15 min at 4,500 rpm and then rinsed with DI water.

UV–vis analysis of the biosynthesis of Ag NPs@CHI and image of the nanocomposite solution (inset).
2.4 Anticancer properties
The cytotoxic efficacies of synthesized Ag NPs@CHI nanocomposite on normal cell (HUVEC) and cutaneous squamous cell carcinoma cells (PM1, MET1, MET 4, SCC T9, SCC IC1MET, SCC IC19, SCC T8, and SCC T11) were evaluated using the MTT assay. To assess the influence of nanoparticles on cell morphology, the first stage consisted of placing 100 nanoparticles into 96-well plates. Following this, the cells were exposed to several dilations of nanoparticles every 72 h. The OLYMPUS model contrast phase microscope was employed to evaluate potential changes in cell morphology after treatment with nanoparticles, in contrast to the control group of untreated cells. The viability and growth of cells were assessed using the trypan blue dye exclusion test and a hemocytometer slide. Afterward, the cells were grown at a density of 106 cells per well. Subsequent to this, the initial plate showed no growth after a day, whereas the second plate was exposed to around 900 μg/mL of methanol as a control for the solvent. Different doses of NPs were introduced to the rest of the plates, and each dosage was replicated three times. The dishes were subsequently transferred to an incubator adjusted to 5% CO2, 37°C, 90% humidity, and 95% oxygen for a duration of 24 h. Subsequently, the dishes were analyzed to determine the IC50 value. Throughout a span of 3 days, MTT solution quantities were added and allowed to incubate for 8 h. Afterward, the formazan crystals were dissolved in 0.1 L of dimethyl sulphoxide, and their absorption at 570 nm was quantified using a Biotech ELISA reader produced in Germany [30,31]:
2.5 Statistical analysis
Minitab-21 was applied to follow the normality of the data. Following this, the non-normal data were corrected. SPSS-22 was used for data variance analysis, and the visual representations were generated using Excel.
3 Results and discussion
The novel hybrid nanomaterial was prepared paved by a bio-inspired green reduction method. A hydrogel solution of A. millefolium extract and chitosan was used as the template to generate the Ag NPs in situ from its precursor salts. Following this pathway the metal ions were reduced promoted by the electron rich organo-functions contained in the green tea-CS hydrogel. The organogel encapsulation additionally facilitated the electronic stabilization of Ag NPs by capping. The so formed Ag NPs@CHI nanocomposites were characterized. Synthesis of the Ag NPs@CHI can be perceived by visible observations only, while changing the solution color from yellow to light-brown, as indicated in Figure 2. The outcome was further validated by a time-dependent UV–vis spectroscopic investigation and consequently a broad hump for Ag NPs emerged at 450 nm, an interpretation of its characteristic surface plasmon resonance. The result is displayed in Figure 2, where the absorption of Ag-nanocomposite can be clearly distinguished from the A. millefolium extract-CS hydrogel. The unmetalled hydrogel extract displays an absorption at 335 nm which diminished gradually in the latter graph when Ag ions got reduced to its NPs over the CS-Achillea hydrogel and a prominent absorption was noticed at 450 nm in 30 min of reaction time.
In order to assess the morphological property of Ag NPs@CHI nanocomposite, FE-SEM and TEM investigations were carried out. Figure 3 shows the FE-SEM image of the prepared nanomaterial as spherical shaped with some aggregated particles due to manual preparation of the sample for recording the analysis.

FE-SEM image of biosynthesized Ag NPs@CHI.
TEM images of Ag NPs@CHI is given in Figure 4, the black dots show the Ag NPs with good distribution as spherical shaped without any aggregation and having sizes around 10–15 nm.

TEM images of Ag NPs@CHI.
Chemical composition of the Ag NPs@CHI was estimated from EDX investigations and the corresponding outcome is presented in Figure 5. It shows Au and Ag as the metallic components and weak signals of O, N, and C at the base regions. The sharp and strong Au signal that appeared at 2.1 keV is related to the gold vapor deposition during the EDX analysis and not contributed from the original sample under investigation. The non-metals are characteristics of the A. millefolium phytochemicals and chitosan association within the nanocomposite sample. The corresponding map sum spectrum is also documented therein as inset which shows the atomic wt% of the elements. Markedly, Ag content in the sample is 36.8%, as compared to C content of 44.1%.

EDX data of biosynthesized Ag NPs@CHI.
Furthermore, the elemental mapping study was done to authenticate the EDX outcome. The Ag NPs@CHI nanomaterial was subjected to SEM investigation and then X-ray scanning of a small segment, as shown in Figure 6. In the end, dispersion of the constituent elemental species is depicted as a collection of colored dots, being evenly distributed throughout the matrix. The absence of Au signals in mapping profile also justifies the discrimination from EDX result. Noticeably, applications of Ag species are greatly impacted by their homogeneity.

SEM-elemental mapping of Ag NPs@CHI.
Finally, Ag NPs@CHI nanocomposite’s crystallinity and nature of phase were confirmed by XRD analysis. The single diffraction pattern shows it as a single entity, as shown in Figure 7. The profile shows four distinct Braggs diffraction signals at 2θ = 38.2°, 44.2°, 64.3°, and 77.3°. These are accredited to the (111), (200), (220), and (311) crystal planes, respectively [32]. The diffraction range of 10–25° corresponds to a broad, non-crystalline region that represents the Achillea-chitosan hydrogel.

XRD pattern of Ag NPs@CHI.
Numerous toxicological studies [33] support the common usage of Ag NPs in the profit-oriented category. Nevertheless, there is still a significant amount of knowledge to acquire regarding their impact on cells derived from mammalian tissues. Ag NPs have been found to cause cell death through various mechanisms such as autophagy, necrosis, or apoptosis. The release of Ag+ ions into the surrounding media can increase H2O2 amounts and initiate apoptosis [34,35]. It is crucial to emphasize that the effectiveness of Ag NPs in harming mammalian cells is influenced by several factors such as temperature duration, concentration, exposure time, shape, size, surface of nanoparticles, and the particular type of cell being examined [36–38].
In the current experiment, the cutaneous squamous cell carcinoma cells viability decreased in the Ag NPs presence. The IC50 of Ag NPs was 182, 158, 177, 178, 177, 99, 62, and 183 µg/mL on PM1, MET1, MET 4, SCC T9, SCC IC1MET, SCC IC19, SCC T8, and SCC T11 cells, respectively (Table 1; Figures 8, 9).
IC50 of nanocomposite in the anti-cutaneous squamous cell carcinoma test
| IC50 | Nanocomposite (µg/mL) |
|---|---|
| PM1 | 182 ± 4 |
| MET1 | 158 ± 6 |
| MET4 | 177 ± 3 |
| SCC T9 | 178 ± 4 |
| SCC IC1MET | 177 ± 5 |
| SCC IC19 | 99 ± 5 |
| SCC T8 | 62 ± 7 |
| SCC T11 | 183 ± 5 |

Anti-cutaneous squamous cell carcinoma efficacy of nanocomposite against PM1 (a), MET1 (b), MET4 (c), and SCC T9 (d) cells.
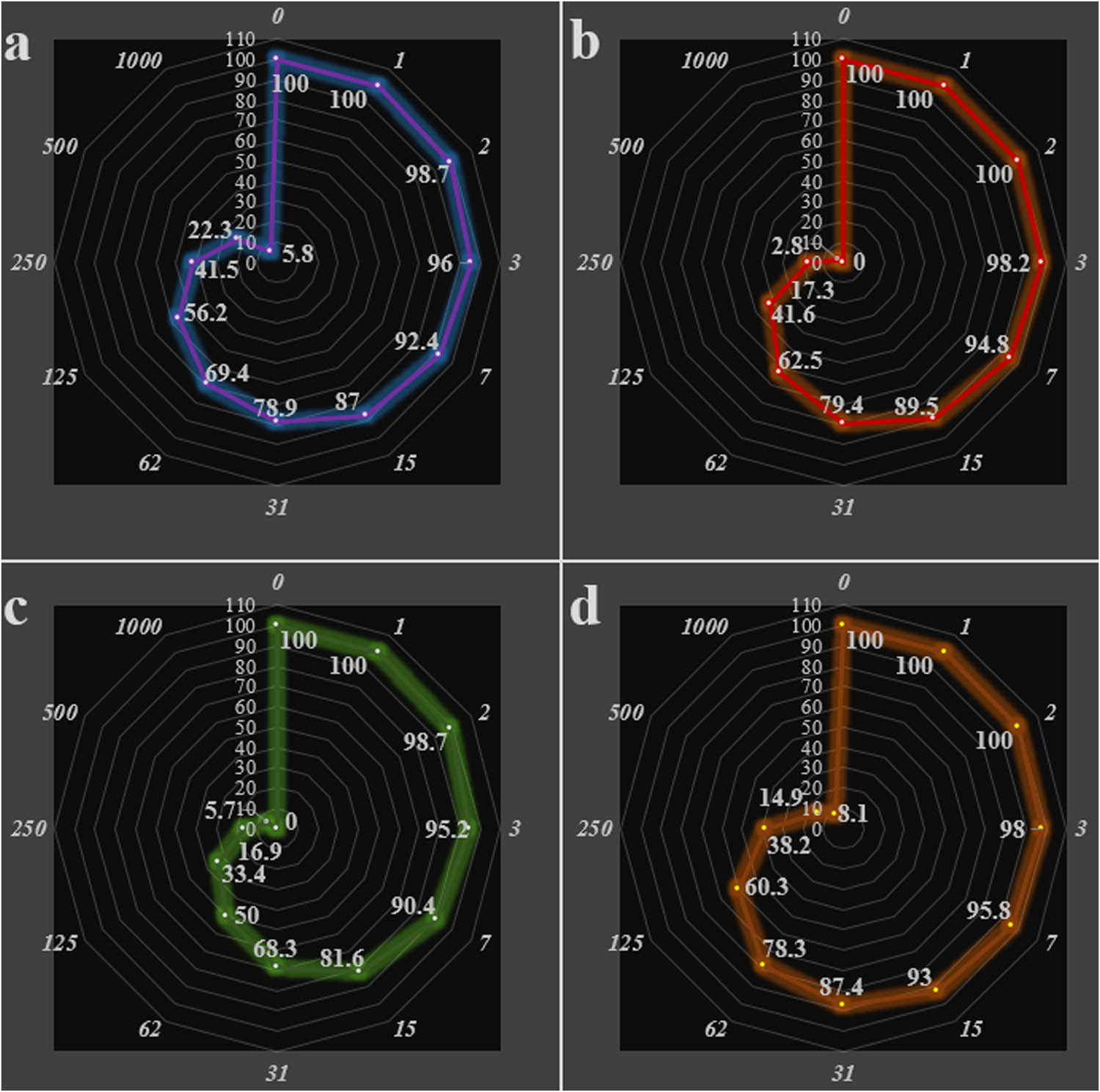
Anti-cutaneous squamous cell carcinoma efficacy of nanocomposite against SCC IC1MET (a), SCC IC19 (c), SCC T8 (c), and SCC T11 (d) cells.
The cell viability examination plays a vital role in toxicology studies. It helps to understand the effects of various toxic substances on cells and offers valuable information on metabolic processes and cell survival [39]. Recent research has shown that the Ag NPs toxicity on HeLa cells rises as the concentration increases [40]. The enhanced toxic effect of Ag NPs on MCF7 cells is linked to decreased viability, increased apoptosis, and reduced cell growth [41]. Franco-Molina et al. discovered that colloidal Ag has a cytotoxic efficacy on breast carcinoma cells [42]. The research aligns with these results, as it is confirmed that Ag NPs cause alterations in the morphology of Hep-2 and BHK-21 cells. Upon microscopic observation, it was evident that cells exposed to Ag NPs exhibited a distinct monolayer loss. A recent study by Liao et al. reported that the cytotoxic effects of Ag NPs are influenced by factors such as dosage, exposure time, and particle size, especially for particles smaller than 10 nm [43].
4 Conclusion
Finally, herein we described an efficient technique for biogenic synthesis of Ag NPs over A. millefolium-chitosan hydrogel in ultrasonic conditions without the use of any toxic or hazardous substances. The unmetalled hydrogel extract displays an absorption at 335 nm which diminished gradually in the latter graph when Ag ions got reduced to its NPs over the CS-Achillea hydrogel and a prominent absorption was noticed at 450 nm in 30 min of reaction time. In the oncological part of the recent research, the IC50 of Ag NPs was 182, 158, 177, 178, 177, 99, 62, and 183 µg/mL against PM1, MET1, MET 4, SCC T9, SCC IC1MET, SCC IC19, SCC T8, and SCC T11 cell lines, respectively.
-
Funding information: Authors state no funding involved.
-
Author contributions: H. Z., L. D. – conceptualization, visualization, writing—original draft, writing—review and editing; Y. L., W. L. – methodology, formal analysis, writing—review and editing; S. Z., C. H. – resources, investigation, writing—review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
[1] Moheghi N, Tavakkol Afshari J, Brook A. The cytotoxic effect of Zingiber officinale in breast cancer (MCF7) cell line. Intern Med Today. 2011;17(3):28–34.Search in Google Scholar
[2] Durak I, Biri H, Devrim E, Sözen S, Avcı A. Aqueous extract of Urtica dioica makes significant inhibition on adenosine deaminase activity in prostate tissue from patients with prostate cancer. Cancer Biol Ther. 2004;3:855–7.10.4161/cbt.3.9.1038Search in Google Scholar PubMed
[3] Konrad L, Müller HH, Lenz C, Laubinger H, Aumüller G, Lichius JJ. Antiproliferative effect on human prostate cancer cells by a stinging nettle root (Urtica dioica) extract. Planta Med. 2000;66:44–7.10.1055/s-2000-11117Search in Google Scholar PubMed
[4] Safarinejad MR. Urtica dioicafor treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5(4):1–11.10.1080/J157v05n04_01Search in Google Scholar
[5] Aydin M, Aslaner A, Zengin A. Using urtica dioica in esophageal cancer: a report of a case. Internet J Surg. 2005;7(2):1–3. https://print.ispub.com/api/0/ispub-article/9543. Accessed February 27, 2017.Search in Google Scholar
[6] Alsemari A, Alkhodairy F, Aldakan A, Al-Mohanna M, Bahoush E, Shinwari Z, et al. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella foenum-graecum. BMC Complement Altern Med. 2014;14:114.10.1186/1472-6882-14-114Search in Google Scholar PubMed PubMed Central
[7] Amin A, Alkaabi A, Al-Falasi S, Daoud SA. Chemopreventive activities of Trigonella foenum graecum (fenugreek) against breast cancer. Cell Biol Int. 2005;29:687–94.10.1016/j.cellbi.2005.04.004Search in Google Scholar PubMed
[8] Abaza MS, Orabi KY, Al-Quattan E, Al-Attiyah RJ. Growth inhibitory and chemo-sensitization effects of naringenin, a natural flavanone purified from Thymus vulgaris, on human breast and colorectal cancer. Cancer Cell Int. 2015;24:15–46.10.1186/s12935-015-0194-0Search in Google Scholar PubMed PubMed Central
[9] Al-Menhali A, Al-Rumaihi A, Al-Mohammed H, Al-Mazrooey H, Al-Shamlan M, AlJassim M, et al. Thymus vulgaris (thyme) inhibits proliferation, adhesion, migration, and invasion of human colorectal cancer cells. J Med Food. 2015;18:54–9.10.1089/jmf.2013.3121Search in Google Scholar PubMed
[10] Applequist WL, Moerman DE. Yarrow (Achillea millefolium L.): a neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ Bot. 2011;65:209–25.10.1007/s12231-011-9154-3Search in Google Scholar
[11] Kowal T, Pic S. Produktywnośćgatunku Achillea millefolium L, w warunkach naturalnych [Productivity of the species Achillea millejolium L. in natural habitats]. Acta Agrobot. 2015;32:91–100.10.5586/aa.1979.009Search in Google Scholar
[12] Stojanović G, Radulović N, Hashimoto T, Palić R. In vitro antimicrobial activity of extracts of four achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol. 2005;101:185–90.10.1016/j.jep.2005.04.026Search in Google Scholar PubMed
[13] Benedek B, Kopp B, Melzig MF. Achillea millefolium L. Sl – is the anti-inflammatory activity mediated by protease inhibition? J Ethnopharmacol. 2007;113:312–7.10.1016/j.jep.2007.06.014Search in Google Scholar PubMed
[14] Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, et al. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–91.10.1002/ptr.1776Search in Google Scholar PubMed
[15] Lemmens-Gruber R, Marchart E, Rawnduzi P, Engel N, Benedek B, Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium sl on isolated guinea-pig ilea. Arzneimittelforschung. 2005;56:582–8.10.1055/s-0031-1296755Search in Google Scholar PubMed
[16] Zangeneh MM, Mohammadi G, Salmani S, Razeghi Tehrani P, Rashidi K, Zangeneh A. A comparative evaluation of nephroprotective property of Urtica dioica L. aqueous extract and glibenclamide in diabetic mice. Res J Pharmacogn. 2020;7(1):31–40.Search in Google Scholar
[17] Nie C, Du P, Zhao H, Xie H, Li Y, Yao L, et al. Ag@TiO2 nanoprisms with highly efficient near-infrared photothermal conversion for melanoma therapy. Chem Asian J. 2020;15:148–55.10.1002/asia.201901394Search in Google Scholar PubMed
[18] Shanmugasundaram T, Radhakrishnan M, Gopikrishnan V, Kadirvelu K, Balagurunathan R. Biocompatible silver, gold and silver/gold alloy nanoparticles for enhanced cancer therapy: in vitro and in vivo perspectives. Nanoscale. 2017;9:16773–90.10.1039/C7NR04979JSearch in Google Scholar PubMed
[19] Swanner J, Sears JJ, Singh R, Hooker A, Donati GL, Furdui CM, et al. Silver nanoparticles selectively treat triple-negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB BioAdv. 2019;1:639–60.10.1096/fba.2019-00021Search in Google Scholar PubMed PubMed Central
[20] Espinosa A, Curcio A, Cabana S, Radtke G, Bugnet M, Kolosnjaj-Tabi J, et al. Intracellular biodegradation of Ag nanoparticles, storage in ferritin, and protection by a Au shell for enhanced photothermal therapy. ACS Nano. 2018;12:6523–35.10.1021/acsnano.8b00482Search in Google Scholar PubMed
[21] Hembram KC, Chatterjee S, Sethy C, Nayak D, Pradhan R, Molla S, et al. Comparative and mechanistic study on the anticancer activity of quinacrine-based silver and gold hybrid nanoparticles in head and neck cancer. Mol Pharm. 2019;16:3011–23.10.1021/acs.molpharmaceut.9b00242Search in Google Scholar PubMed
[22] Liu E, Zhang M, Cui H, Gong J, Huang Y, Wang J, et al. Tat-functionalized Ag-Fe3O4 nano-composites as tissue-penetrating vehicles for tumor magnetic targeting and drug delivery. Acta Pharm Sin B. 2018;8:956–68.10.1016/j.apsb.2018.07.012Search in Google Scholar PubMed PubMed Central
[23] Mohseni MS, Khalilzadeh MA, Mohseni M, Hargalani FZ, Getso MI, Raissi V, et al. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-starch nanocomposite and characterization of mechanical properties of the films. Biocatal Agric Biotechnol. 2020;25:101569.10.1016/j.bcab.2020.101569Search in Google Scholar
[24] Khalilzadeh MA, Borzoo M. Green synthesis of silver nanoparticles using onion extract and their application for the preparation of a modified electrode for determination of ascorbic acid. J Food Drug Anal. 2016;24(4):796–803.10.1016/j.jfda.2016.05.004Search in Google Scholar PubMed PubMed Central
[25] Tang Y, Liang J, Wu A, Chen Y, Zhao P, Lin T, et al. Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl Mater Interfaces. 2017;9:26648–64.10.1021/acsami.7b05292Search in Google Scholar PubMed
[26] Habiba K, Aziz K, Sanders K, Santiago CM, Mahadevan LSK, Makarov V, et al. Enhancing colorectal cancer radiation therapy efficacy using silver nanoprisms decorated with graphene as radiosensitizers. Sci Rep. 2019;9:1–9.10.1038/s41598-019-53706-0Search in Google Scholar PubMed PubMed Central
[27] Chakraborty B, Pal R, Ali M, Singh LM, Rahman DS, Ghosh SK, et al. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell Mol Immunol. 2016;13:191–205.10.1038/cmi.2015.05Search in Google Scholar PubMed PubMed Central
[28] Behnam MA, Emami F, Sobhani Z, Koohi-Hosseinabadi O, Dehghanian AR, Zebarjad SM, et al. Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Adv Pharm Bull. 2018;8:49–55.10.15171/apb.2018.006Search in Google Scholar PubMed PubMed Central
[29] Syafiuddin A, Salmiati, Salim MR, Kueh ABH, Hadibarata T, Nur H. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. J Chin Chem Soc. 2017;64(7):732–56. 10.1002/jccs.201700067.Search in Google Scholar
[30] Lu Y, Wan X, Li L, Sun P, Liu G. Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4-nitrophenol and performance as anti-colorectal carcinoma. J Mater Res Technol. 2021;12:1832–43.10.1016/j.jmrt.2021.03.093Search in Google Scholar
[31] Cai Y, Karmakar B, Salem MA, Alzahrani AY, Bani-Fwaz MZ, Oyouni AAA, et al. Ag NPs supported chitosan-agarose modified Fe3O4 nanocomposite catalyzed synthesis of indazolo[2,1-b]phthalazines and anticancer studies against liver and lung cancer cells. Int J Biol Macromol. 2022;208:20–8.10.1016/j.ijbiomac.2022.02.172Search in Google Scholar PubMed
[32] Yan J, Karmakar B, Zaki MSA, Osman R, Abdalla AM, Shati AA, et al. Introducing a bionanocomposite (ultrasound-assisted synthesis of Ag nanoparticles embedded aloe vera gel) for the treatment of cervical carcinoma. J Exp Nanosci. 2022;17:617–30.10.1080/17458080.2022.2134562Search in Google Scholar
[33] Tortella GR, Rubilar O, Durán N, Diez MC, Martínez M, Parada J, et al. Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater. 2020;390:121974.10.1016/j.jhazmat.2019.121974Search in Google Scholar PubMed
[34] Paciorek P, Żuberek M, Grzelak A. Products of lipid peroxidation as a factor in the toxic effect of silver nanoparticles. Materials. 2020;13:2460.10.3390/ma13112460Search in Google Scholar PubMed PubMed Central
[35] Rohde MM, Snyder CM, Sloop J, Solst SR, Donati GL, Spitz DR, et al. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part Fibre Toxicol. 2021;18:37.10.1186/s12989-021-00430-1Search in Google Scholar PubMed PubMed Central
[36] Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11.10.1186/1743-8977-11-11Search in Google Scholar PubMed PubMed Central
[37] Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55:283–91.10.3349/ymj.2014.55.2.283Search in Google Scholar PubMed PubMed Central
[38] McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27.10.1016/j.jfda.2014.01.010Search in Google Scholar PubMed PubMed Central
[39] Datkhile KD, Durgawale PP, Patil MN. Biogenic silver nanoparticles are equally cytotoxic so as chemically synthesized silver nanoparticles. Biomed Pharmacol J. 2017;10(1):337–44.10.13005/bpj/1114Search in Google Scholar
[40] Jeyaraj M, Rajesh M, Arun R, Mubarak Ali D, Sathishkumar G, Sivanandhan G, An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf B: Biointerfaces. 2013;102:708–17.10.1016/j.colsurfb.2012.09.042Search in Google Scholar PubMed
[41] Abrahamse H, Abdel Harith M, Hussein A, Tynga I. Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int J Nanomed. 2014;9(1):3771.10.2147/IJN.S63371Search in Google Scholar
[42] Franco-Molina MA, Mendoza-Gamboa E, Sierra-Rivera CA, Gómez-Flores RA, Zapata-Benavides P, Castillo-Tello P, et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Cancer Res. 2010;29(1):148.10.1186/1756-9966-29-148Search in Google Scholar PubMed PubMed Central
[43] Liao C, Li Y, Tjong SC. Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci. 2019;20(2):449.10.3390/ijms20020449Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

