Abstract
Sulfadiazine (SDZ) is a commonly used antibiotic in medicine, aquaculture, and animal husbandry. However, its misuse has resulted in its release into soil and water environments, posing a gradual threat to the environment and human health. In this study, cotton pulp, poplar sawdust, and corn stover were chosen as raw materials. Zinc chloride (ZnCl2) was used as a modifier to prepare modified porous carbon through pyrolysis at different carbonization temperatures (400 and 800°C). The objective was to investigate the adsorption effect and mechanism of modified porous carbon on SDZ in aqueous environments, as well as the effect of different biomass fractions of the carbon source on the adsorption effect. The physical and chemical properties of the modified porous carbon were characterized by various means of characterization, and the results showed that the high temperature and modification effects made the adsorbent material possess a larger specific surface area and richer pore structure, higher aromaticity, higher degree of graphitization, etc., which would be beneficial for the adsorption of SDZ. Among them, CCPZ800 showed the highest saturation adsorption of SDZ, Q max = 425.45 mg/g. The adsorption experiments were carried out by changing the initial conditions and fitted with kinetic and isothermal adsorption to further explain the adsorption mechanism of modified porous carbon on SDZ in conjunction with the adsorption of SDZ by hydrothermal carbon materials. The results showed that the adsorption of modified porous carbon on SDZ conformed to the quasi-secondary kinetic and Freundlich isothermal adsorption models. Adsorption mechanism of SDZ on modified porous carbon followed a multimolecular layer adsorption, with chemical adsorption being the dominant process. Both physical adsorption and chemical adsorption occurred simultaneously, with the main adsorption mechanism being π–π conjugation. In addition, compositional distribution of biomass from different carbon sources results in variations in pyrolysis mode and pyrolysis products, which in turn affect adsorption. By analyzing the effect of variability in the composition of biomass on the adsorption effect of SDZ, it can be concluded that higher cellulose content in the carbon source leads to a better adsorption effect of SDZ. The study showcases the effectiveness of ZnCl2-modified porous carbon in removing SDZ from water, offering insights into the selection of raw materials for this adsorbent preparation.
1 Introduction
Sulfonamide antibiotics (SAs) have a long history of use as synthetic antibiotics and are widely consumed in China [1]. Among them, sulfadiazine (SDZ) is commonly used in agriculture and farming as an effective anti-infective drug [2]. However, studies have indicated that SDZ is resistant to degradation and can easily migrate, leading to its accumulation in soil and water bodies [3]. This accumulation poses a significant risk to the ecological environment [4]. Additionally, SDZ can be transferred through the food chain and accumulate in humans, thereby posing a threat to human health [5,6].
For the remediation of SA pollution, various methods are commonly employed, including biodegradation, chemical oxidation, and adsorption [7]. Among these methods, adsorption stands out due to its ease of operation and lower potential for causing secondary pollution [8]. Biochar, a carbon material obtained through high-temperature cracking of waste biomass, is frequently used as an adsorption material due to its affordability and simple preparation process [9]. However, the effectiveness of traditional biochar in adsorption is often unsatisfactory due to factors such as limited pore space, fewer functional groups, and low degree of aromatization [10]. To enhance the adsorption performance of biochar, researchers often employ acid–base modification and metal salt impregnation [11]. These modifications lead to the development of porous carbon with increased pore space, larger specific surface area, more functional groups, and higher aromaticity. Among many modifiers, zinc chloride (ZnCl2) is one of the most effective modifiers for the preparation of porous carbon materials [12], which accelerates the dissolution of cellulose (CE), hemicellulose (HE), and lignin (LI) in biomass constituents, resulting in the formation of a three-dimensional and interconnected porous structure. Some researchers prepared ZnCl2-modified porous carbon materials from coffee grounds, and the results showed that ZnCl2 modification could significantly enhance the adsorption of methylene blue by the adsorbent [13]. ZnCl2 modification can keep more carbon immobilized and increase the specific surface area and porosity of the material, which in turn enhances the adsorption capacity of the material and the ability to adsorb pollutants [14]. Additionally, ZnCl2 disrupts the graphitic structure of biomass-derived carbon, enhancing the degree of graphitization of the biochar [15]. Moreover, zinc serves as a backbone during the carbonization process, improving the degree of carbonization and aromaticity of the biochar [16].
The use of modified porous carbon in the treatment of antibiotic contamination is currently widespread [17]. Porous carbon interacts with pollutants through various adsorption methods, including pore adsorption, electrostatic attraction, π–π conjugation, and hydrogen bonding [18]. The adsorption of porous carbon materials varies significantly depending on the raw materials and methods used for their preparation [19,20]. Therefore, in this study, we utilized cotton pulp, corn stover (CS), and poplar sawdust (PS) as biomass with distinct compositions. Zinc chloride was employed as a modifier to prepare the modified porous carbon at different cracking temperatures. The physicochemical properties and structural differences of the porous carbon were analyzed under different conditions using structural characterization techniques. Additionally, adsorption experiments were conducted using SDZ as a pollutant to elucidate the adsorption mechanism of the porous carbon on SDZ. These experiments aimed to further understand the adsorption behavior of SDZ on porous carbon and analyze the impact of the distribution of biomass components on the adsorption effect. The results of this study will help to deepen the understanding of the adsorption behavior of SDZ in the aqueous environment and provide assistance in the preparation of the initial carbon source selection for the adsorbent materials for the removal of SAs in the aqueous environment, which will help to solve the status quo of SDZ antibiotic pollution, and is of practical significance in the management of antibiotic pollution in the aqueous environment and the sustainable development of the environment.
2 Materials and methods
2.1 Preparation of carbon materials
The raw material cotton pulp (CP) was taken from a university light industry institute, and PS and CS were taken from the park and farmland. The raw materials were cleaned, naturally dried, and then transferred to the oven at 65°C for 2 h, and then crushed and sieved. The raw materials of modified porous carbon should be weighed and mixed with a saturated solution of ZnCl2 according to a certain proportion, then impregnated for 24 h, and then used.
In the experiment, the unmodified carbon materials and modified porous carbon were prepared by the oxygen-limited cracking method, the raw materials were spread into the quartz boat and put into the tube furnace, and the protective gas nitrogen was introduced to make the furnace chamber in an adiabatic state, and the temperature of the furnace chamber was raised to the target temperature (400 or 800°C) and held for 2 h at the rate of 10°C/min after the gas was stabilized, the pyrolysis was finished, and the products were washed by boiling with 1 mol/L hydrochloric acid, and washed to neutral with pure water, transferred to the oven at 90°C for drying, and then ground and bagged for spare use. At the end of pyrolysis, the pyrolysis product was washed with 1 mol/L hydrochloric acid to remove impurities by heating and boiling, washed to neutrality with pure water, transferred to an oven at 90°C for drying, and then ground and bagged for spare use, and labeled according to the raw material, temperature, and modifier as CCP400/800, CPS400/800, CCS400/800, CCPZ400/800, CPSZ400/800, and CCSZ400/800 (CP, PS, CS is the raw material type, Z is ZnCl2 modified, and 400, 800 represents the temperature).
The hydrothermal carbon spheres were prepared by the hydrothermal synthesis method. The raw materials were put into a high-pressure reactor, added 50 mL of pure water, and fully reacted at a temperature of 260°C for 8 h. At the end of the reaction, it was cooled down to room temperature and taken out, and then soaked in ethanol and repeatedly centrifuged to clean for more than three times, and then cleaned until the liquid was colorless and then put into the oven at 90°C to be used for drying, labeled with HCCP, HCPS, and HCCS (CP, PS, and CS represent the raw material types, respectively, and HC is the hydrothermal carbon).
2.2 Characterization of carbon materials
The physical and chemical properties and characteristics of the carbon materials were examined using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), Brunauer, Emmett and Teller, X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). An SEM (Tescan, Czech Republic) was used to observe the micro-morphology and characteristics of the carbon materials. A fully automatic specific surface area analyzer (Quantachrome, USA) was used to analyze the specific surface area and other pore structure parameters of the carbon materials. The FTIR functional group analysis of the carbon materials was carried out at 500–4,000 cm−1 by FTIR spectrometer (Bruker, Germany), and the solid-phase composition of the carbon materials was analyzed by X-ray diffractometer (Bruker, Germany) at a 2θ angle of 10°–70°. X-ray photoelectron spectrometry (Thermo Fisher, USA) was used to radiate the samples with an Alkα X-ray source, with a spot size of 400°–70°. X-ray photoelectron spectrometer (Thermo Fisher, USA) was used to analyze the elemental and structural compositions of the carbon materials using an Alkα X-ray source with a spot size of 400 μm, a fluence energy of 150 eV, and a binding energy range of 1,350–0 eV.
2.3 Carbon material adsorption experiments
The target pollutant SDZ (Aladdin, USA, purity ≥98%) was prepared as a stock solution using deionized water. The modified porous carbon with better adsorption effect on SDZ was preferred, and the effects of adsorbent addition (1–100 mg), pH (2–12), adsorption time (1–2,880 min), and the initial concentration of pollutants (1–50 mg/L) on the adsorption amount of SDZ were investigated, and adsorption kinetics and isothermal adsorption were fitted to analyze the adsorption mechanism.
Batch adsorption experiments were conducted on SDZ using hydrothermal carbon materials prepared from different carbon sources as well as modified porous carbon materials prepared from three components of biomass: CE, HE, and LI (Aladdin, USA), respectively, to further explain the adsorption mechanism and the effect of the differences in the composition of the feedstocks on the adsorption amount.
2.4 Adsorption, kinetics, isothermal equations
The adsorbent adsorption capacity is calculated as
where Q e is the amount of SDZ adsorbed by the porous carbon (mg/g), C 0 represents the initial concentration of SDZ (mg/L), C e denotes the concentration of SDZ after the end of adsorption (mg/L), V represents the volume of the solution (L), and m represents the amount of absorbent added (g).
The adsorption kinetics were fitted to the adsorption process using quasi-primary kinetics, and quasi-secondary kinetics. The quasi-primary adsorption kinetics was modeled as
where Q e (mg/g) is the equilibrium adsorption amount at the adsorption equilibrium of the modified porous carbon, Q t (mg/g) is the adsorption amount at the moment t, and k 1 is the quasi-secondary adsorption kinetic constant. The quasi-secondary adsorption kinetic modeling equation is
where Q e (mg/g) is the equilibrium adsorption of SDZ on porous carbon at adsorption equilibrium, Q t (mg/g) is the adsorption of SDZ at time t, and k 2 is the kinetic constant of quasi-secondary adsorption.
Langmuir isothermal adsorption model and Freundlich isothermal adsorption model were selected for isothermal fitting of the adsorption process of the adsorbent. Langmuir isothermal adsorption model equations are given below:
where Q max is the saturated adsorption of SDZ at adsorption equilibrium (mg/g), Q e is the amount of SDZ adsorbed at different concentrations (mg/g), and C e is the concentration of SDZ (mg/L). K L is the Langmuir’s constant.
The Freundlich isothermal adsorption model equation is given below:
where Q e is the amount of SDZ adsorbed at different concentrations (mg/g), C e is the concentration of SDZ (mg/L) while KF and n are Freundlich adsorption constants.
2.5 Analysis methods
SDZ was detected using liquid chromatography, and the supernatant after adsorption was filtered through 0.22 μm filter membrane and loaded into liquid chromatography vials for determination using HPLC1290 high performance liquid chromatograph (HPLC, 1290, Agilent, USA).
Chromatographic conditions: Column: EclipsePlusC18 (2.1 × 100 mm); Detection wavelength: 280 μm; Injection volume: 20 μL; Mobility V (C2H3N):V (0.01 mol/L H3PO4) = 20:80.
3 Results
3.1 Characterization of the morphology and pore structure of carbon materials
High temperatures and modifications cause significant changes in the microstructure of the carbon material. However, the unmodified materials still retain some of their original structure, such as the fibrous structure of CP and the lamellar structure of PS. The appearance of the ZnCl2-modified porous carbon materials undergoes significant changes, with visible pore structures (Figure 1). This is mainly attributed to the catalytic dehydration of the raw material by ZnCl2. As the temperature increases, the modification process becomes more intense, leading to the destruction, polymerization, and deformation of the original biomass structure. This results in the release of H and O, and the overflow of water vapor, ultimately forming a porous structure [15].

SEM images of carbon materials prepared from three biomasses.
The modified carbon materials exhibited a significant increase in specific surface area and pore volume, with the carbonization temperature playing a crucial role in enhancing these properties (Table 1). CCPZ800 showed the highest total specific surface area of 1663.62 m2/g and pore volume of 1.255 cm3/g. The adsorption/de-adsorption curves of ZnCl2-modified porous carbon exhibited consistent Type IV adsorption isotherm characteristics (Figure 2) with hysteresis loops. The modified porous carbon prepared at a low carbonization temperature of 400°C (Figure 2a–c) displayed H4-type hysteresis loops, primarily attributed to microporous structures. On the other hand, the modified porous carbon prepared at a high carbonization temperature of 800°C (Figure 2d–f) exhibited H2-type hysteresis loops, mainly due to the presence of mesoporous structures. This transition was likely caused by the melting and collapsing of the microporous structures, leading to the formation of mesopores as the temperature increased [21].
Pore structure parameters of raw materials and carbon materials
| Material | Specific surface area (m2/g) | Mesoporous specific surface area (m2/g) | Microporous specific surface area (m2/g) | Total pore volume (cm3/g) | Mesopore pore volume (cm3/g) | Microporous pore volume (cm3/g) | Average pore diameter (nm) |
|---|---|---|---|---|---|---|---|
| CP | 40.67 | 8.62 | 0 | 0.002 | 0.002 | 0 | 8.14 |
| CCP400 | 85.25 | 11.25 | 0 | 0.012 | 0.008 | 0 | 7.64 |
| CCP800 | 110.27 | 75.26 | 0 | 0.027 | 0.008 | 0 | 6.95 |
| CCPZ400 | 935.02 | 53.12 | 803.65 | 0.547 | 0.071 | 0.334 | 2.33 |
| CCPZ800 | 1663.62 | 685.19 | 62.08 | 1.255 | 0.668 | 0.007 | 3.02 |
| PS | 56.47 | 5.16 | 0 | 0.001 | 0.001 | 0 | 8.34 |
| CPS400 | 68.54 | 9.15 | 0 | 0.009 | 0.003 | 0 | 7.62 |
| CPS800 | 91.25 | 52.64 | 0 | 0.021 | 0.015 | 0 | 7.18 |
| CPSZ400 | 352.66 | 66.82 | 116.77 | 0.262 | 0.119 | 0.069 | 2.98 |
| CPSZ800 | 669.26 | 332.65 | 101.64 | 0.764 | 0.555 | 0.039 | 4.56 |
| CS | 43.17 | 2.62 | 0 | 0.001 | 0.001 | 0 | 7.14 |
| CCS400 | 62.16 | 21.62 | 0 | 0.013 | 0.005 | 0 | 6.95 |
| CCS800 | 105.15 | 68.25 | 0 | 0.029 | 0.016 | 0 | 6.44 |
| CCSZ400 | 589.38 | 137.30 | 161.77 | 0.445 | 0.197 | 0.146 | 3.02 |
| CCSZ800 | 741.03 | 496.66 | 26.28 | 0.803 | 0.640 | 0.005 | 4.33 |

N2 adsorption/desorption diagram of modified porous carbon. (a) CCPZ400, (b) CPSZ400, (c) CCSZ400, (d) CCPZ800, (e) CPSZ800, and (f) CCSZ800.
3.2 Characterization of the chemical structure of carbon materials
FTIR analysis revealed that both the raw and unmodified carbon materials (Figure 3a–c) exhibited stretching vibrations of –OH (3,400–3,650 cm−1), (2,978–2,932 cm−1), ester group C–O (1,045–1,100 cm−1), and –C═O double bonds (1,670–1,715 cm−1). The increase in temperature and the modification process resulted in the detachment of binding water in biomass, leading to the breakage of hydroxyl groups and an increase in hydrophobicity [22]. Consequently, the intensity of the –OH group peaks on the surface of the modified porous carbon (Figure 3d–f) gradually weakened or almost disappeared. Simultaneously, the stretching vibration of –COOH appeared on the surface (1,220 cm−1), along with the stretching vibration of C═C double bond (1,540–1,640 cm−1) and the bending vibration of aromatic hydrocarbon –CH (810–820 cm−1). These changes were attributed to the continuous weakening of –CH2, resulting in the formation of an aromatic structure [23]. Additionally, in the fingerprint region below 600 cm−1, a peak at 463 cm−1 (Figure 3e) was observed, indicating the stretching vibration of Zn–O. This peak may be attributed to the generation of Zn-containing oxides during the modification process.

FTIR diagram of carbon material. (a–c) Three feedstocks and unmodified carbon materials and (d–f) Modified porous carbon from three raw materials.
XRD analysis revealed diffraction peaks at 2θ = 15°, 17°, and 22° for CP (Figure 4a), corresponding to the (1-10), (110), and (200) crystal planes of CE CE-I, which aligns with the standard CE crystal structure. Similarly, CS (Figure 4c) also exhibited a peak at 2θ = 22°. PS (Figure 4b) displayed peaks at 2θ = 29.1°, 30.9°, 40.9°, 44.9°, and 51.2°, corresponding to CaMg(CO3)2 (PDF No. 75-1655 and PDF No. 79-1342), respectively. Additionally, the SiO2 peak (PDF No. 85-0539) was observed in both CS and PS, indicating the absorption of silicon elements from raw materials [24]. The high temperature and modification processes led to the disruption of the biomass’s original structure, accelerated CE dissolution, and consequent weakening of the characteristic CE peaks in CP and CS. The SiO2 diffraction peaks in PS were significantly attenuated after warming carbonization and modification, while the appearance of diffraction peaks corresponding to CaSiO3 and Mg2SiO4 (PDF No. 77-5177 and PDF No. 74-1680) suggests the reaction of SiO2 with CaMg(CO3)2 at elevated pyrolysis temperatures, forming silicate. Moreover, CPSZ400/800 exhibited new diffraction peaks at 2θ = 36.3°, 42.1°, and 61.1°, corresponding to (111), (200), and (220) crystal plane reflections of ZnO (PDF No. 77-0191). The presence of Zn-O bonds on the surface of CPSZ400/800 is consistent with the FTIR results. CPSZ800 contains a small amount of elemental Zn on its surface, while no characteristic peaks of Zn were found on the surfaces of CCPZ800 and CCSZ800, which may cause less secondary contamination of elemental Zn in wastewater treatment.

XRD diagram of carbon material. (a) Cotton pulp and its modified and unmodified carbon materials, (b) Poplar sawdust and its modified and unmodified carbon materials and (c) Corn stoever and its modified and unmodified carbon materials.
Modified porous carbon materials exhibit a strong broad peak at 2θ = 25°, which is attributed to the carbon (002) crystal diffraction peak. This peak reflects the level of spatial order of the aromatic carbon lamellae. The peak symmetry and absence of nearby spurious peaks indicate that these carbon materials are composed of ordered and oriented polycyclic aromatic carbon lamellae [25]. Notably, CPSZ400 shows more γ-peaks, which are caused by the presence of aliphatic hydrocarbon chains connected to the edges of the aromatic carbon lamellae. Higher levels of LI in PS are more difficult to break down at low temperatures, as the temperature increases, the pyrolytic fracture of aromatic hydrocarbon side chains leads to a reduction in γ-peaks, resulting in a more regular and orderly structure in CPSZ800 [26]. Additionally, the broader peaks observed at 2θ = 45° in both CP- and CS-modified porous carbons can be attributed to the carbon (101) crystal plane diffraction peaks. The sharper peaks observed in CPSZ800 and CCSZ800 indicate a higher degree of graphitization in these materials [27].
The XPS gross spectra (Figure 5a–c) indicate that all three types of modified porous carbon have high carbon content, with CCPZ800 having the highest carbon content. Additionally, CPSZ800 and CCSZ800 contain a small amount of Si, which is consistent with previous findings. The C1s split-peak fitted spectra (Figure 5d–f) of CCPZ800 reveal the highest C–C/C═C content, followed by CCPZ800 and CCSZ800. It is important to note that a shake-up peak of C1s appears at 291.18 eV next to the main peak of the C1s split-peak map of CCPZ800 (Figure 5d). This peak is primarily caused by the presence of a π–π conjugation system [28]. The existence of conjugated π-bonds in the plane of C atoms leads to the appearance of a companion peak at a higher energy level. This indicates the strong π-electron supplying ability of CCPZ800. Moreover, the companion peak is also a characteristic peak of graphite π-bonding, suggesting that CCPZ800 contains a higher degree of graphite structure and a higher density of π-electrons on its surface [29]. Meanwhile, no significant amount of zinc was detected in the XPS spectra of the three modified porous carbons, suggesting that the material poses a low risk of zinc contamination of wastewater.

XPS diagram of carbon material. (a–c) XPS total mapping of porous carbon modified with three different raw materials and (d–f) C1s spectral profiles of porous carbon modified with three different raw materials.
3.3 Adsorption experiment
Carbon materials with different preparation conditions were selected for the batch adsorption of SDZ to initially investigate the adsorption effect on SDZ (Table 2), and materials with excellent adsorption effects were preferred from each carbon source for the subsequent tests. CCPZ800, CPSZ800, and CCSZ800 were selected to investigate the adsorption of SDZ on modified porous carbon by changing the adsorption conditions.
Adsorption of SDZ by carbon materials with different preparation conditions
| Biomass | Temperature (°C) | Unmodified Q e (mg/g) | Modified Q e (mg/g) |
|---|---|---|---|
| CP | 400 | 5.03 | 279.26 |
| 800 | 19.77 | 425.45 | |
| PS | 400 | 7.52 | 52.76 |
| 800 | 23.67 | 214.99 | |
| CS | 400 | 13.50 | 77.87 |
| 800 | 25.12 | 130.10 |
The three modified porous carbon materials showed the maximum adsorption of SDZ at an additional amount of 1 mg (Figure 6a), which could reach 454.99, 239.50, and 177.27 mg/g, respectively, and the Q e decreased with the increase in the addition amount. This is because the total adsorption capacity increases with the increase in the additional amount, but the amount of the target pollutant is certain, which leads to a decrease in the adsorption capacity per unit mass.
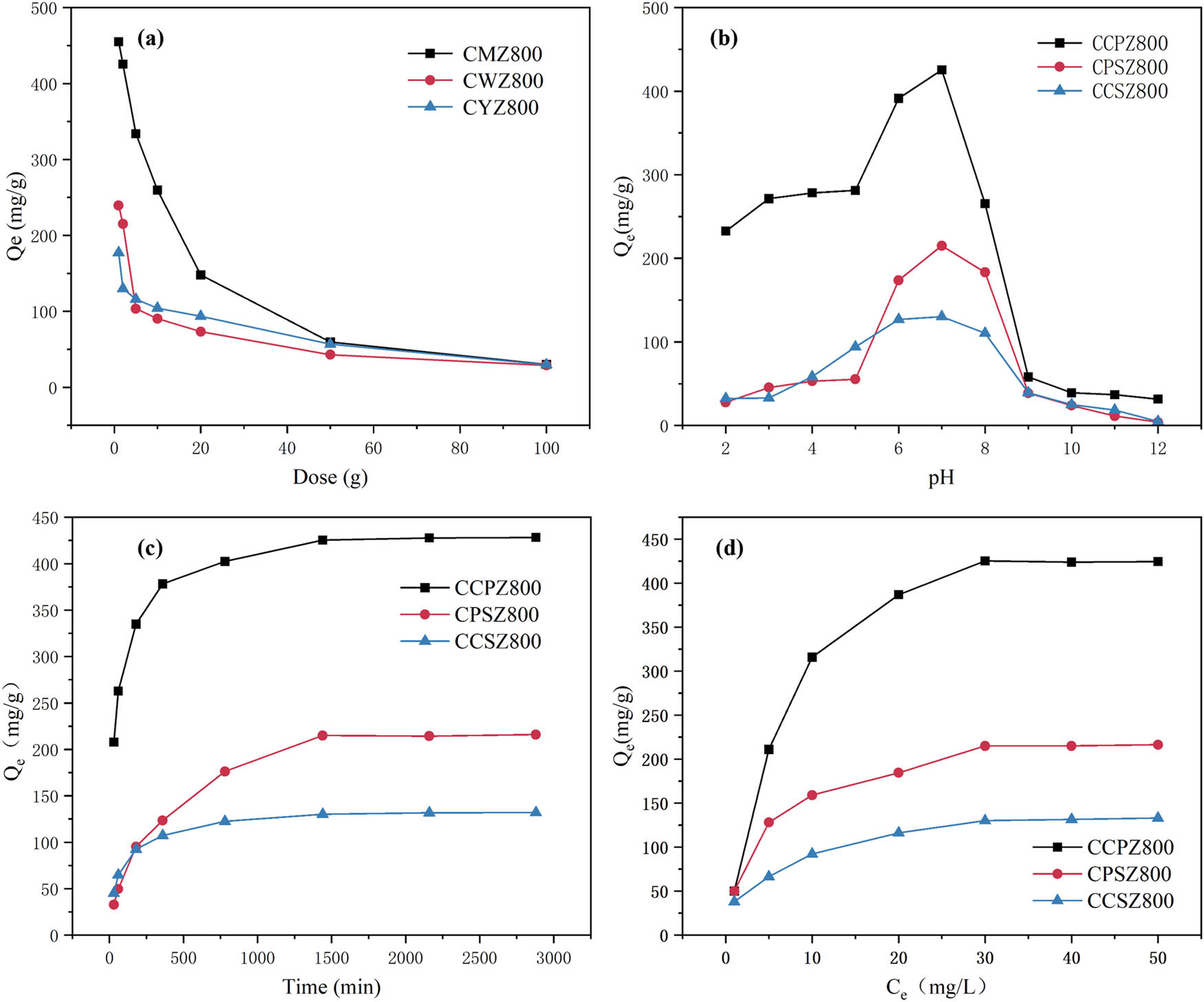
Effect of different conditions on the adsorption of modified porous carbon on SDZ. (a) Dose, (b) pH, (c) time, and (d) concentration.
The adsorption amounts of the three modified porous carbons showed a tendency to increase and then decrease with the increase in pH (Figure 6b). Among them, CCPZ800, CPSZ800, and CCSZ800 all had the largest adsorption amounts at pH = 7, which could reach 425.45, 214.99, and 130.10 mg/g. Changes in acid and alkaline environments can affect the adsorption of SDZ, especially alkaline conditions have the most pronounced effect on SDZ adsorption.
SDZ are ionizable compounds and will exist as cations (SDZ+), anions (SDZ−), and neutral molecules (SDZ0) in different pH backgrounds [30]. Since the zero point charge of pH (pHzpc) of the three modified porous carbons ranges from 5.9 to 6.8 (Table 3), the modified porous carbons are electrostatically repelled from SDZ under acidic conditions (positive repulsion) and alkaline conditions (negative repulsion), thus inhibiting the adsorption [31]. This suggests that electrostatic action was not the primary adsorption mechanism for SDZ, which is consistent with the findings of Tzeng et al. [32].
Modified porous carbon for pHzpc
| Material | pHzpc |
|---|---|
| CCPZ800 | 6.8 |
| CPSZ800 | 5.9 |
| CCSZ800 | 6.4 |
SDZ is an aromatic compound that contains an aromatic heterocyclic ring. The amino and sulfonamide groups on SDZ0 act as strong π-electron acceptors, while the modified porous carbon with aromaticity acts as a strong π-electron donor. The high temperature and modification effects increase the graphitization and hydrophobicity of the material, which results in a higher π-electron density on the surface of the modified porous carbon, which will facilitate the formation of π–π conjugation and thus enhancement of adsorption between the modified porous carbon and SDZ [33]. Acidic and basic conditions lead to a decrease in the π-electron density of SDZ, which can lead to a decrease in the π-electronic properties on the aromatic ring of SDZ, weakening the π–π conjugation with the modified porous carbon, and consequently decreasing the amount of adsorption [31].
In addition, SDZ0 has hydrophobicity, and the modified porous carbon made by high-temperature pyrolytic carbonization has weakened hydrophilicity due to the enhancement of aromaticity, which also suggests that hydrophobicity distribution plays a role in the adsorption of SDZ on the modified porous carbon [34].
The adsorption equilibrium time of the three modified porous carbons on SDZ was about 1,440 min (Figure 6c), and the adsorption amount of SDZ increased rapidly in 0–600 min, and the increase in the adsorption amount slowed down in 600–720 min, and then gradually leveled off in 1,440 min. At the beginning of the reaction, the SDZ concentration was larger, the modified porous carbon possessed more effective adsorption sites, and SDZ would rapidly occupy multiple adsorption sites, resulting in an accelerated adsorption rate. With the decrease in effective adsorption sites, the adsorption rate decreased, the adsorption of SDZ was close to saturation, and the adsorption system reached equilibrium.
At low concentrations (1–30 mg/L), the adsorption of SDZ by the three modified porous carbons gradually increased with the increase in SDZ concentration (Figure 6c), but the adsorption of SDZ by the three modified porous carbons gradually stabilized when the concentration gradually reached 30 mg/L. At low concentrations of SDZ, SDZ could not completely occupy the adsorption sites of porous carbon during the adsorption process. As the concentration increases, the adsorption sites are gradually occupied, resulting in saturation adsorption due to the insufficiency of adsorption sites, and the adsorption amount tends to stabilize gradually [35].
3.4 Kinetics and isothermal adsorption
To further analyze the interaction between adsorbent and SDZ, it is important to analyze the adsorption kinetics and isothermal adsorption. The adsorption process of SDZ on modified porous carbon with quasi-primary, quasi-secondary kinetics fitting results (Figure 7) showed that the quasi-secondary kinetics R 2 = 0.9807, 0.9882, and 0.9935 for CCPZ800 (Figure 7a), CPSZ800 (Figure 7b), and CCSZ800 (Figure 7c) were greater than the quasi-primary kinetics of R 2 = 0.8658, 0.9753, 0.9894 (Table 4). And the quasi-secondary kinetic Q e (CCPZ800 = 426.63 mg/g, CPSZ800 = 242.64 mg/g, CCSZ800 = 134.25 mg/g) was closer to the experimental values (CCPZ800 = 425.45 mg/g, CPSZ800 = 214.99 mg/g, CCSZ800 = 130.10 mg/g), which indicates that the quasi-secondary model is more consistent with the adsorption process of SDZ by these three modified porous carbons than the quasi-primary model. The quasi-primary kinetic model is commonly used to describe the initial stage of adsorption, specifically focusing on single-layer physical adsorption, It is obtained by parametric integration of the Lagergren equation, which has some limitations in practical applications. On the other hand, the quasi-secondary adsorption kinetic equation is mainly based on chemisorption, describes the whole process of adsorption, and contains multilayer adsorption with the joint action of various mechanisms (surface adsorption, intra-particle diffusion, etc.).

Kinetic fitting of SDZ adsorption by modified porous carbon.
Adsorption kinetics fitting parameters
| Model parameter | CCPZ800 | CPSZ800 | CCSZ800 | |
|---|---|---|---|---|
| Quasi-primary kinetic model | K 1 | 0.0190 | 0.0028 | 0.0001 |
| Q e (mg/g) | 403.0871 | 213.0637 | 133.6503 | |
| R 2 | 0.8658 | 0.9753 | 0.9894 | |
| Quasi-secondary kinetic model | K 2 | 0.0006 | 0.0001 | 0.0001 |
| Q e (mg/g) | 426.6309 | 242.6377 | 134.2449 | |
| R 2 | 0.9807 | 0.9882 | 0.9935 | |
At the same time, the quasi-secondary adsorption kinetic model suggests that chemisorption may be an important factor in the whole adsorption process, and the adsorption process may be controlled by the interactions generated by adsorbent and adsorbate as well as by the exchange of electrons [36]. Therefore, the quasi-secondary kinetic equations of the three adsorbents were highly fitted, and the adsorption of SDZ on the three modified porous carbons could be preliminarily judged to be the result of multiple adsorption mechanisms, including chemisorption or physicochemical adsorption.
The isothermal adsorption model can well describe the distribution of adsorbent in the solid–liquid phase at adsorption equilibrium, and then explain the adsorption mechanism. The adsorption of SDZ by CCPZ800 (Figure 8a), CPSZ800 (Figure 8b), and CCSZ800 (Figure 8c) showed good linear relationships with the fits of both Langmuir isothermal adsorption model and Freundlich isothermal adsorption model, but the correlation coefficients of Freundlich isothermal adsorption model R 2 (CCPZ800 = 0.9815, CPSZ800 = 0.9639, and CCSZ800 = 0.9753) were higher than the correlation coefficients R 2 of the Langmuir isothermal adsorption model (CCPZ800 = 0.9684, CPSZ800 = 0.8966, and CCSZ800 = 0.8864) (Table 5), and the adsorption of SDZ on the three kinds of modified porous carbons by the Freundlich isothermal adsorption model showed good linear relationships.

Isothermal adsorption fitting of modified porous carbon for SDZ adsorption. (a) CCPZ800 Q e, (b) CPSZ800 Q e, and (c) CCSZ800 Q e.
Isothermal adsorption fitting parameters
| Model parameter | CCPZ800 | CPSZ800 | CCSZ800 | |
|---|---|---|---|---|
| Langmuir | K L | 1.0559 | 0.3199 | 0.2543 |
| Q max (mg/g) | 429.7380 | 234.3726 | 144.0729 | |
| R 2 | 0.9684 | 0.8966 | 0.8864 | |
| Freundlich | K F | 247.4364 | 126.3029 | 53.5503 |
| n | 6.2710 | 7.0048 | 3.9957 | |
| R 2 | 0.9815 | 0.9639 | 0.9753 | |
The Langmuir isothermal adsorption model assumes the presence of numerous adsorption active sites on the surface of the adsorbent. Saturation adsorption is achieved when all the active sites are occupied. This model primarily describes monomolecular layer adsorption. On the other hand, the Freundlich isothermal adsorption model assumes that the adsorption process takes place on a surface that is not uniform [37]. The amount of adsorption increases as the concentration of the target pollutant increases. This model mainly describes multimolecular layer adsorption. This is supported by the adsorption constants (n) in the Freundlich isothermal adsorption model, which are greater than 1 for all three modified porous carbons. The higher values of n suggest a stronger force between the modified porous carbons and SDZ, making the adsorption process easier to occur. This type of adsorption is known as favorable adsorption, adsorption of SDZ by modified porous carbon is a multimolecular layer adsorption and is a chemisorption-dominated adsorption mechanism [38].
Table 5 shows that the saturated adsorption Q max of the three modified porous carbons were CCPZ800 = 429.7380 mg/g > CPSZ800 = 234.3726 mg/g > CCSZ800 = 144.0729 mg/g. Compared to other similar adsorbents in the literature (Table 6), CCPZ800 showed better adsorption capacity for SDZ removal.
3.5 Hydrothermal carbon and raw material composition adsorption experiment
The modified porous carbon is expected to have oxygen-containing functional groups that can form hydrogen bonding interactions with the amino groups of SDZ, it is possible that hydrogen bonding is also one of the main adsorption mechanisms Hydrothermal carbonization is a process that converts biomass into carbon nanomaterials in an aqueous phase, using specific temperature and pressure conditions [42]. The resulting hydrothermal carbon nanomaterials possess a significant amount of oxygen-containing functional groups on their surface. These functional groups can form hydrogen bonds with polar groups, such as amino groups, present on the surface of SDZ and enhance the adsorption effect on SDZ [43]. The adsorption Q e of SDZ by the hydrothermal carbon materials HCCP, HCPS, and HCCS prepared by hydrothermal synthesis of the three raw materials were 25.37, 18.17, and 16.48 mg/g, respectively (Table 7). The adsorption capacity of the hydrothermal carbon material was significantly lower than that of the modified porous carbon. The limited adsorption of SDZ by the hydrothermal carbon material, which was enriched with oxygen-containing functional groups, suggests that the hydrogen-bonding effect produced by these groups on SDZ is not the primary adsorption mechanism.
Adsorption of SDZ by hydrothermal carbon
| Hydrothermal carbon | Q e (mg/g) |
|---|---|
| HCCP | 25.37 |
| HCPS | 18.17 |
| HCCS | 16.48 |
There were differences in the adsorption capacity of the modified porous carbon from different carbon sources, and the distribution of the major constituents of the carbon source biomass might also affect the adsorption effect.
CE, HE, and LI are the primary constituents of biomass, along with a few other organic compounds and inorganic elements [44]. CE acts as the “skeleton,” HE functions as the “filler,” and LI acts as the “binder,” collectively forming a comprehensive biomass [45]. CP contains the highest amount of CE (>90%), PS contains more LI, and CS contains more HE than the other raw materials [46]. Modified porous carbon was prepared using CE, HE, and LI as carbon sources (same method as in 2.1 of this article) CCEZ400/800, CHEZ400/800, and CLIZ400/800, (400/800 stands for carbonization temperature), and SDZ was adsorbed to further investigate the effect of carbon source composition on adsorption.
Table 8 shows that the CE-modified porous carbon prepared at 800°C showed the highest adsorption, followed by LI and HE-modified porous carbon with 307.97, 256.33, and 156.83 mg/g, respectively. The variability in adsorption is due to the different behaviors of the constituent components during pyrolysis. CE in the lower temperature (<400°C) under the partial decomposition of CO2, CO, H2O, and other forms of release and produce coke and small molecules (hydroxyacetone, monosaccharides, etc.) and the formation of a certain pore structure, with the increase in temperature, while at high temperatures (>650°C), the small molecules through the decarbonylation and arylation of a large number of aromatic hydrocarbons, coke will be arylated into an aryl ring, and a large number of mesopores will be generated. The pyrolysis process of HE is similar to that of CE. It produces a significant amount of light-oxygenated compounds (such as acetic acid, propionic acid, and other compounds), coke, and a small amount of pore structure at temperatures below 400°C. At temperatures above 650°C, the coke undergoes further polymerization to form an aromatic ring [47]. Additionally, the oxygenated compounds undergo arylation, resulting in the generation of aromatic hydrocarbons along with some CO and H2. This process also leads to the formation of mesoporous structure. In contrast, LI is structurally more stable than CE because of its numerous aromatic rings. It primarily undergoes dehydration and softening reactions at low temperatures (<400°C), resulting in the production of intermediate products and a poor pore structure [48]. However, at higher temperatures (>650°C), the LI β–O-4 linkage breaks down, leading to the formation of carbon nanosheets within the aromatic units. Additionally, the coke produced by LI enhances its aromaticity through the arylation reaction, resulting in the generation of a larger number of micropores [49]. The pyrolysis products of CE, HE, and LI produced at higher carbonization temperatures exhibit greater aromaticity compared to those produced at lower temperatures. This increased aromaticity enhances the adsorption of SDZs through π–π conjugation. Therefore, modified porous carbon materials carbonized at 800°C demonstrate a superior adsorption.
Adsorption of SDZ by modified porous carbon prepared from raw material components
| Material | Temperature (°C) | Q e (mg/g) |
|---|---|---|
| CCEZ400 | 400 | 94.74 |
| CCEZ800 | 800 | 307.97 |
| CHEZ400 | 400 | 49.45 |
| CHEZ800 | 800 | 156.80 |
| CLIZ400 | 400 | 17.53 |
| CLIZ800 | 800 | 256.33 |
Due to its lower degree of polymerization compared to CE, HE is more easily decomposed [50]. At high temperatures, HE undergoes almost complete decomposition, resulting in a weaker aromaticity in the CE product. This weaker aromaticity can potentially reduce the adsorption of SDZ. This explains the difference in SDZ adsorption by CCPZ/CCEZ and CCSZ/CHEZ [51].
The modifier and high-temperature effects can disrupt the graphitic structure of CE-derived carbon. This disruption is beneficial as it increases the graphitization of the carbon. Higher graphitization, in turn, results in a higher π-electron density on the surface of the carbon. This higher π-electron density potentially promotes the π–π conjugation of modified porous carbon to SDZ, enhancing the adsorption effect [52]. It is possible that this is the reason why CCPZ presents better adsorption properties to SDZ [53].
The distribution of biomass raw materials and the pyrolysis temperatures have different effects on the adsorption of SDZ by the pyrolysis products of porous carbon. Under lower temperature preparation conditions, the adsorption effects of the modified pyrolysis products’ three components on SDZ were as follows: CE > HE > LI. However, under higher temperature preparation conditions, the adsorption effects of the three components on SDZ were: CE > LI > HE. The adsorption effect of the modified pyrolysis products’ three components on SDZ was found to be highest at higher temperature preparation conditions, with CE having the strongest effect. Therefore, when selecting a carbon source for the preparation of porous carbon for SDZ adsorption, biomass with higher CE content should be chosen. Additionally, if the carbon source biomass has a higher LI content, it should be subjected to elevated carbonization temperature to improve the experimental outcome.
4 Conclusion
The modified porous carbon was prepared using ZnCl2 as a modifier and different carbon sources were selected at varying temperatures through the oxygen-limited cracking method. The physical and chemical properties of the carbon were characterized, and its adsorption performance was investigated using SDZ as the target pollutant. The aim of this study was to explain the adsorption mechanism and the reasons for the variability in adsorption among different carbon sources. The results of the study are as follows:
High temperature and modification can lead to the destruction of the original morphology of feedstock biomass, resulting in significant changes and reorganization of its microstructure. This process also leads to the formation of a pore structure in the modified porous carbon, which has a large specific surface area and pore volume. The modified porous carbon prepared at the higher carbonization temperature (800°C) was dominated by mesoporous structure, while the modified porous carbon prepared at the lower carbonization temperature (400°C) was dominated by microporous structure. High temperatures and modifications help to increase the functional group structure of the porous carbon surface and enhance the aromaticity and graphitization of the porous carbon. Among the modified porous carbons prepared at 800°C, CCPZ800 has the highest degree of carbonization and graphitization, indicating a higher carbon content and its adsorption of SDZ was the largest, reaching 425.45 mg/g, followed by CPSZ800 and CCSZ800. The results showed that higher carbonization temperature and the modification effect of ZnCl2 could enhance the adsorption of SDZ on porous carbon.
CCPZ800, CPSZ800, and CCSZ800 exhibited the highest adsorption capacity for SDZ at pH = 7. The adsorption equilibrium time was approximately 1,440 min, and the adsorption process followed the quasi-secondary adsorption kinetic model and Freundlich isothermal adsorption model. The saturated adsorption amounts were as follows: CCPZ800 (429.74 mg/g) > CPSZ800 (234.37 mg/g) > CCSZ800 (144.07 mg/g). The Q max of CCPZ800 for SDZ is slightly better than other adsorbent materials prepared in the literature. The poor adsorption of SDZ on hydrothermal carbon materials enriched with oxygen-containing functional groups suggests that hydrogen bonding of SDZ by oxygen-containing functional groups on the surface is not the main adsorption mechanism. The variation in the distribution of carbon source biomass components resulted in differences in pyrolysis products, thereby impacting the adsorption process. Specifically, a higher biomass CE content led to improved adsorption efficiency of SDZ by the modified porous carbon material. The stronger aromaticity, higher degree of carbonization and graphitization of modified porous carbon enhance the π–π conjugation and thus the adsorption of sulphadiazine, which may be the reason for the differences in the adsorption of sulphadiazine by modified porous carbon prepared from different raw materials and methods.
The adsorption mechanism of SDZ on ZnCl2-modified porous carbon was chemisorption-dominated multimolecular layer adsorption, it contains a cooperative interaction of physical pore adsorption, electrostatic attraction, hydrogen bonding, and π–π conjugation, and π–π conjugation played the most significant role. Additionally, hydrophobicity partitioning also contributed to the adsorption process.
5 Limitations and future research
In this study, ZnCl2-modified porous carbon was successfully prepared using different raw materials and modification conditions, and its physical and chemical property characteristics were investigated to study the adsorption effect and mechanism of SDZ in the aqueous environment, and to explore the effects of different carbon sources and preparation conditions on the adsorption effect, which provides theoretical and technological help to carry out the remediation work of SDZ pollution.
However, there are parts of this study that need to be improved and thought deeply, based on which this study still needs to improve and explore the experiments and contents for the following elements to make the results more perfect and scientific.
In this study, SDZ was selected as a single target pollutant, but wastewater often contains a variety of pollutants, so it is necessary to conduct adsorption experiments when a variety of common pollutants are mixed in subsequent experiments, and to examine the selective adsorption and competitive adsorption by modified porous carbon.
Alternatively, in the actual treatment of contaminated wastewater by adsorbent materials, it is particularly important to prevent secondary pollution, which will directly affect the effect of adsorbent materials to treat wastewater, in the subsequent experiments should be optimized preparation process, as far as possible to reduce the modifiers and other exogenous substances on the water environment caused by secondary pollution.
The long-term stability and regeneration of the adsorbent material is particularly important, and subsequent experiments are needed to demonstrate how the modified porous carbon behaves over multiple use cycles and whether the adsorption capacity changes over time, loaded with magnetic material to facilitate separation from the water column, which will be beneficial in demonstrating the value of the material for practical applications.
The preparation temperature of modified porous carbon in this experiment was only up to 800°C, the experimental results of this study show that a higher carbonization temperature can enhance the adsorption of SDZ on porous carbon, so a higher carbonization temperature (>900°C) can be chosen to investigate the adsorption performance of the pollutants in the subsequent experiments on the preparation of modified porous carbon.
This study mainly focuses on the adsorption of SDZ in the aqueous environment, while antibiotic contamination usually involves the water and soil environments, and the remediation of SDZ-contaminated soils can be considered in subsequent experiments by using this experimental material.
-
Funding information: This research was funded by Shaanxi Province Key Research Program Project (2023-ZDLSF-28) and Shaanxi Land Engineering Construction Group internal scientific research project (DJNY-YB-2023-27, DJNY2024-21, and DJNY-YB-2023-21).
-
Author contributions: Methodology: J.W. and L.Z.; software: T.C.; validation: H.Z. and Y.S.; formal analysis: Y.W. and C.Y.; data curation: Y.H.; writing – original draft: J.W.; writing – review and editing: L.Z. and T.C.; supervision: H.Z. and Y.S. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors J.W., L.Z., T.C., H.Z., Y.S., Y.W., C.Y., and Y.H. were employed by the company Shaanxi Provincial Land Engineering Construction Group Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
[1] Ponka D. Approach to managing patients with sulfa allergy: Use of antibiotic and nonantibiotic sulfonamides. Can Fam Physician Med de Famille Canadien. 2006;52(11):1434–8.Search in Google Scholar
[2] Guo X, Li J, Yang F, Yang J, Yin D. Prevalence of sulfonamide and tetracycline resistance genes in drinking water treatment plants in the Yangtze River Delta, China. Sci Total Environ. 2014 Sep;493:626–31.10.1016/j.scitotenv.2014.06.035Search in Google Scholar PubMed
[3] Fahad MM, Al-Khuzaie MGA. Recent advances in sulfadiazine’s preparation, reactions and biological applications. Euras Chem Commun. 2021;3:383–91.Search in Google Scholar
[4] Zhou H, Cui J, Li X, Wangjin Y, Pang L, Li M, et al. Antibiotic fate in an artificial‐constructed urban river planted with the algae Microcystis aeruginosa and emergent hydrophyte. Water Environ Res. 2022;94(1):e1670.10.1002/wer.1670Search in Google Scholar PubMed
[5] Ting-Ting Z, Zong-Cai TU, Ping-Ping T, Lu Z, Xiao-Mei S, Hui W. Histopathology of carassius auratus gibelio after sulfadiazine exposure in water environment. Acta Hydrobiol Sin. 2018;42:47–56.Search in Google Scholar
[6] Wehrhan A, Streck T, Groeneweg J, Vereecken H, Kasteel R. Long-term sorption and desorption of sulfadiazine in soil: experiments and modeling. J Environ Qual. 2010;39(2):654–66.10.2134/jeq2009.0001Search in Google Scholar PubMed
[7] Zhang WW, Gong AJ, Lina Q, Cao YQ, Yuan XT. Processes of degradation and removal methods of antibiotics from waste water. Chin J Antibiot. 2013;38(9):401–10.Search in Google Scholar
[8] Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG, et al. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol. 2015;175:391–5. 10.1016/j.biortech.2014.10.104.Search in Google Scholar PubMed
[9] Dong J, Li P, Ji X, Kang Y, Yuan X, Tang J, et al. Electrons of d-orbital (Mn) and p-orbital (N) enhance the photocatalytic degradation of antibiotics by biochar while maintaining biocompatibility: A combined chemical and biological analysis. J Hazard Mater. 2023Jun;451:131083.10.1016/j.jhazmat.2023.131083Search in Google Scholar PubMed
[10] Zhu X, Li C, Li J, Xie B, Lü J, Li Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour Technol. 2018;475–82.10.1016/j.biortech.2018.05.041Search in Google Scholar PubMed
[11] Munir R, Ali K, Naqvi SAZ, Muneer A, Bashir MZ, Maqsood MA, et al. Green metal oxides coated biochar nanocomposites preparation and its utilization in vertical flow constructed wetlands for reactive dye removal: Performance and kinetics studies. J Contam Hydrol. 2023 May;256:104167.10.1016/j.jconhyd.2023.104167Search in Google Scholar PubMed
[12] He X, Ling P, Yu M, Wang X, Zheng M. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors. Electrochim Acta. 2013;105(26):635–41.10.1016/j.electacta.2013.05.050Search in Google Scholar
[13] Ifi DZ, Aydn N. Comparison of H3PO4 and ZnCl2 activated filtered coffee waste carbon-based adsorbents in methylene blue removal by using ultrasonic-assisted adsorption. Arab J Sci Eng. 2022;48(7):8641–53.10.1007/s13369-022-07248-9Search in Google Scholar
[14] Benmahdi F, Oulmi K, Khettaf S, Kolli M, Merdrignac-Conanec O, Mandin P. Synthesis and characterization of microporous granular activated carbon from Silver berry seeds using ZnCl2 activation. Fuller Nanotube Carbon Nanostruct. 2021;9(29):657–69.10.1080/1536383X.2021.1878154Search in Google Scholar
[15] Minaei S, Benis KZ, Mcphedran KN, Soltan J. Evaluation of a ZnCl2-modified biochar derived from activated sludge biomass for adsorption of sulfamethoxazole. Chem Eng Res Des. 2023;190:407–20.10.1016/j.cherd.2022.12.038Search in Google Scholar
[16] Xia D, Tan F, Zhang C, Jiang X, Chen Z, Li H, et al. ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Appl Surf Sci. 2016;377:361–9.10.1016/j.apsusc.2016.03.109Search in Google Scholar
[17] Sudhakar MP. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel. 2021;285:119205.10.1016/j.fuel.2020.119205Search in Google Scholar
[18] Yu S, Zhou J, Ren Y. Excellent adsorptive-photocatalytic performance of zinc oxide and biomass derived N, O-contained biochar nanocomposites for dyes and antibiotic removal. Chem Eng J. 2022;451:138959.10.1016/j.cej.2022.138959Search in Google Scholar
[19] Zheng H, Wang Z, Zhao J, Herbert S, Xing B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ Pollut. 2013;181(1):60–7.10.1016/j.envpol.2013.05.056Search in Google Scholar PubMed
[20] Teixidó M, Pignatello JJ, Beltrán JL, Granados M, Peccia J. Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar). Environ Sci Technol. 2011;45(23):10020.10.1021/es202487hSearch in Google Scholar PubMed
[21] Sing KSW, Williams RT. Physisorption hysteresis loops and the characterization of nanoporous materials. London, England: SAGE Publications; 2004. p. 773–82.10.1260/0263617053499032Search in Google Scholar
[22] Wang L, Wang X, Zou B, Ma X, Qu Y, Rong C, et al. Preparation of carbon black from rice husk by hydrolysis, carbonization and pyrolysis. Bioresour Technol. 2011;102(17):8220–4. 10.1016/j.biortech.2011.05.079. Search in Google Scholar
[23] Wang P, Tang L, Wei X, Zeng G, Zhou Y, Deng Y, et al. Synthesis and application of iron and zinc doped biochar for removal of p-nitrophenol in wastewater and assessment of the influence of co-existed Pb(II). Appl Surf Sci. 2017;392:391–401. 10.1016/j.apsusc.2016.09.052.Search in Google Scholar
[24] Wang Z, Yang X, Qin T, Liang G, Li Y, Xie X. Efficient removal of oxytetracycline from aqueous solution by a novel magnetic clay–biochar composite using natural attapulgite and cauliflower leaves. Environ Sci Pollut Res Int. 2019;26(8):7463–75. 10.1007/s11356-019-04172-8.Search in Google Scholar PubMed
[25] Wen Y, Tang X, Li J, Hao J, Wei L, Tang X. Impact of synthesis method on catalytic performance of MnOx–SnO2 for controlling formaldehyde emission. Catal Commun. 2009;10(8):1157–60.10.1016/j.catcom.2008.12.033Search in Google Scholar
[26] Sonibare OO, Haeger T, Foley SF. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy (Oxf). 2010;35(12):5347–53. 10.1016/j.energy.2010.07.025.Search in Google Scholar
[27] Huang Q, Chen H, Xu L, Lu D, Tang L, Jin L, et al. Visible-light-activated photoelectrochemical biosensor for the study of acetylcholinesterase inhibition induced by endogenous neurotoxins. Biosens Bioelectron. 2013;45:292–9. 10.1016/j.bios.2013.01.075.Search in Google Scholar PubMed
[28] Luo L, Shen X, Song L, Zhang Y, Zhu B, Liu J, et al. MoS2/Bi2S3 heterojunctions-decorated carbon-fiber cloth as flexible and filter-membrane-shaped photocatalyst for the efficient degradation of flowing wastewater. J Alloy Compd. 2019;779:599–608. 10.1016/j.jallcom.2018.11.154.Search in Google Scholar
[29] Ji L, Chen W, Zheng S, Xu Z, Zhu D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir. 2009;25(19):11608–13. 10.1021/la9015838.Search in Google Scholar PubMed
[30] Ma D, Wang J, Feng K. A green strategy from waste red mud to Fe∼0-based biochar for sulfadiazine treatment by peroxydisulfate activation. Chem Eng J. 2022;446:441P–6P.10.1016/j.cej.2022.136944Search in Google Scholar
[31] Xie M, Chen W, Xu Z, Zheng S, Zhu D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ Pollut. 2014;186:187–94.10.1016/j.envpol.2013.11.022Search in Google Scholar PubMed
[32] Tzeng T, Liu Y, Deng Y, Hsieh Y, Tan C, Wang S, et al. Removal of sulfamethazine antibiotics using cow manure-based carbon adsorbents. Int J Environ Sci Technol. 2016;13(3):973–84.10.1007/s13762-015-0929-4Search in Google Scholar
[33] Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang J, et al. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol. 2012;118:536–44.10.1016/j.biortech.2012.05.042Search in Google Scholar PubMed
[34] Zhao H, Liu X, Cao Z, Zhan Y, Shi X, Yang Y, et al. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J Hazard Mater. 2016;310:235–45. 10.1016/j.jhazmat.2016.02.045.Search in Google Scholar PubMed
[35] Zhang Z, Sun L, Pei Z, Li H, Wang L, Ma J, et al. New insight into the adsorption of sulfadiazine on graphite-like biochars prepared at different pyrolytic temperatures. J Clean Prod. 2023 Aug;413:137468.10.1016/j.jclepro.2023.137468Search in Google Scholar
[36] Nam S, Choi D, Kim S, Her N, Zoh K. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J Hazard Mater. 2014;270:144–52. 10.1016/j.jhazmat.2014.01.037.Search in Google Scholar PubMed
[37] Santos RKS, Nascimento BF, De ACMB, Cavalcanti JVFL, Bruckmann FS, Rhoden CRB, et al. Removal of chloroquine from the aqueous solution by adsorption onto acaí-based biochars: Kinetics, thermodynamics, and phytotoxicity. J Mol Liq. 2023;383:122162.10.1016/j.molliq.2023.122162Search in Google Scholar
[38] Yang W, Lu Y, Zheng F, Xue X, Li N, Liu D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem Eng J. (Lausanne, Switzerland: 1996) 2012;179(1):112–8. 10.1016/j.cej.2011.10.068.Search in Google Scholar
[39] Xia S, Deng L, Liu X, Yang L, Pei Y. Fabrication of magnetic nickel incorporated carbon nanofibers for superfast adsorption of sulfadiazine: Performance and mechanisms exploration. J Hazard Mater. 2021;423(Pt B):127219.10.1016/j.jhazmat.2021.127219Search in Google Scholar PubMed
[40] Li CXHY. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J Mol Liq. 2019;274:353–61.10.1016/j.molliq.2018.10.142Search in Google Scholar
[41] Zhong J, Feng Y, Li JL, Yang B, Ying GG. Removal of sulfadiazine using 3D interconnected petal-like magnetic reduced graphene oxide (MrGO) nanocomposites. Water. 2020;12(7):1933.10.3390/w12071933Search in Google Scholar
[42] Zhurinsh A. Hydrothermal carbonization vs pyrolysis: effect on the porosity of the activated carbon materials. Sustainability. 2022;14:15982.10.3390/su142315982Search in Google Scholar
[43] Peng B, Liang S, Wang H, Meng Z, Guo M, Wang H, et al. In situ synthesis of low-valence MnO/Mn3O4 catalyst via carbon-acid hydrothermal strategy for NO removal. Mater Lett. 2022;327:133044.10.1016/j.matlet.2022.133044Search in Google Scholar
[44] Kostryukov SG, Matyakubov HB, Masterova YY, Kozlov AS, Pryanichnikova MK, Pynenkov AA, et al. Determination of lignin, cellulose, and hemicellulose in plant materials by FTIR spectroscopy. J Anal Chem. 2023;78(6):718–27.10.1134/S1061934823040093Search in Google Scholar
[45] Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci. 2008;9(9):1621–51. 10.3390/ijms9091621.Search in Google Scholar PubMed PubMed Central
[46] Díez D, Uruea A, Piero R, Barrio A, Tamminen T. Determination of hemicellulose, cellulose, and lignin content in different types of biomasses by thermogravimetric analysis and pseudocomponent kinetic model (TGA-PKM method). Processes. 2020;8(9):1048.10.3390/pr8091048Search in Google Scholar
[47] Rojas M, Ruano D, Orrego-Restrepo E, Chejne F. Non-isothermal kinetics of cellulose, hemicellulose, and lignin degradation during cocoa bean shell pyrolysis. Biomass Bioenergy. 2023;177:106932.10.1016/j.biombioe.2023.106932Search in Google Scholar
[48] Ge L, Zhao C, Zuo M. Effects of Fe addition on pyrolysis characteristics of lignin, cellulose and hemicellulose. J Energy Inst. 2023;107:101177.10.1016/j.joei.2023.101177Search in Google Scholar
[49] Liu Z, Zhang F, Wu J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel (Guildf). 2010;89(2):510–4. 10.1016/j.fuel.2009.08.042.Search in Google Scholar
[50] Li C, Sun Y, Yi Z, Zhang L, Zhang S, Hu X. Co-pyrolysis of coke bottle wastes with cellulose, lignin and sawdust: Impacts of the mixed feedstock on char properties. Renewable Energy. 2022;181:1126–39.10.1016/j.renene.2021.09.103Search in Google Scholar
[51] Xue P, Liu M, Yang H, Zhang H, Chen Y, Hu Q, et al. Mechanism study on pyrolysis interaction between cellulose, hemicellulose, and lignin based on photoionization time-of-flight mass spectrometer (PI-TOF-MS) analysis. Fuel. 2023;338:127276.10.1016/j.fuel.2022.127276Search in Google Scholar
[52] Ma L, Syed-Hassan SSA, Tong Y. Interactions of cellulose-and lignin-derived radicals during pyrolysis: An in-situ electron paramagnetic resonance (EPR) study. Fuel Process Technol. 2023;239:107536.10.1016/j.fuproc.2022.107536Search in Google Scholar
[53] Deng J, Xiong T, Wang H, Zheng A, Wang Y. Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustainable Chem Eng. 2016;4(7):3750–6. 10.1021/acssuschemeng.6b00388.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

