Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
-
Farah Aabouch
, Mohammed Kara
, Riaz Ullah
Abstract
Natural substances extracted from plants have been increasingly studied and recognized, recently. Essential oils (EOs), for example, possess antioxidant and antibacterial properties, enabling their application across different sectors like agro-food, pharmaceuticals, and cosmetics. In Morocco, exceptional plant diversity, mirroring the diversity of ecosystems, has not yet revealed all its secrets. Therefore, the aim of this study is to determine the chemical composition and evaluate the antibacterial and antioxidant activities of EOs from Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh. collected in the El Hoceima and Mamora regions, respectively. The EOs were extracted by hydrodistillation employing a Clevenger-type apparatus. Gas chromatography/mass spectrometry (GC/MS) analyses identified 54 constituents representing 92.65% of the total for T. zygis and 55 components representing 99.60% for E. camaldulensis. The primary components found in the EO of T. zygis are δ-terpineol (27.64%), followed by δ-3-carene (15.7%), thymol (14.17%), and dehydrolinalool (4.99%). The main compounds in E. camaldulensis EO are 1,8-cineole (43.61%), γ-terpinene (11.71%), α-terpineol (10.58%), and p-cymene (4.93%). The antioxidant properties of these oils were investigated by utilization of the 2,2-diphenyl-1-picrylhydrazyl method and the ferric reducing antioxidant power (FRAP) test. The antibacterial activity was assessed against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative bacteria (Micrococcus luteus and Escherichia coli). Both EOs showed significant antioxidant activity but were less effective than reference antioxidants quercetin and catechin. Antibacterial studies demonstrated strong activity of T. zygis and E. camaldulensis EOs against the studied bacteria, as well as good inhibitory properties (minimum inhibitory concentration).
1 Introduction
Today, concerns regarding public health issues related to foodborne diseases are significant on a global scale. The primary cause for the development of these diseases is the deterioration of food caused by microbial contamination and oxidation of foodstuffs [1]. Presently, the food industries use synthetic preservatives to hinder the oxidation and microbial contamination of packaged food products. Nevertheless, there is a current trend in these industries toward embracing natural antioxidant and antimicrobial compounds, plant extracts, as substitutes for synthetic preservatives. This shift is driven by the adverse impacts of synthetic preservatives on both the human health and the environment [2].
Essential oils (EOs) contain secondary plant metabolites. Distinguished by their strong odor and complex volatile composition, they act as chemical signals controlling a plant’s environment [3]. These EOs, comprising a blend of volatile compounds, exhibit a wide range of properties, including antifungal, antibacterial, antimicrobial, and antioxidant activities [4,5,6]. Due to these biological characteristics, proposals to use for food preservation have been suggested, whether by incorporating them directly into foods or in packaging materials [7]. In Morocco, various plant species, including Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh., have been the subject of extensive chemical and pharmacological studies.
T. zygis is a perennial plant of the Lamiaceae family, known in Morocco as “Zaâitra, whose natural range is restricted to Morocco and the Iberian Peninsula,” primarily found in the Mediterranean region, North Africa, Asia, and southern Europe. In Morocco, it is mainly localized in the regions of High Atlas, Anti-Atlas, Middle Atlas, and Middle Atlantic. It is commonly used to treat respiratory infections, colds, acute bronchial conditions, as a food preservative, and in traditional medicine [8,9]. Studies have shown that it improves the sensory properties of milk, reducing lipid oxidation, and increases its nutritional value, including proteins, lipids, dry matter, polyunsaturated fatty acids, as well as cheese [10]. The EOs of this plant are widely used as antiseptic agents in various pharmaceutical fields due to their antioxidant, antimicrobial, anti-inflammatory, antiseptic, anticoagulant, and antispasmodic properties [11]. These activities are attributed to phenolic compounds, particularly thymol, and alcoholic compounds such as δ-terpineol [12].
E. camaldulensis Dehnh. originates from continental Australia and is a member of the Myrtaceae family [13]. It is widely cultivated worldwide and is in the process of naturalization in Morocco [8]. The trees can reach impressive heights, with some exceeding 100 m; the typical height for the most prevalent species is between 40 and 50 m, while others of this genus are more modest in size. Eucalyptus leaves are entire, leathery, and possess a distinct cuticle; they are persistent and emit an aromatic fragrance. Essences of the Eucalyptus genus are acknowledged as significant repositories of secondary metabolites, several of which demonstrate diverse biological activities. Eucalyptus leaves are used as antispasmodics and antipyretics, frequently employed to alleviate respiratory ailments [14,15]. EOs obtained from Eucalyptus have long been used in pharmacy for the production of antiseptics [16]. The chemical analysis of its EOs has revealed the presence of various compounds, such as 1,8-cineole, γ-terpinene, α-terpineol, and p-cymene. These compounds indicate that the EOs may have antibacterial, antioxidant, anti-inflammatory, and analgesic properties [17,18,19,20].
In this context, the aim of this study is to analyze the chemical composition of the EOs from T. zygis and E. camaldulensis, as well as to assess their antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and the ferric reducing antioxidant power (FRAP). Additionally, the study aims to assess their antibacterial potency against four pathogenic strains, namely Escherichia coli, Micrococcus luteus, Bacillus subtilis, and Staphylococcus aureus. This is part of a broader effort to explore active natural substances for food preservation, targeting oxidative processes, microbial contamination, and prolonging the freshness of food products.
2 Materials and methods
2.1 Plant material
Samples of aerial parts of T. zygis and E. camaldulensis were collected in June 2021 from the El Hoceima region (Northern Morocco: latitude 35°08′09.8“N; longitude 4°05′10.7“W) and Mamora (Northwest Morocco: latitude 34°16′16.0“N; longitude 6°25′28.1“W), respectively. Species identification was done at the Scientific Institute of Rabat (Morocco) by Dr. Mohammed Sghir Taleb (a Research Professor at the Scientific Institute. His research interests are botany, plant ecology, aromatic, and socioeconomy).
2.2 Extraction of EOs
The aerial parts of T. zygis and E. camaldulensis were subjected to hydrodistillation using a Clevenger-type apparatus [21]. Three distillations were carried by boiling 200 g of plant material with 2 L of distilled water for 3 h each. The resulting EOs were transferred into securely sealed glass bottles and stored at a temperature of 4°C until they were needed for subsequent use. The yields of EO from the samples were determined using the following formula proposed by Marion et al. [22].
All experiments were conducted in triplicate.
2.3 Gas chromatography/mass spectrometry (GC/MS) analysis
The EOs were subject to gas chromatographic analysis using a Hewlett-Packard gas chromatograph (HP 6890) equipped with an HP-5 capillary column (30 m × 0.25 mm, film thickness: 0.25 µm), an FID detector, and a fixed injector at 275°C. The oven temperature was set to 50°C for 5 min and then ramped up to 250°C at a rate of 4°C/min. Nitrogen gas was used as the carrier gas at a flow rate of 1.8 mL/min, with a split ratio of 1/50 and a flow rate of 72.1 mL/min. Samples were diluted 1/50 in methanol, and manual injection was performed with a volume of 1 µL.
The chemical composition was determined through GC/MS, conducted on a Hewlett-Packard gas chromatograph (HP 6890) linked to a mass spectrometer (HP 5973). The column employed was a capillary column filled with HP-5MS (5% phenyl methyl siloxane) (30 m × 0.25 mm, film thickness: 0.25 µm). The column temperature was held at 50°C, and the water temperature was set to 0.5°C. The temperature was then increased to 250°C at a rate of 2°C/min. Helium was used as the carrier gas at a flow rate of 1.5 mL/min, and a fraction of the sample was introduced into the system. The split ratio was 1/74.7, with a flow rate of 112 mL/min. Identification of components through MS was confirmed using the NIST 98 spectral library. MS parameters included an ionization voltage of 70 eV, an ion source temperature of 230°C, and a mass scan range of 35–450 m/z. Component identification was further validated by comparing their elution order with their reported retention indices in the literature.
3 Antioxidant activity
3.1 DPPH radical scavenging activity
The trapping capacity of the DPPH radical was assessed using the standard method as reported by Lopes-Lutz et al. [23]. In this method, 1 mL of each methanolic solution of the tested EOs at various concentrations (62.5–1,000 μg/mL) was mixed with 1 mL of a methanolic solution of DPPH (0.3 mM). The mixture was vortexed and then incubated in the dark for 30 min. Absorbance was measured using a spectrophotometer at 517 nm. A positive control was represented by a solution of a standard antioxidant, quercetin, whose absorbance was measured under the same conditions as the samples. The obtained data were used to determine the sample concentration required to trap 50% of DPPH-free radicals (IC50), by plotting the percentage of inhibition against the sample concentrations.
The antioxidant activity was evaluated using the following equation:
where AA is the antioxidant activity; Abs is the absorbance at 517 nm.
3.2 FRAP assay
This assay is based on the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+). The reducing capacity of various EOs was determined following the method established by Oyaizu [24]. Various concentrations of samples and the positive control (catechin) were prepared. In each test tube, 1 mL of each sample (1.5–20 µg/mL), 2.5 mL of phosphate buffer (0.2 M, pH = 6.6), and 2.5 mL of potassium ferricyanide complex (1% w/v) (K3Fe(CN)6) were added. The mixture was incubated in a water bath at 50°C for 20 min. After incubation, 2.5 mL of trichloroacetic acid (10% w/v) was added to stop the reaction. Then, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of ferric chloride (0.1% w/v) (FeCl3). Absorbance was measured at a wavelength of 700 nm using a spectrophotometer. EC50 represents the concentration value of the oil giving an absorbance of 0.5. All experiments were repeated three times.
3.3 Organisms tested
Bacterial strains E. coli (ATCC 8739), M. luteus (ATCC 9341), S. aureus (ATCC 6538), and B. subtilis (ATCC 6633) were obtained from the Microbiology Laboratory Collection at the Center for Innovation, Research, and Training in Rabat, Morocco. The strains were inoculated from a mother culture maintained on agar at 4°C, onto nutrient agar plates and incubated at 37°C for 24 h.
3.4 Antibacterial activity of EOs
The antibacterial activity of EOs was evaluated by the disc diffusion method, recognized for its reliability and reproducibility. This method involves placing a sterile disc, previously impregnated with EO, on a growing bacterial lawn and measuring the area where bacteria cannot grow; this is indicated by the diameter value of the inhibition zone, signifying the antibacterial activity of the EOs.
To accomplish this, 15 mL of TSB (tryptic soy broth) agar medium was poured into each Petri dish, and 100 µL of bacterial suspension with a density equivalent to 0.5 McFarland standard (108 CFU/mL) was deposited. Sterile filter paper discs of 6 mm diameter were impregnated with 5 μL of EO and placed on the inoculated Petri dishes. Additionally, ampicillin (100 µg/mL), penicillin (100 µg/mL), and tetracycline (300 µg/mL) were employed as positive reference standards to ascertain the susceptibility of the tested strains. The Petri dishes were then incubated at 37°C for 24 h. Following incubation, the diameter of the inhibition zone was measured in millimeters. All experiments were conducted in triplicate [25].
3.5 Determining the minimum inhibitory and bactericidal concentrations
The minimum inhibitory concentration (MIC) of EOs was assessed through the microdilution method using 96-well microplates [26]. It corresponds to the lowest concentration of the EO that entirely inhibits the visible growth of the tested microorganism following incubation. Accordingly, from stock solutions of EOs, various dilutions of each EOs were prepared in TSB medium to achieve a final volume of 50 µL for each well. Then, 50 µL of microbial inoculum with a concentration of 108 CFU/mL (equivalent to 0.5 McFarland) was added to a series of dilution concentrations. After 24 h of incubation at 37°C, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was added to each well as an indicator of bacterial growth. After another incubation at 37°C for 30 min, microbial growth was revealed by a change in the color from yellow to violet. Minimum bactericidal concentrations (MBCs) were determined by streaking negative wells on TSB agar. The MBC value represents the concentration of the EO at which no growth is observed upon subculture after incubation at 37°C for 24 h [27].
3.6 Statistical analysis
Statistical analysis was carried out using GraphPad Prism version 8.0 for Windows (Graphpad Software Inc., San Diego, California, USA). The data presented are expressed as mean ± standard deviation derived from three independent extractions. A one-way analysis of variance (ANOVA) was performed to evaluate the data, followed by Tukey’s test for determining differences in mean values, with the significance level set as p ≤ 0.05.
4 Results and discussion
4.1 EO yields
The yield of EOs extracted from T. zygis and E. camaldulensis through hydrodistillation is 0.57 and 3.18% EOs, respectively. Different yields have been obtained for T. zygis EOs in previous studies: in the Meknes region in Northeast Morocco, the yield is 1.3% [28]; in Spain, the yield ranges from 0.4 to 0.8% [29]; while it is higher in Khénifra in the Middle Atlas (Morocco) at approximately 3.87% [30]. Similarly, for E. camaldulensis, the EO yield varies from one region to another: in Iran, it is 2.10% [24]; in Egypt, it is only 0.9% [31]; and it is even lower in Italy, at 0.31% [32].
4.2 Chemical composition of EOs
Chromatographic analyses using GC and GC/MS identified 54 compounds in the EO of T. zygis (Table 1), accounting for 99.65% of the total composition. The monoterpene fraction was predominant (94.18%) compared to the sesquiterpene one (5.47%). It exhibited a high content of δ-terpineol (27.64%), followed by δ-3-carene (15.7%), thymol (14.17%), dehydrolinalool (4.99%), trans-carvone oxide (4.13%), and α-pinene (3.98%) as major constituents. The EOs of T. zygis from the Rif region of Morocco (El Hoceima) are notably distinct from other previously studied sources. For instance, the EOs of T. zygis from Ifrane to Tigrigra (Middle Atlas) are characterized by predominantly thymol (1.8–47.10%), p-cymene (14.8–19%), carvacrol (12–57.5%), and γ-terpinene (3.1–11.90%) [33]. In Serbia, the EO of this species is marked by high concentrations of thymol (35%) and p-cymene (24.1%) [34]. Conversely, the EOs of T. zygis from Spain are predominantly linalool (39.37%), terpinen-4-ol (15.92%), β-myrcene (7.95%), γ-terpinene (6.36%), and borneol (5.17%) [35]. Research conducted in Spain similarly revealed that thymol (48.59%), p-cymene (18.79%), γ-terpinene (22.85%), and linalool (4.31%) constitute the primary components in the EOs of T. zygis [36]. However, other authors have found that T. zygis EO from Serbia has predominantly linalool (38.0%), 4-terpineol (10.1%), p-cymene (6.9%), and β-myrcene (4.9%) [37].
Chemical composition of EOs of T. zygis and E. camaldulensis
| No | Compounds | RI | Area % | |
|---|---|---|---|---|
| Thymus zygis L. ssp. gracilis | E. camaldulensis Dehnh. | |||
| 1 | Heptanal | 901 | 0.14 | — |
| 2 | Tricyclene | 921 | 1.45 | — |
| 3 | α-Thujene | 924 | 0.05 | 3.49 |
| 4 | α-Pinene | 932 | 3.98 | 0.32 |
| 5 | Norbornen-2-ol | 939 | 0.11 | — |
| 6 | Verbenene | 961 | 1.65 | — |
| 7 | Sabinene | 968 | 0.13 | 0.25 |
| 8 | trans-Pinane | 969 | 0.13 | — |
| 9 | β-Pinene | 974 | 0.24 | 0.35 |
| 10 | Myrcene | 988 | 0.07 | — |
| 11 | 2-Octanol | 994 | — | 0.1 |
| 12 | δ-2-Carene | 1,001 | 0.37 | 0.1 |
| 13 | δ-3-Carene | 1,008 | 15.7 | 0.06 |
| 14 | α-Terpinene | 1,014 | 0.42 | — |
| 15 | p-Cymene | 1,020 | 2.44 | 4.93 |
| 16 | 1,8-Cineol | 1,026 | — | 43.61 |
| 17 | E-β-Ocymene | 1,044 | 1.85 | — |
| 18 | γ-Terpinene | 1,054 | 0.72 | 11.71 |
| 19 | cis-Sabinene hydrate | 1,065 | — | 0.13 |
| 20 | cis-Linalool oxide | 1,067 | 0.6 | — |
| 21 | Camphenilone | 1,078 | 1.38 | 0.85 |
| 22 | Terpinoline | 1,086 | 0.48 | 2.3 |
| 23 | Linalool | 1,095 | 0.16 | — |
| 24 | trans-Sabinene hydrate | 1,098 | — | 0.27 |
| 25 | 6-Camphenol | 1,111 | 0.23 | — |
| 26 | Dehydrolinalool | 1,131 | 4.99 | — |
| 27 | trans-Dihydro β-terpineol | 1,134 | — | 0.39 |
| 28 | cis-Pinene hydrate | 1,139 | — | 1.42 |
| 29 | δ-Terpineol | 1,162 | 27.64 | 1.16 |
| 30 | Thujanol | 1,164 | 1.12 | — |
| 31 | cis-Linalool oxide | 1,170 | 0.93 | — |
| 32 | Terpinene-4-ol | 1,174 | — | 3.91 |
| 33 | iso-Verbanol | 1,176 | 0.31 | — |
| 34 | neo-Verbanol | 1,182 | 0.84 | — |
| 35 | α-Terpineol | 1,186 | — | 10.58 |
| 36 | γ-Terpineol | 1,199 | — | 0.22 |
| 37 | Verbinone | 1,204 | 0.05 | — |
| 38 | trans-Piperitol | 1,207 | — | 0.11 |
| 39 | trans-Carveol | 1,215 | 2.14 | — |
| 40 | cis-Sabinene hydrate acetate | 1,219 | 0.37 | |
| 41 | cis-Carveol | 1,226 | 1.77 | — |
| 42 | Pulegone | 1,233 | — | 1.02 |
| 43 | Carvone | 1,239 | — | 0.23 |
| 44 | trans-Sabinene hydrate acetate | 1,253 | — | 0.13 |
| 45 | iso-3-Thujanol acetate | 1,267 | — | 0.33 |
| 46 | trans-Carvone oxide | 1,273 | 4.13 | 0.6 |
| 47 | neo-iso-3-Thujanol acetate | 1,281 | — | 0.2 |
| 48 | Thymol | 1,289 | 14.17 | — |
| 49 | p-Cymen-7-ol | 1,290 | — | 1.35 |
| 50 | trans-Verbenyl acetate | 1,291 | 0.06 | — |
| 51 | iso-Verbanol acetate | 1,308 | 0.31 | — |
| 52 | δ-Terpinyl acetate | 1,316 | — | 0.08 |
| 53 | δ-Elemene | 1,335 | — | 0.95 |
| 54 | α-Terpinyl acetate | 1,346 | — | 0.51 |
| 55 | α-Copaene | 1,374 | 0.08 | — |
| 56 | β-Longipinene | 1,400 | 0.07 | — |
| 57 | Longifoline | 1,407 | — | 0.05 |
| 58 | E-Caryophyllene | 1,417 | 2.75 | 0.35 |
| 59 | Carvone hydrate | 1,422 | — | 0.26 |
| 60 | γ-Elemene | 1,434 | 0.07 | — |
| 61 | Aromadendrene | 1,439 | — | 0.24 |
| 62 | α-Humulene | 1,452 | 0.12 | 0.23 |
| 63 | Sesquisabinene | 1,457 | 0.17 | — |
| 64 | 9-epi-E-Caryophyllene | 1,464 | — | 0.09 |
| 65 | β-Thujaplicin | 1,475 | 0.16 | — |
| 66 | γ-Muurolene | 1,478 | — | 0.25 |
| 67 | β-Selinene | 1,489 | 0.39 | — |
| 68 | δ-Selinene | 1,492 | — | 0.2 |
| 69 | α-Muurolene | 1,500 | — | 0.38 |
| 70 | β-Bisabolene | 1,505 | 0.13 | — |
| 71 | γ-Cadinene | 1,513 | 0.27 | 0.07 |
| 72 | δ-Cadinene | 1,522 | — | 0.09 |
| 73 | Elemol | 1,548 | — | 2.11 |
| 74 | β-Calacorene | 1,564 | — | 0.22 |
| 75 | Caryophyllenyl alcohol | 1,570 | 0.21 | — |
| 76 | Germacrene D-4-ol | 1,574 | — | 0.07 |
| 77 | trans-Sesquisabinene hydrate | 1,577 | 0.22 | — |
| 78 | Caryophyllene oxide | 1,582 | 2.24 | 0.3 |
| 79 | Davanone | 1,587 | — | 1.1 |
| 80 | cis-β-Elemenone | 1,589 | 0.12 | — |
| 81 | Widdrol | 1,599 | — | 0.23 |
| 82 | trans-β-Elemenone | 1,601 | 0.09 | — |
| 83 | cis-Isologifolanone | 1,612 | — | 0.08 |
| 84 | trans-Isologifolanone | 1,625 | — | 0.28 |
| 85 | α-Acorenol | 1,632 | — | 0.15 |
| 86 | cis-Cadin-4-en-7-ol | 1,635 | 0.69 | — |
| 87 | epi-α-Muurolol | 1,644 | — | 0.18 |
| 88 | β-Eudesmol | 1,649 | 0.35 | — |
| 89 | α-Eudesmol | 1,652 | 0.28 | — |
| 90 | Dihydroeudesmol | 1,661 | — | 0.54 |
| 91 | 14-Hydroxy-Z-caryophyllene | 1,666 | 0.39 | — |
| 92 | Davanol acetate | 1,689 | 0.09 | — |
| 93 | 2E,6Z-Farnesol | 1,714 | — | 0.1 |
| Total | 99.65% | 99.6% | ||
| Monoterpenes | 94.18% | 90.57% | ||
| Sesquiterpenes | 5.47% | 9.03% | ||
| Phenols | 19.73% | 0.11% | ||
| Aldehydes | 0.14% | — | ||
| Ketones | 7.42% | 4.04% | ||
| Alcohols | 37.55% | 22.22% | ||
| Ethers | — | 43.61% | ||
| Hydrocarbons | 34.93% | 29.03% | ||
| Esters | 0.06% | 0.59% | ||
No: In order of elution on HP-5ms; Components: Components identified based on retention indices and mass spectra; RI: Retention indices are calculated experimentally using a homologous series of C9–C28 alkanes; –: Not detected.
Furthermore, in the EO of E. camaldulensis, 55 constituents were identified, comprising 99.60% of the total compounds. The EO of E. camaldulensis extracted in this study is rich in 1,8-cineole (43.61%), γ-terpinene (11.71%), α-terpineol (10.58%), p-cymene (4.93%), terpinen-4-ol (3.91%), and α-thujene (3.49%), with a high monoterpene content (90.57%) compared to sesquiterpenes (9.03%) (Table 1). It is neither qualitatively nor quantitatively comparable to previous research on the same species. Indeed, the EO extracted from E. camaldulensis collected from Algeria is characterized by high contents of eucalyptol (24.260–72.718%), aromadendrene (2.655–8.796%), globulol (1.871–8.247%), and spathulenol (0.940–7.304%) [38]. For instance, the EO of E. camaldulensis from Nicosia is predominantly characterized by β-phellandrene (30.6%), α-phellandrene (10.3%), spathulenol (9.3%), p-cymene (8.2%), and bicyclogermacrene (6.1%) [39]. Meanwhile, the oil from Taounate in Northern Morocco is co-dominated by 1,8-cineole (34.16%), (−)-spathulenol (21.21%), α-pinene (6.73%), and α-guajene (5.51%) [40]. In Senegal, the EO of this species revealed high contents of 1,8-cineole (52.6%), α-terpineol (6.6%), cis-p-mentha-1-(7)-8-dien-2ol (5.1%), and trans-p-mentha-1-(7)-8-dien-2ol (4.8%) [41]. Conversely, a research in Malaysia [42] showed that the EO of this species is rich in γ-terpinene (57.4%), terpinen-4-ol (16.2%), and o-cymene (15.7%). The highest levels of 1,8-cineole (76.93%) were reported in Brazil, followed by β-pinene (11.49%) and α-pinene (7.15%) [18].
The variability in the chemical composition of the EOs of T. zygis and E. camaldulensis can be ascribed to various factors such as climate, soil type, harvest season, humidity levels, temperature, exposure duration, water stress, solar radiation, as well as preservation and extraction methods, which play a significant role. Additionally, the plant’s genetic characteristics and growth cycle also contribute to this chemical diversity [18,43,44].
4.3 Antioxidant activity
Antioxidants play a vital role by competing with free radicals and inhibiting the propagation of oxidation reactions [45]. In this study, we examined the EOs of T. zygis and E. camaldulensis using two antioxidant testing methods: DPPH radical scavenging and FRAP assays. DPPH is commonly utilized to assess the ability of radical scavenging property of various studied substances (Figures 1 and 2). It is a straightforward, rapid, and highly replicable technique [46]. The results presented in Table 2 indicate that the antioxidant efficacy of T. zygis EO surpasses that of E. camaldulensis, with respective values of 57.292 ± 0.001 µg/mL and 238.851 ± 0.001 µg/mL, while quercetin (reference antioxidant) exhibited higher activity with IC50 = 5.499 ± 0.019 µg/mL. Our results demonstrate higher antioxidant activity than those obtained in previous studies, such as in Spain, where the antioxidant activity of T. zygis on DPPH yielded an IC50 value of 0.90 ± 0.03 mg/mL [47]. Conversely, in another study conducted in Spain, different tests were employed to evaluate the antioxidant capacity of T. zygis EOs containing a high proportion of thymol, showing strong antioxidant activity with an IC50 value of 27.7 ± 0.3 µmol/g [48]. For E. camaldulensis, our findings are consistent with previous studies investigating the antioxidant potential of E. camaldulensis growing in Tunisia, where a reported IC50 value was 342 µg/mL [49]. In Pakistan, EOs obtained from the leaves of this tree, extracted by two different methods, showed IC50 values of 16.21 ± 0.97 µg/mL by hydrodistillation and 19.89 ± 0.79 µg/mL by supercritical fluid extraction [50]. Similarly, the EO extracted from E. camaldulensis in Turkey revealed stronger activity, exhibiting an IC50 value of 4.096 ± 0.724 µg/mL [51].

Inhibition percentage of the DPPH radical as a function of different concentrations of T. zygis L. EO and the positive control quercetin.
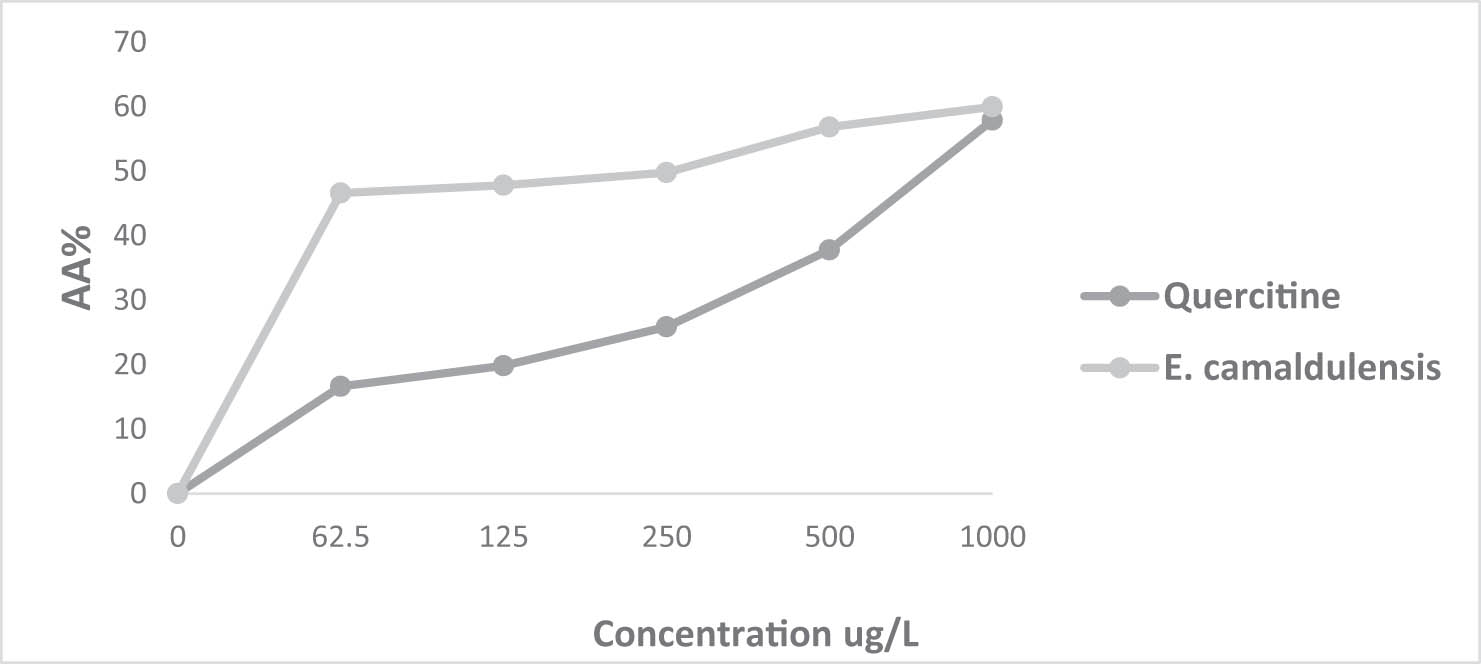
Percentage inhibition of the DPPH radical in relation to different concentrations of E. camaldulensis Dehnh. EO and the positive control quercetin.
Evaluation of the antioxidant activity of essential oils, as assessed by DPPH radical scavenging and FRAP tests
| DPPH (IC50 = µg/mL) | FRAP (EC50 = µg/mL) | |
|---|---|---|
| Thymus zygis L. ssp. Gracilis | 57.292 ± 0.001 | 7.207 ± 0.001 |
| E. camaldulensis Dehnh. | 238.851 ± 0.001 | 11.834 ± 0.001 |
| Quercitin | 5.499 ± 0.019 | |
| Catechin | 13.904 ± 0.023 |
The AA% of T. zygis remains significantly higher than the AA% of quercetin even at high concentrations, while that of E. camaldulensis at a concentration of 1,000 µg/mL approaches that of quercetin.
The FRAP test was utilized to evaluate the reducing capacity of EOs. This method was developed to quantify the EOs’ capability to reduce the ferric iron (F3+) in the K3Fe(CN)6 complex into ferrous iron (Fe2+) (Figures 3 and 4). The iron reduction capacity is directly correlated with the increase in the sample concentration [52]. The reducing power of T. zygis and E. camaldulensis through the FRAP test yielded EC50 values of 7.207 ± 0.001 µg/mL and 11.834 ± 0.001 µg/mL, respectively (Table 2). These values differ from those of the natural antioxidant (catechin), which exhibits an EC50 value of 13.904 ± 0.023 µg/mL (Table 2). Some previous studies have also reported a strong reducing power of T. zygis EOs from Spain against the K3Fe(CN)6 complex with an EC50 value of 49.56 ± 0.09 mg/mL [47]. Studies conducted in Burkina Faso on E. camaldulensis EO showed significant reducing power with an EC50 value of 6.47 ± 1.34 mg/mL [53].
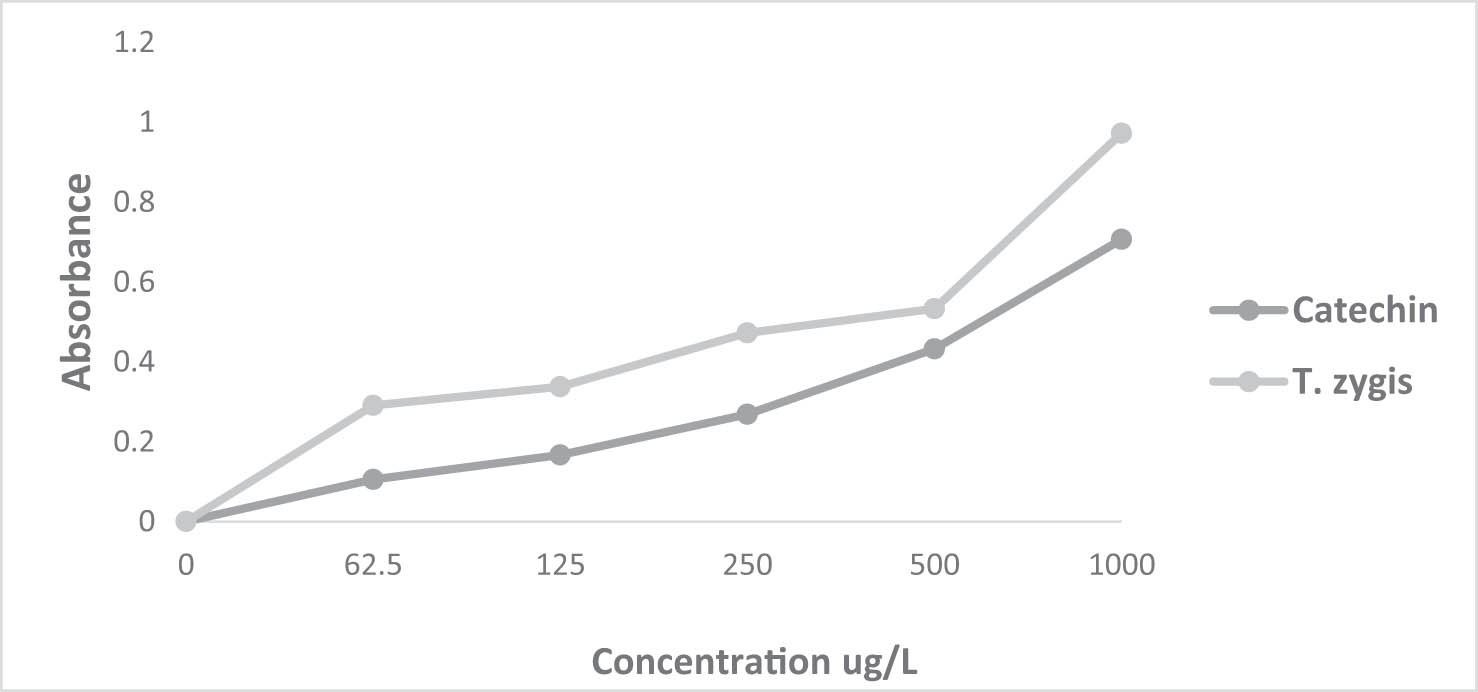
Reducing ability of T. zygis L. EO and the positive control catechin.
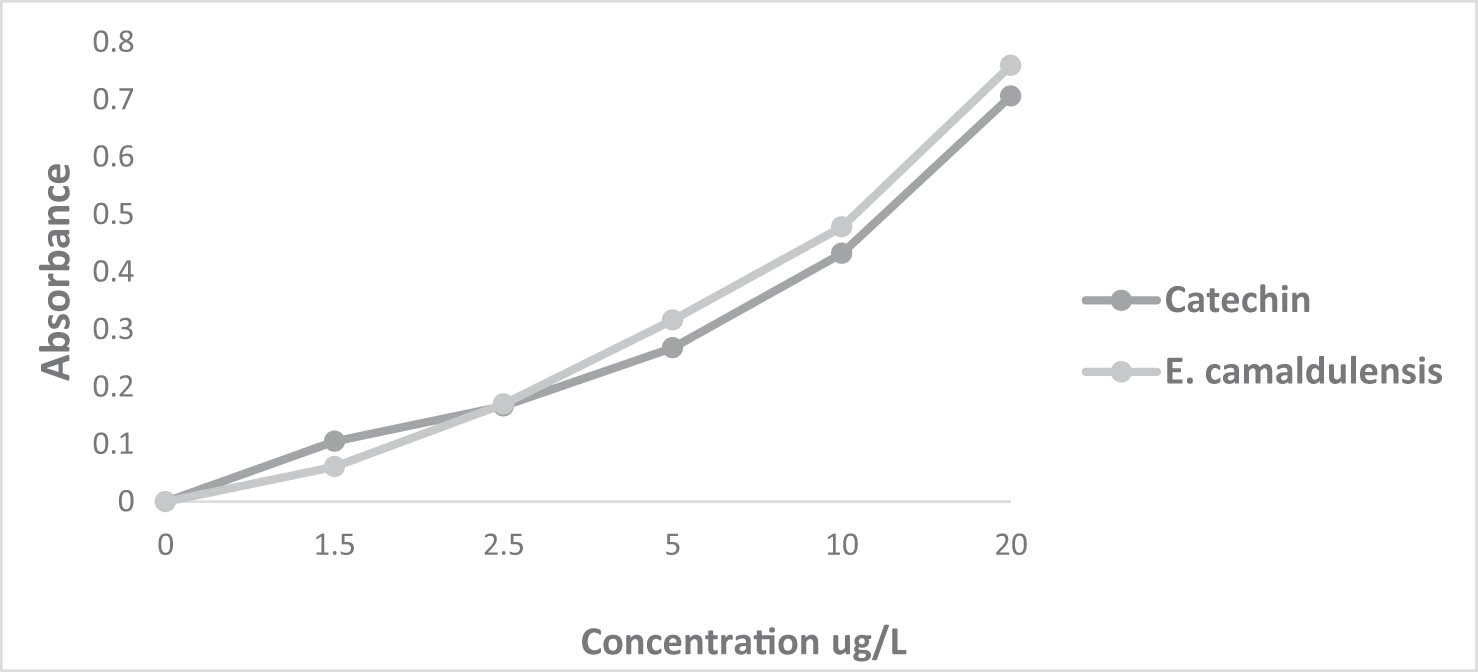
Reducing power of E. camaldulensis Dehnh. EO and the positive control catechin.
The antioxidant activity of EOs predominantly depends on their chemical composition. The reported antioxidant activity for T. zygis and E. camaldulensis EOs may be linked to their elevated percentage of monoterpenes. The difference in the antioxidant capacity observed between T. zygis and E. camaldulensis EOs stems from their different chemical profiles, as they possess complex chemical behavior and different functional groups and polarities [54]. Certainly, the antioxidant activity of T. zygis EO is primarily attributed to its elevated levels of phenols and alcohols, such as δ-terpineol and thymol, which are recognized for their robust antioxidant properties [48].
4.4 Antibacterial activity of EOs
The objective of this study is to determine whether the EOs extracted from T. zygis and E. camaldulensis possess antibacterial activity against four pathogenic bacteria (E. coli, M. luteus, B. subtilis, and S. aureus) and subsequently compare it to that of antibiotics. The diameters of the inhibition zones against the tested bacteria were measured and are presented in Table 3. The EOs of T. zygis and E. camaldulensis exhibited potent antibacterial activity, as the inhibition zones exceeded 15 mm for all studied bacteria. Additionally, Gram-positive bacteria (S. aureus and B. subtilis) showed stronger sensitivity in contrast to Gram-negative bacteria (E. coli and M. luteus). However, the tested bacteria showed no sensitivity to antibiotics such as ampicillin and penicillin, as no inhibition zones were detected. On the other hand, all tested strains exhibited very strong sensitivity to the antibiotic tetracycline. These results align with those reported by other researchers, notably Smahane et al. [55], who showed that the EO of T. zygis effectively inhibits the growth of bacteria like S. aureus (37.33–46.33 mm), E. coli (28.67–29.33 mm), and Pseudomonas aeruginosa (10 mm). Furthermore, Ballester-Costa et al. [47] demonstrated that the EO of T. zygis exhibits stronger antibacterial effects against strains of Listeria innocua (45.37 mm), Serratia marcescens (16.96 mm), Pseudomonas fragi (26.61 mm), Pseudomonas fluorescens (27.72 mm), Aeromonas hydrophila (19.35 mm), Shewanella putrefaciens (20.85 mm), Achromobacter denitrificans (23.92 mm), Enterobacter amnigenus (17.98 mm), Enterobacter gergoviae (13.92 mm), and Alcaligenes faecalis (33.85 mm).
Antimicrobial activities of EOs against various bacterial strains assessed using the disk diffusion method
| ID (mm) | ||||||
|---|---|---|---|---|---|---|
| Microorganisms | T. zygis L. ssp. gracilis (5 µl/disc) | E. camaldulensis Dehnh. (5 µl/disc) | Tetracycline | Ampicillin | Penicillin | |
| Gram-negative bacteria | E. coli (ATCC 8739) | 16.11 ± 0.96 | 19.11 ± 0.30 | 24 ± 0.33 | NZ | NZ |
| M. luteus (ATCC 9341) | 16,22 ± 0.30 | 21.78 ± 0.30 | 20.67 ± 0.44 | NZ | NZ | |
| Gram-positive bacteria | B. subtilis (ATCC 6633) | 17,11 ± 0.30 | 22.56 ± 0.59 | 21.67 ± 0.44 | NZ | NZ |
| S. aureus (ATCC 6538) | 18.33 ± 0.89 | 25.00 ± 0.22 | 25.5 ± 0.33 | NZ | NZ | |
NZ: no measurable zone of inhibition; ID: inhibition diameter; antibiotics: tetracycline, ampicillin, and penicillin.
Moreover, Diriye et al. [56] reported that the EO extracted from E. camaldulensis showed notable efficacy against E. coli (21 mm), S. aureus (24 mm), Salmonella typhi (23 mm), and Klebsiella pneumoniae (18 mm). Additionally, the EO derived from this tree exhibited antibacterial activity against various pathogenic strains associated with dental caries, including Streptococcus mutans (18.8 mm), Lactobacillus rhamnosus (19.7 mm), and Actinomyces viscosus (21.3 mm) [57].
4.5 MICs and MBCs
The results derived from the disc diffusion method were corroborated by those obtained using the MIC method (Table 4). The EOs of T. zygis and E. camaldulensis exhibited a potent inhibitory effect against the tested strains, particularly B. subtilis and S. aureus; these strains showed high sensitivity to T. zygis EO with MIC values of 0.062 and 0.125, respectively. On the other hand, strains of M. luteus and E. coli demonstrated higher resistance, with corresponding MIC values of 0.25 and 0.5. Furthermore, the MIC of E. camaldulensis EO against the growth of the tested bacteria (S. aureus and B. subtilis) falls within the range of 0.25 (Table 4); these bacteria proved to be highly sensitive. In contrast, strains of M. luteus and E. coli exhibited resistance, with respective MIC values of 0.5 and 1.
MIC and MBC of T. zygis and E. camaldulensis EOs against S. aureus, B. subtilis, M. luteus, and E. coli
| CMI/CMB (µL/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| M. luteus (ATCC 9341) | B. subtilis (ATCC 6633) | E. coli (ATCC 8739) | S. aureus (ATCC 6538) | |||||
| CMI | CMB | CMI | CMB | CMI | CMB | CMI | CMB | |
| T. zygis L. ssp. gracilis | 0.25 | 4 | 0.062 | 2 | 0.5 | 8 | 0.125 | 2 |
| E. camaldulensis Dehnh. | 0.5 | — | 0.25 | 4 | 1 | — | 0.25 | 8 |
Indeed, the inhibitory activity of T. zygis EO has been previously studied. Its inhibitory effect has been demonstrated by Afonso et al. [58] against P. aeruginosa, S. aureus, E. coli, Salmonella typhimurium, and Staphylococcus epidermidis. Later, this activity was confirmed by Radi et al. [59], who tested this EO against Acinetobacter baumannii, S. aureus, Enterobacter cloacae, Shigella dysenteriae, E. coli, and S. typhi. Previous studies have documented the inhibitory activity of E. camaldulensis EO against S. aureus and E. coli [60,16]. Research on the antimicrobial activity of E. camaldulensis EO containing a high proportion of 1,8-cineole has shown strong antibacterial activity. Additional studies have determined the MIC of this compound against the bacteria under investigation, with values of 3.125 µL/mL for E. coli and 6.25 µL/mL against S. aureus, as well as 6.25 µL/mL against Salmonella enteritidis [61]. Furthermore, the MIC of the identical compound against the bacterium S. aureus was assessed to be 23.43 µg/mL [62].
The EO of T. zygis exhibits a significant bactericidal effect against both Gram-positive bacteria S. aureus and B. subtilis, with an MBC value of 2 µL/mL. Regarding the Gram-negative bacteria, M. luteus is sensitive to the bactericidal effect of T. zygis EO, with an MBC value of 4 µL/mL, but E. coli shows resistance at these concentrations. On the contrary, the EO of E. camaldulensis demonstrates moderate bactericidal activity against both Gram-positive bacteria B. subtilis and S. aureus, with an MBC value of 8 µL/mL. Additionally, it exhibits a bacteriostatic effect on the Gram-negative bacteria M. luteus and E. coli.
The extensive and noteworthy antimicrobial efficacy observed in the tested T. zygis EO could be ascribed to its elevated concentrations of δ-terpineol (27.64%) and thymol (14.17%), which are the principal constituents of T. zygis EO. Indeed, thymol functions by disrupting the bacterial cell wall and plasma membrane, as well as by interacting with membrane proteins. Specifically, it disrupts ATP production within the cell by inhibiting the Krebs cycle, thereby impeding its ability to return to normal function following exposure to thymol [10].
On the other hand, the results of the study conducted by Li et al. [61] revealed that 1,8-cineole, the primary component of E. camaldulensis EO, induces significant degradation of the outer membrane, decrease in the cytoplasm, and can alter the physical characteristics of both Gram-negative bacteria and Gram-positive. Furthermore, research conducted by Jaradat et al. [63] demonstrated that p-cymene causes changes in intracellular pH and ATP levels, suppressing the microbial growth. δ-Terpineol, terpinol-4-ol, and α-terpineol result in the disruption of membrane and cell wall integrity, which changes the permeability and leads to the leakage of intracellular substances [12]. Therefore, the antibacterial effects observed in the EOs of T. zygis and E. camaldulensis could be elucidated by the molecular interaction between the functional groups of their constituents and bacterial cell walls. Moreover, the considerable antibacterial efficacy could be attributed to the potential synergistic interaction among these constituents.
Indeed, Gram-negative bacteria possess a rigid outer membrane characterized by a high content of lipopolysaccharides and a complex structure, which restricts the diffusion of hydrophobic compounds through this membrane. Conversely, Gram-positive bacteria lack this membrane, which accounts for the resistance of Gram-negative bacteria and the sensitivity of Gram-positive bacteria [40].
Phenols and alcohols are recognized as highly potent chemical compounds with broad-spectrum antibacterial properties. Indeed, phenols and terpene alcohols constitute roughly 50% of the overall composition of T. zygis EO. These molecules are widely recognized for their high effectiveness as antimicrobial agents [33,58,59]. Even though the EO of E. camaldulensis leaves lacks phenolic compounds and contains a low concentration of alcoholic compounds, it may still exhibit significant antibacterial activity. The active principle, 1,8-cineole, belonging to the ether group, is known for its interesting antibacterial properties [16,50].
5 Conclusions
This study aimed to characterize the chemical composition of EOs from two Moroccan plants, T. zygis and E. camaldulensis, and assess their antioxidant and antibacterial properties. The results indicate a more significant antioxidant efficacy for T. zygis compared to E. camaldulensis. Additionally, both EOs exhibited substantial antibacterial properties. Among the four tested strains, S. aureus and B. subtilis exhibited the highest sensitivity. The antibacterial activity of T. zygis and E. camaldulensis EOs surpassed that of antibiotics such as ampicillin and penicillin, which showed no inhibition zones. However, all tested strains displayed strong sensitivity to tetracycline antibiotics. The antibacterial activity of T. zygis and E. camaldulensis EOs is contingent upon their concentration, chemical composition, and specific strains under investigation. The extensive utilization of antibiotics has prompted substantial adaptability among bacterial strains and the emergence of multi-resistant strains, explaining the resistance of bacteria to ampicillin and penicillin.
Nevertheless, natural antioxidants like T. zygis and E. camaldulensis EOs could provide a beneficial value to prevent undesirable health issues compared to potentially hazardous synthetic antioxidants. The effectiveness of T. zygis and E. camaldulensis EOs makes them a potential solution for preserving food products against oxidation and microbial contamination. They might find applications in pharmaceutical, cosmetic, and food industries to extend the shelf life of various products.
Acknowledgement
The authors wish to thank the Researchers Supporting Project Number (RSP2024R346) at King Saud University Riyadh Saudi Arabia for financial support.
-
Funding information: This research was financially supported by the Researchers Supporting Project Number (RSP2024R346) at King Saud University Riyadh Saudi Arabia.
-
Author contributions: F.A., B.S., S.A., M.A., I.E., M.G., A.F., and M.O.: methodology and formal analysis. F.A., A.A., and M.K: writing – review and editing. F.A., B.S., A.A., and M.K.: software, project administration, and resources. F.A., R.U., and A.B.: writing – original. J.D.: supervision, review, and editing. F.A., S.A., J.D., R.U., and A.B.: conceptualization. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: All related data are given in the manuscript.
-
Ethical approval: The conducted research is not related to either human or animal use.
References
[1] Bag A, Chattopadhyay RR. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS One. 2015;10(7):e0131321. 10.1371/journal.pone.0131321.Search in Google Scholar PubMed PubMed Central
[2] Sharmaa K, Guleria S, Razdan KV, Babu V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind Crop Prod. 2020;154:112569. 10.1016/j.indcrop.2020.112569.Search in Google Scholar
[3] Chraibi M, Fadil M, Fikri-Benbrahim K, Farah A, Benkhaira N, Lebrazi S. Simplex-centroid design as innovative approach in the optimization of antimicrobial effect of Thymus satureioides, Myrtus communis and Artemisia herba alba essential oils against Escherichia coli, Staphylococcus aureus and Candida tropicalis. experimental. Parasitology. 2023;247:108472.10.1016/j.exppara.2023.108472Search in Google Scholar PubMed
[4] Harfouch RM, Darwish M, Al-Asadi W, Mohammad AF, Gharib NM, Haroun M. Antibacterial activity of essential oils of Rosmarinus officinalis, Salvia officinalis and Anthemis nobilis widespread in the Syrian coast. Res J Pharm Technol. 2019;12(7):3410–2.10.5958/0974-360X.2019.00576.6Search in Google Scholar
[5] Qaralleh H, Khleifat MK, Khlaifat MA, Al-limoun M. chemical composition, antioxidant and inhibitory effect of Cupressus sempervirens essential oils and methanolic extract on beta-lactamase producing isolates. Res J Pharm Tech. 2021;14(9):4673–9. 10.52711/0974-360X.2021.00812.Search in Google Scholar
[6] Mohammed K, Saghrouchni H, Abdali YE, Amine A, Haoudi N, Fadili ME, et al. Phytochemical and physicochemical studies of different apple varieties grown in Morocco. Open Chem. 2024 Jan;22(1):20230205. [cited 2024 Apr 25] https://www.degruyter.com/document/doi/10.1515/chem-2023-0205/html.10.1515/chem-2023-0205Search in Google Scholar
[7] Ouedrhiri W, Balouiri M, Harki El-H, Moja S, Greche H. Synergistic antimicrobial activity of two binary combinations of marjoram, lavender and wild thyme essential oils. Int J Food Prop. 2017;20(12):3149–58. 10.1080/10942912.2017.1280504.Search in Google Scholar
[8] Fennane M, Ibn Tattou M, Ouyahya A, El Oualidi J. Flore Pratique du Maroc, vol. 2. Trav Inst Sci sér Bot. 2007;38:636.Search in Google Scholar
[9] Li X, He T, Wang X, Shen M, Yan X, Fan S, et al. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem Biodivers. 2019;16(9):e1900254.10.1002/cbdv.201900254Search in Google Scholar PubMed
[10] Gourich AA, Bencheikh N, Bouhrim M, Regragui M, Rhafouri R, Drioiche A, et al. Comparative analysis of the chemical composition and antimicrobial activity of four Moroccan North Middle Atlas medicinal plants’ essential oils: Rosmarinus officinalis L., Mentha pulegium L., Salvia officinalis L., and Thymus zygis subsp. gracilis (Boiss.) R. Morales. Chemistry. 2022;4:1775–88. 10.3390/chemistry4040115.Search in Google Scholar
[11] Tagnaout I, Zerkani H, Hadi N, El Moumen B, El Makhoukhi F, Bouhrim M, et al. Chemical composition, antioxidant and antibacterial activities of Thymus broussonetii Boiss and Thymus capitatus (L.) Hoffmann and link essential oils. Plants. 2022;11:954.10.3390/plants11070954Search in Google Scholar PubMed PubMed Central
[12] Huang J, Yang L, Zou Y, Luo S, Wang X, Liang Y, et al. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021;66:59–67.10.1007/s12223-020-00818-0Search in Google Scholar PubMed
[13] El-Baha AM, El-Sherbiny AA, Salem MZM, Sharrawy NMM, Mohamed NH. Toxicity of essential oils extracted from Corymbia citriodora and Eucalyptus camaldulensis leaves against Meloidogyne inc. Pak J Nematol. 2017;35(1):93–104. 10.18681/pjn.v35.i01.p93-104.Search in Google Scholar
[14] Ben Akka F, Benkhnigue O, Salhi S, El Hilah F, Dahmani J, Douira A, et al. Ethnobotany study of medicinal plants used in the treatment of respiratory diseases in the middle region of Oum Rbai. Int J Environ Agric Biotechnol. 2017;2(4):238815. 10.22161/ijeab/2.4.3.Search in Google Scholar
[15] Chaachouay N, Benkhnigue O, Douira A, Zidane L. Poisonous medicinal plants used in the popular pharmacopoeia of the Rif, Northern Morocco. Toxicon. 2021;189:24–32. 10.1016/j.toxicon.2020.10.028.Search in Google Scholar PubMed
[16] Ez-Zriouli R, ElYacoubi H, Imtara H, Mesfioui A, ElHessni A, Al Kamaly O, et al. Chemical composition, antioxidant and antibacterial activities and acute toxicity of Cedrus atlantica, Chenopodium ambrosioides and Eucalyptus camaldulensis essential oils. Molecules. 2023;28(7):2974.10.3390/molecules28072974Search in Google Scholar PubMed PubMed Central
[17] Beyaoui A, Jlizi S, Ascrizzi R, Flamini G, Harrath, A H, et al. Chemical profiling and biological assessment of trunk bark essential oil from Eucalyptus camaldulensis: In vitro study coupled with chemoinformatics calculations. J Mol Struct. 2024;1300:137120.10.1016/j.molstruc.2023.137120Search in Google Scholar
[18] Chaves TP, Pinheiro REE, Melo ES, Soares MJDS, Souza JSN, de Andrade TB, et al. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind Crop Prod. 2018;112:70–4.10.1016/j.indcrop.2017.10.048Search in Google Scholar
[19] Sabo VA, Knezevic P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind Crop Prod. 2019;132:413–29. 10.1016/j.indcrop.2019.02.051.Search in Google Scholar PubMed PubMed Central
[20] Sobhy M, Ali SS, Cui H, Lin L, El-Sapagh S. Exploring the potential of 1, 8-cineole from cardamom oil against food-borne pathogens: antibacterial mechanisms and its application in meat preservation. Microb Pathog. 2023;184:106375. 10.1016/j.micpath.2023.106375.Search in Google Scholar PubMed
[21] Clevenger JF. Apparatus for the determination of volatile oil. J Am Pharm Assoc. 1928;17:346–51.10.1002/jps.3080170407Search in Google Scholar
[22] Marion C, Pelissier Y, Sabatier R, Andary C, Bessiere JM. Calculation of essential oil yield without prior extraction application to the Genus Forsythia Vahl. (Oleaceae). J Essent Oil Res. 1994;6:379–87.10.1080/10412905.1994.9698403Search in Google Scholar
[23] Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of artemisia essential oils. Phytochemistry. 2008;69(8):1732–8.10.1016/j.phytochem.2008.02.014Search in Google Scholar PubMed
[24] Oyaizu M. Studies on products of browning reaction. antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44(6):307–15.10.5264/eiyogakuzashi.44.307Search in Google Scholar
[25] Jaber H, Oubihi A, Ouryemchi I, Boulamtat R, Oubayoucef A, Bourkhiss B, et al. Chemical composition and antibacterial activities of eight plant essential oils from Morocco against Escherichia coli strains isolated from different Turkey organs. Biochem Res Int. 2021;1–9.10.1155/2021/6685800Search in Google Scholar PubMed PubMed Central
[26] Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharma Anal. 2016;6:71–9.10.1016/j.jpha.2015.11.005Search in Google Scholar PubMed PubMed Central
[27] Fadil M, Fikri-Benbrahim K, Rachiq S, Ihssane B, Lebrazi S, Chraibi M, et al. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur J Pharm Biopharm. 2017;126:211–20. 10.1016/j.ejpb.2017.06.002.Search in Google Scholar PubMed
[28] Lemrhari A, Zouhair R, El Kahkahi R, Elidrissi M, Amchrouk A, Elhourri M. Chemical composition and differentiation of essential oils of Morocco’s different varieties of thyme. Glob J Pure Appl Chem Res. 2016;4(1):30–5.Search in Google Scholar
[29] Cutillas AB, Carrasco A, Martinez-Gutierrez R, Tomas V, Tudela J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J Food Drug Anal. 2018;26(2):529–44. 10.1016/j.jfda.2017.05.004.Search in Google Scholar PubMed PubMed Central
[30] Zerkani H, Kharchoufa L, Tagnaout I, Fakchich J, Bouhrim M, Amalich S, et al. Chemical composition and bioinsecticidal effects of Thymus zygis L., Salvia officinalis L. and Mentha suaveolens Ehrh. essential oils on medfly Ceratitis capitata and tomato leaf miner Tuta absoluta. Plants. 2022;11(22):3084.10.3390/plants11223084Search in Google Scholar PubMed PubMed Central
[31] Ebadollahi A, Setzer NW. Analysis of the essential oils of Eucalyptus camaldulensis Dehnh. and E. viminalis Labill. as a contribution to fortify their insecticidal application. Nat Product Commun. 2020;15(9):1–10. 10.1177/1934578X20946248.Search in Google Scholar
[32] Barra A, Coroneo V, Dessi S, Cabrasa P, Angionia A. Chemical variability, antifungal and antioxidant activity of Eucalyptus camaldulensis essential oil from Sardinia. Nat Product Commun. 2010;5(2):329–35.10.1177/1934578X1000500232Search in Google Scholar
[33] Drioiche A, Radi F, Zair T. Correlation between the chemical composition and the antimicrobial properties of seven samples of essential oils of endemic Thymes in Morocco against multi-resistant bacteria and pathogenic fungi. Saudi Pharm J. 2022;30:1200–14. 10.1016/j.jsps.2022.06.022.Search in Google Scholar PubMed PubMed Central
[34] Marinkovića J, Mitić Ćulafićb D, Nikolićb B, Đukanovićb S, Markovićc T, Tasića G, et al. Antimicrobial potential of irrigants based on essential oils of Cymbopogon martinii and Thymus zygis towards in vitro multispecies biofilm cultured in ex vivo root canals. Arch Oral Biol. 2020;117:104842. 10.1016/j.archoralbio.2020.104842.Search in Google Scholar PubMed
[35] Park SY, Raka RN, Hui XL, Song Y, Sun JL, Xiang J, et al. Six spain thymus essential oils composition analysis and their in vitro and in silico study against Streptococcus mutans. BMC Complement Med Ther. 2023;23(1):106. 10.1186/s12906-023-03928-7.Search in Google Scholar PubMed PubMed Central
[36] Ballester-Costa C, Sendra E, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind Crop Prod. 2013;50:304–11. 10.1016/j.indcrop.2013.07.052.Search in Google Scholar
[37] Vuki´c MD, Cmiková N, Hsouna AB, Saad RB, Garzoli S, Schwarzová M, et al. Thymus zygis, Valuable Antimicrobial (in vitro and in situ) and antibiofilm agent with potential antiproliferative effects. Plants. 2023;12:3920. 10.3390/plants12233920.Search in Google Scholar PubMed PubMed Central
[38] Belhachemi A, Maatoug MH, Canela-Garayoa R. GC-MS and GC-FID analyses of the essential oil of Eucalyptus camaldulensis grown under greenhouses differentiated by the LDPE cover-films. Ind Crop Prod. 2022 Apr 1;178:114606.10.1016/j.indcrop.2022.114606Search in Google Scholar
[39] Hanoğlu DY, Hanoğlu A, Adediran BS, Can Baser KH, ÖzkumYavuz Ö. The essential oil compositions of two Eucalyptus sp. (E. camaldulensis Dehnh. and E. torquata Luehm.) naturalized to Cyprus. J Essent Oil Res. 2022;35(2):136–42. 10.1080/10412905.2022.2147592.Search in Google Scholar
[40] Ouaritini ZB, EL Hajjaji MA, Braoul S, Benkhaira N, Fikri-Benbrahim K. Ethnopharmacological study, phytochemical quality and antibacterial activity of the essential oils of Chenopodium ambrosioides, Eucalyptus camaldulensis and Origanum majorana from Taounate region (Morocco). Asian J Chem. 2023;35(9):2048–54. 10.14233/ajchem.2023.26688.Search in Google Scholar
[41] NdiayeEl., Gueye MT, Ndiaye I, Mbacké Diop S, Bakar Diop M, Fauconnier M-L, et al. Chemical composition of essential oils and hydrosols of three Eucalyptus species from Senegal: Eucalyptus alba Renv, Eucalyptus camaldulensis Dehnh and Eucalyptus tereticornis Hook. Am J Essent Oils Nat Products. 2017;5(1):1–7.Search in Google Scholar
[42] Mubarak EE, Ali LZ, Ahmed IFA, Ahmed ABA, Taha RM. Essential oil compositions and cytotoxicity from various organs of Eucalyptus camaldulensis. Int J Agric Biol. 2015;17(2):320–6.Search in Google Scholar
[43] Amarti F, El Ajjouri M, Ghanmi M, Satrani B, Aafi A, Farah A, et al. Composition Chimique, Activité Antimicrobiennne et Antioxydante de l’huile Essentielle de Thymus zygis Du Maroc. Phytothérapie. 2011;9:149. 10.1007/s10298-011-0625-6.Search in Google Scholar
[44] El Ajjouri M, Satrani B, Ghanmi M. Activité antifongique des huiles essentielles de Thymus bleicherianus Pomel et Thymus capitatus (L.) Hoffm. & Link contre les champignons de pourriture du bois d’œuvre. Biotechnol Agron Soc Environ. 2008;12:345–51.Search in Google Scholar
[45] Coimbra A, Miguel S. Thymus zygis essential oil: Phytochemical characterization, bioactivity evaluation and synergistic effect with antibiotics against Staphylococcus aureus. Antibiotics. 2022;11:146. 10.3390/antibiotics11020146.Search in Google Scholar PubMed PubMed Central
[46] Ez Zoubi Y, El Ouali Lalami A. Chemical composition, antioxidant and antimicrobial activities of the essential oil and its fractions of Lavandula stoechas L. from Morocco. Int J Curr Pharm Rev Res. 2017;8(1):60–7.Search in Google Scholar
[47] Ballester-Costa C, Sendra E, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Assessment of antioxidant and antibacterial properties on meat homogenates of essential oils obtained from four Thymus species achieved from organic growth. Foods. 2017;6(8):59. 10.3390/foods6080059.Search in Google Scholar PubMed PubMed Central
[48] Carrasco A, Ortiz-Ruiz V, Martinez-Gutierrez R, Tomas V, Tudela J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind Crop Prod. 2015;73:16–27. 10.1002/ffj.3283.Search in Google Scholar
[49] Sliti S, Ayadi S, Kachouri F, Khouja MA, Abderrabba M, Bouzouita N. Leaf essential oils chemical composition, antibacterial and antioxidant activities of Eucalyptus camaldulensis and E. rudis from korbous (Tunisia). J Mater Environ Sci. 2015;6:743–8.Search in Google Scholar
[50] Abbas A, Anwar F, Alqahtani SM, Ahmad N, Al-Mijalli SH, Shahid M, et al. Hydro-distilled and supercritical fluid extraction of Eucalyptus camaldulensis essential oil: Characterization of bioactives along with antioxidant, antimicrobial and antibiofilm activities. Dose-Response. 2022;20(3):15593258221125477.10.1177/15593258221125477Search in Google Scholar PubMed PubMed Central
[51] Sahin Basak S, Candan FERDA. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh. Essential oil. J Iran Chem Soc. 2010;7:216–26.10.1007/BF03245882Search in Google Scholar
[52] Bentabet N, Boucherit-Otmani Z, Boucherit K. Composition chimique et activité antioxydante d’extraits organiques des racines de Fredolia aretioides de la région de Béchar en Algérie. Phytothérapie. 2014;364–71. 10.1007/s10298-014-0834-x.Search in Google Scholar
[53] Kiendrebeogo M, Coulibaly AY, Nebie RC, Zeba B, Lamien CE, Lamien-Meda A, et al. Antiacetylcholinesterase and antioxidant activity of essential oils from six medicinal plants from Burkina Faso. Rev Brasileira de Farmacogn. 2011;21:63–9. 10.1590/S0102-695X2011005000008.Search in Google Scholar
[54] Baali F, Boumerfeg S, Napoli E, Boudjelal A, Righi N, Deghima A, et al. Chemical composition and biological activities of essential oils from two wild Algerian medicinal plants: Mentha pulegium L. and Lavandula stoechas L. J Essent Oil Bear Plants. 2019;22(3):821–37.10.1080/0972060X.2019.1642800Search in Google Scholar
[55] Smahane B, Mounyr B, Faisl B, Stephane M, Sghir TM, Dalila B. Antimicrobial activities of essential oil of five plant species from Morocco against some microbial strains. Int J Pharmacogn Phytochem Res. 2016;8(11):1901–6.Search in Google Scholar
[56] Diriye AM, Ali MM, Ishag OA, Abdi Mohamed M. Chemical composition and antimicrobial activity of essential oils extracted from Eucalyptus Camaldulensis leaves grown in Sudan. Red Sea Univ J Basic Appl Sci. 2017;2:244–53.Search in Google Scholar
[57] Etemadi R, Moghadam P, Yousefi F. Evaluation of chemical composition and antimicrobial activities of Eucalyptus camaldulensis essential oil on dental caries pathogens. J Basic Res Med Sci. 2020;7(1):43–9.10.2174/1874285802014010142Search in Google Scholar
[58] Afonso AF, Pereira OR, Válega M, Silva AM, Cardoso SM. Metabolites and biological activities of Thymus zygis, Thymus pulegioides, and Thymus fragrantissimus grown under organic cultivation. Molecules. 2018;23(7):1514. 10.3390/molecules23071514.Search in Google Scholar PubMed PubMed Central
[59] Radi FZ, Bouhrim M, Mechchate H, Al-Zahrani M, Qurtam AA, Aleissa AM, et al. Phytochemical analysis, antimicrobial and antioxidant properties of Thymus zygis L. and Thymus willdenowii Boiss. essential oils. Plants. 2021;11(1):15. 10.3390/plants11010015.Search in Google Scholar PubMed PubMed Central
[60] Khalaf ZZ, Zahra LA. Evaluation of the activity of essential oil and hydrosol from eucalyptus camaldulensis against some bacterial species. Iraqi J Sci. 2020;61(6):1282–8. 10.24996/ijs.2020.61.6.5.Search in Google Scholar
[61] Li L, Li ZW, Yin ZQ, Wei Q, Jia RY, Zhou LJ, et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int J Clin Exp Med. 2014;7(7):1721.Search in Google Scholar
[62] Farhanghi A, Aliakbarlu J, Tajik H, Mortazavi N, Manafi L, Jalilzadeh‐Amin G. Antibacterial interactions of pulegone and 1, 8‐cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci Nutr. 2022;10(8):2659–66. 10.1002/fsn3.2870.Search in Google Scholar PubMed PubMed Central
[63] Jaradat N, Al-Maharik N, Hawash M, Qadi M, Issa L, Anaya R, et al. Eucalyptus camaldulensis Dehnh leaf essential oil from palestine exhibits antimicrobial and antioxidant activity but no effect on porcine pancreatic lipase and α-Amylase. Plants. 2023;12(22):3805. 10.3390/plants12223805.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

