Abstract
Plant-derived bioactive compounds displayed major therapeutic and chemo-preventive roles in the pathogenesis of numerous chronic malignancies such as cancer and enhanced oxidative stress and inflammation. Antioxidants found in food, such as genkwanin, may reduce oxidative stress and the release of cytokines or pathways that promote inflammation. The goal of this work is to summarize the potential for anticancer effects of genkwanin, a methoxyflavone that is present in a variety of plant species. This review examined and analyzed numerous research studies on identifying, isolating, measuring, and analyzing anticancer properties of genkwanin. The mechanisms involved cellular and molecular activities at various levels, including apoptosis induction and cancer cell growth and proliferation inhibition. Preclinical studies have demonstrated genkwanin’s effects and mechanism of action; however, further research is required to investigate its therapeutic potential thoroughly. Additional research is needed to further our understanding of the pharmacodynamic effects of genkwanin. Additional toxicological study is necessary to evaluate the clinical efficacy and safety of genkwanin, which would help scientists to elucidate a potent drug candidate for cancer management.
1 Introduction
Epidemiological studies have associated flavonoids with several health benefits, including a decreased chance of acquiring many cancers. Even though a large variety of flavonoids have been established to possess notable anticancer effects, only a few of these substances have proven effective enough to be tested as therapies in clinical trials [1]. The flavonoid family consists of 3-phenylchromen-4-one-based secondary metabolites of polyphenols. Numerous scientific studies have suggested that natural antioxidants may have beneficial biochemical effects against various diseases [2]. Many studies have focused on flavonoids among polyphenols because of their strong antioxidative, anti-inflammatory, anti-carcinogenic, or enzyme-inhibiting properties. They are subdivided into isoflavones, flavones, flavonols, anthocyanins, and flavanones [3]. A non-glycosylated flavone, genkwanin is isolated and present in various plant matrices, including Rosmarinus officinalis, Salvia officinalis, Leonurus sibiricus, and Genkwa Flos [4]. Inflammation and oxidative stress contribute to the onset and development of many chronic illnesses, such as diabetes, obesity, cancer, autoimmune diseases, cardiometabolic and neurological diseases, and critical contributors to the aging process [5,6]. Due to its potent action against pro-inflammatory mediators such as TNF-α, IL-1β, IFNγ, and IL-6 cytokines, as well as its inhibition of protein kinases and down-regulation of the p38, JNK, and microRNA-101-mediated AP-1 signaling pathway, genkwanin has been demonstrated to be a potential anticancer agent. These biomolecules have been shown to have both pro-tumor and antitumor effects in cancer via affecting cell proliferation, metastasis, and tumor microenvironment [7].
Genkwanin has been shown in this context to exert anticancer effects through the inhibition of PARP1, Bcl-2, and Bcl-xL proteins, as well as an increase in host immunity and the reduction of inflammatory factor level, all of which lead to apoptosis induction in human breast cancer cell lines MCF-7, hepatocellular carcinoma HepG-2, and colon cancer cell lines HT-29, HCT-116, and SW-480 [8]. Among the isoflavonoid group of chemicals, genistein is present in large quantities in soy. Genistein has several different anticancer characteristics that have been discovered. According to several research, genistein suppresses angiogenesis, stops the cell cycle, promotes apoptosis, and so forth. In addition, genistein has been suggested to improve glucose metabolism and alleviate menopausal symptoms. In addition, genistein has been proposed as a beneficial natural remedy for many chronic conditions, including diabetes, rheumatoid arthritis, and cardiovascular disease. In women suffering from illnesses and menopausal symptoms, genistein has been shown to regulate the action of estrogen [9].
Moreover, it can alter proteins and pathways linked to the development of tumors, including Bcl-2, Bax, NF-κB, MAPK, P13K/Akt, and KIF20A. For improved treatment, it can also make cancer cells more sensitive to chemotherapeutic medications such as adriamycin and tamoxifen [10]. By controlling the metabolism of fats and carbohydrates, genistein aids in treating nonalcoholic fatty liver disease [11]. According to reports, female adult mice treated with genistein dietary supplements for their lives exhibit methylation of the BRAC 1 cytosine-guanine dinucleotide and reduced activation of the aryl hydrocarbon receptor in their offspring’s mammary tissue. Upregulated expression of ERα was observed upon genistein administration. Antagonism of aryl hydrocarbon receptor demethylates BRCA1 upon genistein treatment. It also decreases the expression of Cyp1b1, a target for the ARH receptor [12]. Genistein also induces apoptosis significantly when its concentration is increased. It also arrests the cell cycle post-72 h in a dose-dependent manner, along with decreased levels of Notch-1, Bcl-2, Bcl-xL, and cyclin-1 expressions [13]. To our knowledge, this is the first thorough analysis outlining genkwanin’s potential to cause cancer. Consequently, this review can direct future research to create efficient techniques for locating, isolating, and evaluating this flavonoid, which may help with future pharmaceutical industry and medical application applications.
2 Structure and biosynthesis of genkwanin
The O-methylated flavone genkwanin (4′,5-dihydroxy-7-methoxyflavone) has one hydroxyl group that is methylated [14] (Figure 1a). In vitro and in vivo research studies have displayed health benefits of including flavonoid- and flavone-rich diet [15]. Genkwanin has been shown to have therapeutic potential in several conditions, including type 2 diabetes, cancer, cardiometabolic diseases, and neurodegenerative illnesses (Figure 1b). The beneficial impacts of genkwanin have been linked to its ability to regulate apoptosis, cellular cycle arrest, oxidative stress, and inflammation [16].

(a) Structure of genkwanin and its (b) medicinal potential.
Numerous plant matrices have been shown to contain genkwanin. It was the most significant compound that was separated from the Vernonia fasciculata leaf chloroform extract that was obtained from the United States, the Eremanthus elaeagnus hydromethanolic extract stem parts, and the Daphne genkwa flower that was gathered from China and Korea. In addition, the high concentration of genkwanin identified the propolis of Apis mellifera honeybees (Table 1).
Genkwanin isolated from plant species
| Plant part | Plant species | Family | Country | Reference |
|---|---|---|---|---|
| Leaf (chloroform extract) | Vernonia fasciculate | Asteraceae | United States | [17] |
| Stem (hydromethanolic extract) | Eremanthus elaeagnus | Asteraceae | China | [18,19,20,21] |
| Flower | Daphne genkwa | Thymelaeaceae | Korea | [22] |
| Leaves | Ocimum basilicum | Lamiaceae | Brazil | [23] |
| Aerial parts | Baccharis trimera | Asteraceae | Brazil | [24] |
| Stems | Daphne gnidium | Thymelaeaceae | Italy | [25,26] |
| Seeds | Salvia officinalis | Lamiaceae | Portugal and Tunisia | [27,28] |
| Flowers, leaves, roots, and stems (aqueous and methanolic extracts) | Rosmarinus officinalis | Lamiaceae | Spain | [29,30,31,32,33] |
| Leaves | Nepeta | Lamiaceae | Iranian | [34] |
| aerial parts (methanolic extract) | Artemisia iwayomogi | composite asteraceae | Korea | [35] |
| Leaves and roots (hydromethanolic extract) | Rumex induratus | Polygonaceae | Portugal | [36] |
| Seeds | Alnus glutinosa | Betulaceae | United Kingdom | [37] |
| Leaves | Combretum erythrophyllum | Combretaceae | South Africa | [38] |
| Leaves | Aquilaria crassna | Thymeleaceae | Japan | [39] |
| Leaves | Phegopteris decursive-pinnata | Thelypteridaceae | Bangladesh | [40] |
| Whole plant (methanolic extract) | Tinospora crispa | Menispermaceae | Bangladesh | [40] |
Phenylalanine is the precursor for synthesizing additional flavonoid groups in the genkwanin biosynthesis pathway (Figure 2). This synthesis requires several enzymes, including chalcone synthase, chalcone isomerase, flavone synthase, phenylalanine ammonia lyase, cinnamic acid4-hydroxylase, 4-coumarate-CoA ligase, and flavonoid-7-O-methyltransferase. While apigenin itself has several biological properties, such as anti-inflammatory, antidepressant, and anticancer properties [41–44], regioselective O-methylation of apigenin (to produce genkwanin) confers additional biological properties, such as antibacterial, antiplasmodial, radical scavenging, chemopreventive, and inhibiting 17β-hydroxysteroid dehydrogenase type 1 activities [25,45–49].
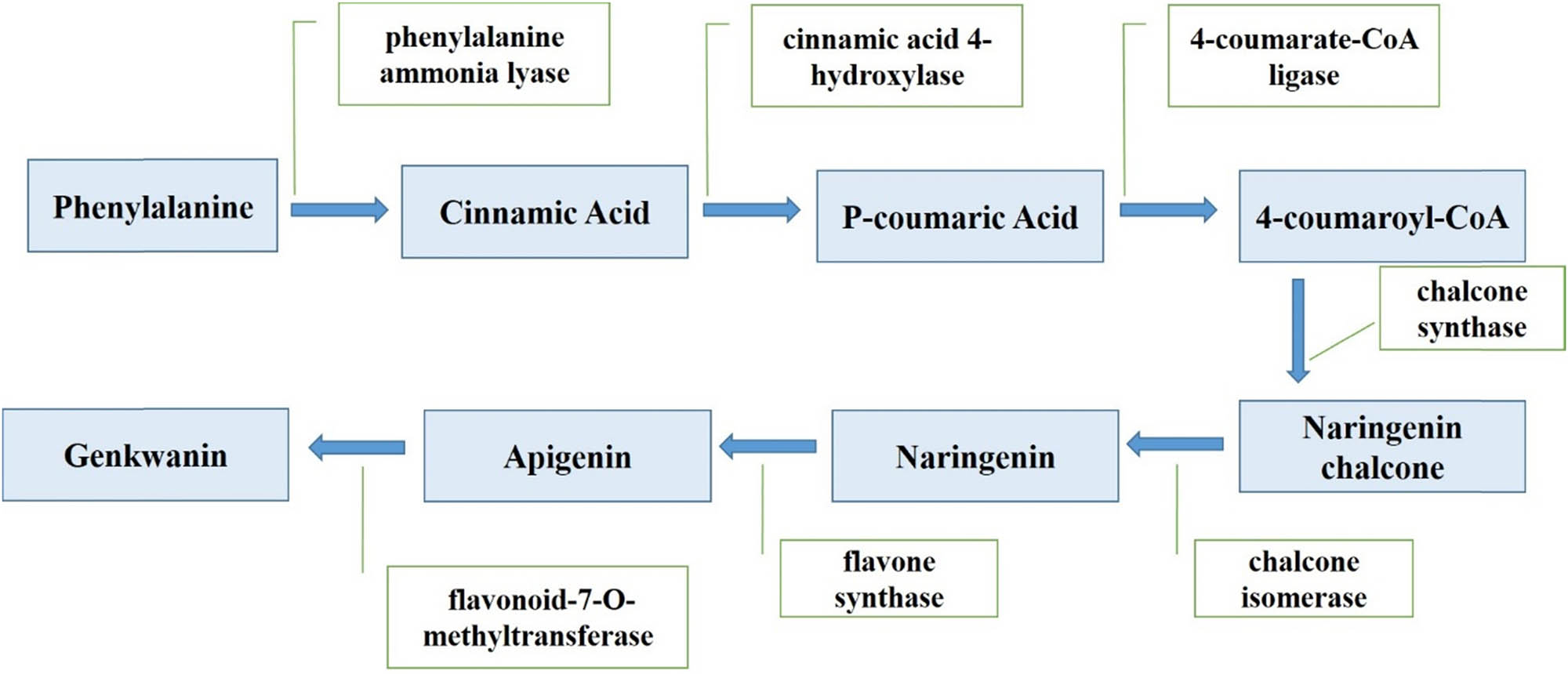
Biosynthesis pathway of genkwanin.
3 Pharmacological potential and sources of genkwanin
Flavonoids are effective antioxidants and can scavenge free radicals because of their polyphenolic structure. Table 2 enumerates the genkwanin compound’s physical and chemical characteristics that enhance its potential for therapeutic development.
Physical and chemical properties of genkwanin
| Physicochemical properties | |
| Molecular weight | 284.070 |
| Number of hydrogen bond acceptors | 5 |
| Number of hydrogen bond donors | 2 |
| Number of rotatable bonds | 2 |
| Number of rings | 3 |
| Number of heteroatoms | 5 |
| Topological polar surface area | 79.900 |
| Log of the aqueous solubility | −3.724 |
| Log of octanol/water partition coefficient | 3.670 |
| GI absorption | High |
| PPP-Permeant | No |
| P-gp (P-glycoprotein) substrate | No |
| CYP1A2 (member of cytochrome P450 superfamily of enzymes) inhibitor | Yes |
| CYP2C19 (enzyme involved in the hepatic metabolism of drug) inhibitor | No |
| CYP2C9 (enzymes that break steroids and fatty acids) inhibitor | Yes |
| CYP2D6 (enzyme expressed in liver)inhibitor | Yes |
| CYP3A4 (cytochrome P450 isoforms) inhibitor | Yes |
| Toxicity profiling | |
| hERG blockers (human ether-a-go-go related gene) | Excellent (no blockage to hERG gene associated with cardiac regulation) |
| H-HT (human hepatotoxicity) | Excellent (no toxicity to liver) |
| DILI (drug-induced liver injury) | Poor (toxic) |
| AMES toxicity | Poor (mutagenic) |
| Rat oral acute toxicity | Excellent (low toxicity) |
| FDAMDD (toxic dose threshold of chemicals in humans) | Poor (toxic) |
| Skin sensitization | Poor (sensitizer) |
| Carcinogenicity | Medium (non-carcinogen) |
| Eye corrosion and irritation | Excellent (non-corrosives/non-irritants chemicals) |
| Respiratory toxicity (a major cause of drug withdrawal) | Good (non-respiratory toxicants) |
| Nuclear receptor (NR) signaling pathway | |
| AR (androgen receptor) dependent pathway: steroid/nuclear hormone receptor | AR agonist |
| Ligand-binding domain (LBD) of androgen receptor | Significant binding efficacy with LBD |
| AhR (aryl hydrocarbon receptor) signaling pathway | Activator (mediator of cellular response to environmental pollutants) |
| Aromatase: catalyzes the conversion of androgen to estrogen (maintains the androgen and estrogen balance) | Aromatase inhibitor |
| Estrogen receptor (ER): nuclear hormone receptor | Poor binding with ER receptor |
| ER-LBD (ER ligand binding domain) | Inactive |
| Stress response pathways | |
| p53 (tumor suppressor protein) | Activates p53, leading to cancer cell death |
| Mitochondrial membrane potential (effect on mitochondrial potential) | Active |
| Heat shock factor response element (HSE) | Activation of heat shock response |
| ATPase family AAA domain-containing protein 5 | Active (more DNA Damage to cancer cells) |
4 Genkwanin: a potential antitumor bioactive compound against several human carcinomas
As a typical bioactive, non-glycosylated flavonoid, genkwanin (GKA) is used as a representative marker to ensure the quality of medicines prescribed by traditional Chinese medicine. According to earlier research, GKA possesses several pharmacological actions, such as expectorant, anti-inflammatory, antibacterial, antiplasmodial, chemopreventive, and radical scavenging properties [50]. Furthermore, reports have indicated that GKA possesses a specific anti-tumor activity. Wang et al. found that GKA reduced the levels of inflammatory cytokines and enhanced host immunity to some extent, which hindered the multiplication of tumor cells [51]. According to Androutsopoulos et al., GKA reduced the in vitro proliferation of MDA-MB-468 breast cancer cells at micromolar doses. Additionally, GKA demonstrated anti-proliferative action against granulomas generated by cotton pellets and B16F10 melanoma cells [52]. The potential of nanosuspensions to make insoluble pharmaceuticals more soluble and their adaptability to different delivery methods have made them a popular new class of nanoscale drug delivery technology [53]. Genkwanin nanosuspensions showed a constant drug release pattern and stronger cytotoxicity compared to free genkwanin in 4T1, MCF-7, HeLa, A549, HepG2, A549, BT474, and MDA-MB-453 cells. Additionally, in tumor-bearing nude mice genkwanin nanosuspensions (60 mg/kg, i.v.) showed better safety (at a minimal dose of 320 mg/kg) and therapeutic efficacy (62.09% vs 61.27%) in comparison to paclitaxel doses (8 mg/kg, i.v.) [54]. Hydroxy genkwanin nanosuspensions showed more potent cytotoxicity than the hydroxyl genkwanin solution in MCF-7 breast cancer cell [55]. In female nude mice and Kumingy mice, hydroxy genkwanin nanosuspensions (40 mg/kg) exhibited therapeutic efficacy similar to paclitaxel (8 mg/kg) injection. The highest dose of Hydroxy genkwanin nanosuspensions (360 mg/kg) presented 100% survival and safety in mice models [8].
Genkwanin treatment attenuated lung cancer progression and repressed cell proliferation and migration via modulating PI3K/PKB (phosphatidylinositol 3-kinase/protein kinase B) cell signaling pathway in A549 and H69AR cancer cells [56]. Genkwanin-loaded self-nano emulsifying drug delivery system formulation displayed boosted oral bioavailability and tremendous anti-colitis-associated colorectal cancer efficacy in AOM/DSS-induced C57BL/6J mice model [57]. Further, genkwanin induced reduction in melanin synthesis via inhibition of tyrosinase activity in B16F10 melanoma cells [58]. Genkwanin displayed better antitumor efficacy partly via augmenting host immunity and reducing expression levels of inflammatory cytokines APCMin/+ mice. Hydroxygenkwanin (HGK) suppressed NSCLC progression by enhancing EGFR degradation in TKI-resistant NSCLC cells [59].
The antitumor impact of genkwanin on colorectal cancer was studied in vivo on APCMin/+ mice and in vitro on human colorectal cancer lines HT-29 and SW-480 [59]. While genkwanin effectively suppressed the proliferation of human colorectal cancer cells HT-29 and SW-480 and the release of the inflammatory cytokine IL-8, six different inflammatory cytokines in a cell culture system boosted the development of two cancer cells in a concentration-dependent manner. After receiving 12.5 and 25 mg/kg/day of genkwanin orally, the body weights, spleen and thymus indexes, and immune cytokine secretions in the APCMin/+ mice were dramatically improved [51]. In addition, two groups treated with genkwanin showed significantly reduced inflammatory cytokine levels and tumor multiplicity alterations. Besides, there was an apparent amelioration of the dysplastic adenomatous modifications in the gut histology. Together, these data suggested that genkwanin’s superior anticancer efficacy was partly caused by raising host immunity and lowering inflammatory cytokine levels. Genkwanin regulated tumor necrosis factor-α-induced HaCaT cancer cell proliferation and inflammatory cytokines in Psoriasis via regulating nuclear factor-kappa B cell signaling pathway in human immortal keratinocyte HaCaT cells [60]. Treating colorectal cancer with genkwanin may be a successful chemotherapeutic approach [51]. Figure 3 projects a possible mechanism associated with the anticancerous efficacy of genkwanin in several human carcinomas.
![Figure 3
Proposed mechanism associated with the anticancerous potential of genkwanin. Pharmacokinetic analyses showed that GKA-SNEDDS has a 353.28% higher relative bioavailability when compared to GKA suspension. In addition, GKA-SNEDDS (self-nanoemulsifying drug delivery system) significantly reduces the histological scores of inflammatory cytokine levels and enhances the disease activity index (DAI). In the AOM/DSS-induced CAC mice model, it also prevents weight loss and inhibits the formation of colon tumors by inducing tumor cell apoptosis. Improved oral bioavailability and superior anti-CAC activity were demonstrated by the generated GKA-SNEDDS. In conclusion, GKA-SNEDDS can be used as a possible drug delivery method to enhance the therapeutic application of GKA since it uses lipid nanoparticles as the drug delivery carrier [62].](/document/doi/10.1515/chem-2024-0003/asset/graphic/j_chem-2024-0003_fig_003.jpg)
Proposed mechanism associated with the anticancerous potential of genkwanin. Pharmacokinetic analyses showed that GKA-SNEDDS has a 353.28% higher relative bioavailability when compared to GKA suspension. In addition, GKA-SNEDDS (self-nanoemulsifying drug delivery system) significantly reduces the histological scores of inflammatory cytokine levels and enhances the disease activity index (DAI). In the AOM/DSS-induced CAC mice model, it also prevents weight loss and inhibits the formation of colon tumors by inducing tumor cell apoptosis. Improved oral bioavailability and superior anti-CAC activity were demonstrated by the generated GKA-SNEDDS. In conclusion, GKA-SNEDDS can be used as a possible drug delivery method to enhance the therapeutic application of GKA since it uses lipid nanoparticles as the drug delivery carrier [62].
With an average diameter of 261.1 ± 4.8 nm, a narrow particle size distribution (PDI of 0.12 ± 0.01), spherical morphology, high drug-loading content (39.9 ± 2.3%, w/w), and good stability in several physiological mediums, the resulting HGK nanosuspensions (HGK-NSps) were observed. The obtained nanosuspensions of HGK slowly released HGK, and HGK-NSps proved safe for intravenous injection at low concentrations. In vitro, HGK-NSps demonstrated more significant cytotoxicity against several tumor cells than free HGK. The IC50 value was 5-fold lower than the HGK solution, specifically against MCF-7 cells, at 1.0 μg/mL. The therapeutic efficacy of HGK-NSps (40 mg/kg) in the in vivo antitumor activity trial was comparable to that of the paclitaxel injection (8 mg/kg). According to the preliminary acute toxicity test, HGK-NSps had 100% of the mice survived even at the highest dose of 360 mg/kg (iv), and every mouse was in good condition, indicating a maximum tolerable dose of more than 360 mg/kg. HGK-NSps have shown a robust antitumor effect and good tolerance, suggesting that they could potentially develop into a safe and valuable antitumor medication in the future for the treatment of breast cancer [55].
Another study reported a new flavonoid called HGK, which selectively kills all of the NSCLC cells we tested [61]. The present investigation evaluated the anticancer activity of HGK on TKI-resistant NSCLC cells using a xenograft mouse model and NSCLC cells with EGFR mutations. In vitro and in vivo suppression of cancer cell viability was demonstrated by HGK, according to the data. According to whole-transcriptome research, the alterations in gene expression caused by HGK may include EGFR as an upstream regulator. We provided proof that HGK blocked many EGFR downstream signaling and decreased the amount of EGFR to corroborate this analysis. These findings suggest that HGK’s anticancer action against TKI-resistant NSCLC cells may be mediated by improving EGFR degradation [61].
Further anticancerous efficacy of genkwanin has been investigated in lung cancer cells. MTT assay was then employed to assess the antiproliferative effects of genkwanin (20, 40, and 80 μM) against H69AR cells. Quantitative real time PCR and Western blot experiments further reported inhibition of the PI3K/Akt signaling pathway, significantly reducing PI3K/Akt mRNA protein and mRNA expression levels. Transwell test further reported inhibition of invasion and migration levels in genkwanin-treated cancer cells via inhibiting the PI3K/Akt pathway. Transwell test further reported inhibition of invasion and migration levels in genkwanin-treated cancer cells via inhibiting the PI3K/Akt pathway. Hence, genkwanin displayed significant inhibition of lung cancer cell growth, invasion, proliferation, and migration via inhibition of PI3K/Akt pathway, thus presenting a solid alternative therapy for lung tumor growth and metastasis [60].
Gnidia latifolia, Gnidia glaucus, Dendrostellera lessertii, Daphne odorata, and Daphne genkwa are the primary sources of genkwadaphnin, a daphnane diterpene ester molecule. Research has indicated that genkwadaphnin may be used therapeutically to treat leukemia, squamous cell carcinoma, human colon cancer, and hepatocellular carcinoma. It also plays an integral part in melanogenesis, skeletal disorders, inflammatory cytokines, natural killer cells, and innate immunity. On the other hand, the current work also covered pharmacokinetic and metabolomics features of genkwadaphnin. Moreover, additional scientific information on human clinical studies is required to ensure the safety and efficacy of genkwadaphnin in medicine [16].
Genkwanin exhibited prominent anti-cancerous efficacy against B16F10 melanoma cells by inducing growth arrest at G0/G1 phases after 24 and 48 h of incubation. Furthermore, genkwanin treatment also reduced melanin synthesis via inhibition of tyrosinase activity. This research has also projected the potential of genkwanin in cosmetic formulations as a skin-whitening agent [58].
At submicromolar and micromolar doses, respectively, sinensetin and genkwanin were demonstrated to cause a higher number of metabolites and to strongly inhibit the in vitro proliferation of MDA-MB-468 cells while having no discernible effect on the viability of MCF-10A cells. On the other hand, genkwanin and sinensetin suppressed MDA-MB-468 cell proliferation more than chrysin, baicalein, and scutellarein. CYP1A1 and CYP1B1 promoted the metabolism of chrysin to baicalein and genkwanin to apigenin. When the findings are combined, they indicate that CYP1 family enzymes increase the antiproliferative action of dietary flavonoids in breast cancer cells by converting them into more potent forms [52]. AFB1-induced damages in the rats were wholly reversed by genkwanin therapy. Combining its antioxidant, anti-inflammatory, and anti-apoptotic qualities, the current work highlights the possible application of GKA as a therapeutic drug to stop AFB1-induced testicular damage [63].
Another study found that oral genkwanin treatment prevented mice from developing colitis caused by oral DSS administration, as shown by decreased weight loss, colon lengthening, and histopathological scores. Moreover, genkwanin reduced the synthesis of proinflammatory cytokines and alleviated oxidative stress. In vitro experiments showed that genkwanin treatment resulted in enhanced mitochondrial functioning and reduced ROS generation in human intestinal epithelial cells. In addition, genkwanin increased the expression of SIRT1, and the protective effect of genkwanin against oxidative stress and mitochondrial dysfunction was partially reversed by lentivirus-mediated SIRT1 knockdown. Clinical trials using genkwanin as a therapy for IBD have a strong foundation thanks to results from cell culture and murine model studies [64].
Flavonoids obtained from Tephroseris kirilowii (Turcz.) Holub were tested for their anticancer properties in human cancer cells. Three of the eight flavonoids from T. kirilowii (IH: isorhamnetin, GN: genkwanin, and Aca: acacetin) were extracted and identified for the first time to have the ability to suppress the proliferation of various human cancer cell lines. These potent flavonoids promoted apoptosis and autophagy in human breast cancer cells, producing cell cycle arrest at the G2/M phase. According to molecular docking analysis, these flavonoids dock in the ATP binding pocket of PI3Kγ. The administration of these flavonoids resulted in a significant reduction in the concentrations of PI3Kγ-p110, phospho-PI3K, phospho-AKT, phospho-mTOR, phospho-p70S6K, and phospho-ULK. Flavonoids-mediated inactivation of p70S6K, AKT, ULK, mTOR, p70S6K, and apoptosis was enhanced by pretreatment with the PI3Kγ-specific inhibitor AS605240. Together, these results provide a unique method by which these flavonoids-induced cell cycle arrest at the G2/M phase, apoptosis, and autophagy may be largely dependent on the downregulation of PI3Kγ-p110 and the subsequent disruption of the PI3K/Akt/mTOR/p70S6K/ULK signaling network. This research offers fresh perspectives on the anticancer properties of particular flavonoids and how they might be applied in anticancer treatment [65]. Another study developed an animal model of acute lung damage generated by cecal ligation and puncture (CLP). Genkwanin decreased inflammation, apoptosis, and lung edema (or damage) in CLP mice. Furthermore, these data mechanically verified that genkwanin ameliorated inflammatory injury in CLP mice by controlling the NF-κB signaling pathway. Hence, these findings supported the possibility that genkwanin would be a helpful medication in the management of acute lung injury brought on by sepsis [66].
5 Conclusion
Genkwanin is a group of readily accessible flavonoids with minimal toxicity that have demonstrated notable health advantages for humans, including specific anticancer characteristics. Genkwanin can improve well-being, prolong life, and have anti-aging effects by reducing ROS and inflammatory cytokine levels, blocking the NF-κB signaling pathway and carcinogenesis, and altering enzymes necessary for brain function. The current review has also demonstrated that anticancer flavonoids and genkwanin nanoparticles can be combined to create new and powerful antitumor medications. In contrast to free GKA, GKA-NSps have overcome the limited solubility of genkwanin and successfully increased anticancer efficacy against numerous tumor cell lines. Furthermore, GKA-NSps enhanced the bioavailability by long-lasting drug release and robust stability in various physiological mediums without hemolysis. More investigation is necessary to ascertain genkwanin’s safety and efficacy in people through conducting clinical trials. Further research will delve deeper into the beneficial properties of genkwanin and help develop strategies for preventing and managing oxidative stress and inflammatory conditions.
Acknowledgements
We want to thank the Saveetha Institute for providing me with all kinds of support in writing this manuscript.
-
Funding information: Authors state no funding.
-
Author contributions: Methodology and writing – original manuscript; P.P., S.R., M.V., I.R., F.K., and M.A.S., project validation; S.R., M.V., I.R., F.K., and M.A.S., investigation; S.R., M.V., I.R., F.K., and M.A.S reviewing; S.R., M.V., I.R., F.K., and M.A.S . All the authors agreed on the final version of the manuscript.
-
Conflict of interest: All authors declare no conflict of interest in publishing this manuscript.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Xiaolong JI, Jianhang GU, Jingyuan TI, Ke MA, Yanqi LI. Research progress on degradation methods and product properties of plant polysaccharides. J Light Ind. 2023 Jun;38(3):55.Search in Google Scholar
[2] Wei S, Sun T, Du J, Zhang B, Xiang D, Li W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol Rep. 2018;40(6):3213–22.10.3892/or.2018.6723Search in Google Scholar PubMed PubMed Central
[3] Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022 Jul;383:132531.10.1016/j.foodchem.2022.132531Search in Google Scholar PubMed
[4] Isaev IM, Agzamova MA, Isaev MI. Genkwanin and iridoid glycosides from Leonurus turkestanicus. Chem Nat Compd. 2011 Mar;47:132–4.10.1007/s10600-011-9857-9Search in Google Scholar
[5] Gao TH, Liao W, Lin LT, Zhu ZP, Lu MG, Fu CM, et al. Curcumae rhizoma and its major constituents against hepatobiliary disease: Pharmacotherapeutic properties and potential clinical applications. Phytomedicine. 2022 Jul;102:154090.10.1016/j.phymed.2022.154090Search in Google Scholar PubMed
[6] Xiang J, Mlambo R, Shaw I, Seid Y, Shah H, He Y, et al. Cryopreservation of bioflavonoid-rich plant sources and bioflavonoid-microcapsules: Emerging technologies for preserving bioactivity and enhancing nutraceutical applications. Front Nutr. 2023;10:1232129.10.3389/fnut.2023.1232129Search in Google Scholar PubMed PubMed Central
[7] Sahu RK, Aboulthana WM, Mehta DK. Phyto-phospholipid complexation as a novel drug delivery system for management of cancer with better bioavailability: Current perspectives and future prospects. Anti-Cancer Agents Med Chem (Former Curr Med Chem-Anti-Cancer Agents). 2021 Jul;21(11):1403–12.10.2174/1871520620999201110191741Search in Google Scholar PubMed
[8] El Menyiy N, Aboulaghras S, Bakrim S, Moubachir R, Taha D, Khalid A, et al. Genkwanin: An emerging natural compound with multifaceted pharmacological effects. Biomed Pharmacother. 2023 Sep;165:115159.10.1016/j.biopha.2023.115159Search in Google Scholar PubMed
[9] Thangavel P, Puga-Olguín A, Rodríguez-Landa JF, Zepeda RC. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules. 2019 Oct;24(21):3892.10.3390/molecules24213892Search in Google Scholar PubMed PubMed Central
[10] Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015 Jul;6(4):408–19.10.3945/an.114.008052Search in Google Scholar PubMed PubMed Central
[11] Xin X, Chen C, Hu YY, Feng Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed Pharmacother. 2019 Sep;117:109047.10.1016/j.biopha.2019.109047Search in Google Scholar PubMed
[12] Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Epigenetic activation of BRCA1 by genistein in vivo and triple negative breast cancer cells linked to antagonism toward aryl hydrocarbon receptor. Nutrients. 2019 Oct;11(11):2559.10.3390/nu11112559Search in Google Scholar PubMed PubMed Central
[13] Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med. 2012 Aug;30(2):337–43.10.3892/ijmm.2012.990Search in Google Scholar PubMed
[14] Kim BG, Jung BR, Lee Y, Hur HG, Lim Y, Ahn JH. Regiospecific flavonoid 7-O-methylation with Streptomyces avermitilis O-methyltransferase expressed in Escherichia coli. J Agric Food Chem. 2006 Feb;54(3):823–8.10.1021/jf0522715Search in Google Scholar PubMed
[15] Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012 Jun;31:323–51.10.1007/s10555-012-9347-ySearch in Google Scholar PubMed
[16] Patel DK, Patel K. Herbal medicines genkwadaphnin as therapeutic agent for cancers and other human disorders: A review of pharmacological activities through scientific evidence. Curr Tradit Med. 2024 Aug;10(4):21–7.10.2174/2215083810666230523155650Search in Google Scholar
[17] Narain NK. Spectroscopic studies of a less abundant flavone, genkwanin. Spectrosc Lett. 1976 Jan;9(12):865–75.10.1080/00387017608067478Search in Google Scholar
[18] Tang W, Eisenbrand G. Chinese drugs of plant origin: Chemistry, pharmacology, and use in traditional and modern medicine. Berlin: Springer-Verlag; 1992. p. 429–35.10.1007/978-3-642-73739-8Search in Google Scholar
[19] Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti‐complement activity. Phytother Res: An Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2006 Jul;20(7):610–3.10.1002/ptr.1915Search in Google Scholar PubMed
[20] Li YN, Yin LH, Xu LN, Peng JY. A simple and efficient protocol for large‐scale preparation of three flavonoids from the flower of Daphne genkwa by combination of macroporous resin and counter‐current chromatography. J Sep Sci. 2010 Jul;33(14):2168–75.10.1002/jssc.201000054Search in Google Scholar PubMed
[21] Shu Y, Liang Y, Liang Z, Zhao X, Zhu X, Feng W, et al. Studies on a simple and efficient method for large-scale preparation of genkwanin from daphne genkwa sieb. et zucc. using normal-phase flash chromatography. J Liq Chromatogr Relat Technol. 2014 Apr;37(6):773–85.10.1080/10826076.2012.749501Search in Google Scholar PubMed PubMed Central
[22] Kim MK, Park G, Ji Y, Lee YG, Choi M, Go SH, et al. Design of experiments-based optimization of flavonoids extraction from Daphne genkwa flower buds and flavonoids contents at different blooming stages. Plants. 2022 Mar;11(7):925.10.3390/plants11070925Search in Google Scholar PubMed PubMed Central
[23] Grayer RJ, Bryan SE, Veitch NC, Goldstone FJ, Paton A, Wollenweber E. External flavones in sweet basil, Ocimum basilicum, and related taxa. Phytochemistry. 1996 Nov;43(5):1041–7.10.1016/S0031-9422(96)00430-XSearch in Google Scholar
[24] Nakasugi T, Komai K. Antimutagens in the Brazilian folk medicinal plant Carqueja (Baccharis t rimera Less.). J Agric Food Chem. 1998 Jul;46(7):2560–4.10.1021/jf9711045Search in Google Scholar
[25] Cottigli F, Loy G, Garau D, Floris CO, Caus M, Pompei RA, et al. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine. 2001 Jan;8(4):302–5.10.1078/0944-7113-00036Search in Google Scholar PubMed
[26] Deiana M, Rosa A, Casu V, Cottiglia F, Bonsignore L, Dessi MA. Chemical composition and antioxidant activity of extracts from Daphne gnidium L. J Am Oil Chemists’ Soc. 2003 Jan;80(1):65–70.10.1007/s11746-003-0652-xSearch in Google Scholar
[27] Fernando Rolim de Almeida L, Elena Delachiave M, Sannomiya M, Vilegas W, Campaner dos Santos L, Mancini E, et al. In vitro allelopathic potential of Leonurus sibiricus L. leaves. J Plant Interact. 2008 Mar;3(1):39–48.10.1080/17429140701749906Search in Google Scholar
[28] Santos-Gomes PC, Seabra RM, Andrade PB, Fernandes-Ferreira M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci. 2002 Jun;162(6):981–7.10.1016/S0168-9452(02)00052-3Search in Google Scholar
[29] Borrás-Linares I, Stojanović Z, Quirantes-Piné R, Arráez-Román D, Švarc-Gajić J, Fernández-Gutiérrez A, et al. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int J Mol Sci. 2014 Nov;15(11):20585–606.10.3390/ijms151120585Search in Google Scholar PubMed PubMed Central
[30] Del Bano MJ, Lorente J, Castillo J, Benavente-García O, Del Rio JA, Ortuño A, et al. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J Agric Food Chem. 2003 Jul;51(15):4247–53.10.1021/jf0300745Search in Google Scholar PubMed
[31] del Baño MJ, Lorente J, Castillo J, Benavente-García O, Marín MP, Del Río JA, et al. Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Postulation of a biosynthetic pathway. J Agric Food Chem. 2004 Aug;52(16):4987–92.10.1021/jf040078pSearch in Google Scholar PubMed
[32] Jordán MJ, Moñino MI, Martínez C, Lafuente A, Sotomayor JA. Introduction of distillate rosemary leaves into the diet of the Murciano-Granadina goat: Transfer of polyphenolic compounds to goats’ milk and the plasma of suckling goat kids. J Agric Food Chem. 2010 Jul;58(14):8265–70.10.1021/jf100921zSearch in Google Scholar PubMed
[33] Pérez-Fons L, Aranda FJ, Guillén J, Villalaín J, Micol V. Rosemary (Rosmarinus officinalis) diterpenes affect lipid polymorphism and fluidity in phospholipid membranes. Arch Biochem Biophys. 2006 Sep;453(2):224–36.10.1016/j.abb.2006.07.004Search in Google Scholar PubMed
[34] Jamzad Z, Grayer RJ, Kite GC, Simmonds MS, Ingrouille M, Jalili A. Leaf surface flavonoids in Iranian species of Nepeta (Lamiaceae) and some related genera. Biochem Syst Ecol. 2003 Jun;31(6):587–600.10.1016/S0305-1978(02)00221-1Search in Google Scholar
[35] Kim AR, Zou YN, Park TH, Shim KH, Kim MS, Kim ND, et al. Active components from Artemisia iwayomogi displaying ONOO− scavenging activity. Phytother Res: Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2004 Jan;18(1):1–7.Search in Google Scholar
[36] Ferreres F, Ribeiro V, Izquierdo AG, Rodrigues MÂ, Seabra RM, Andrade PB, et al. Rumex induratus leaves: Interesting dietary source of potential bioactive compounds. J Agric Food Chem. 2006 Aug;54(16):5782–9.10.1021/jf0613233Search in Google Scholar PubMed
[37] Kumarasamy Y, Cox PJ, Jaspars M, Nahar L, Sarker SD. Bioactivity of hirsutanolol, oregonin and genkwanin, isolated from the seeds of Alnus glutinosa (Betulaceae). Nat Product Commun. 2006 Aug;1(8):1934578X0600100808.10.1177/1934578X0600100808Search in Google Scholar
[38] Regnier TC, Kouekam CR, Leonard CM, Mokgalaka NS, Weiersbye IM. Chemical analysis and potential use of the tree Combretum erythrophyllum grown on gold and uranium mine tailings seepage. In Mine Closure 2009: Proceedings of the Fourth International Conference on Mine Closure 2009 Sep 9. Australian Centre for Geomechanics; p. 539–47.10.36487/ACG_repo/908_42Search in Google Scholar
[39] Ito T, Kakino M, Tazawa S, Oyama M, Maruyama H, Araki Y, et al. Identification of phenolic compounds in Aquilaria crassna leaves via liquid chromatography-electrospray ionization mass spectroscopy. Food Sci Technol Res. 2012;18(2):259–62.10.3136/fstr.18.259Search in Google Scholar
[40] Watanabe M, Watanabe T, Devkota HP. Phenolic compounds from the leaves of Phegopteris decursivepinnata (HC Hall) Fée. Biochem Syst Ecol. 2018 Jun;78:81–3.10.1016/j.bse.2018.04.002Search in Google Scholar
[41] Rakib A, Ahmed S, Islam MA, Haye A, Uddin SN, Uddin MM, et al. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci Nutr. 2020 Jan;8(1):547–56.10.1002/fsn3.1339Search in Google Scholar PubMed PubMed Central
[42] Elsisi NS, Darling-Reed S, Lee EY, Oriaku ET, Soliman KF. Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci Lett. 2005 Feb;375(2):91–6.10.1016/j.neulet.2004.10.087Search in Google Scholar PubMed
[43] Nakazawa T, Yasuda T, Ueda J, Ohsawa K. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol Pharm Bull. 2003;26(4):474–80.10.1248/bpb.26.474Search in Google Scholar PubMed
[44] Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF‐1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005 Mar;19(3):342–53.10.1096/fj.04-2175comSearch in Google Scholar PubMed
[45] Martini ND, Katerere DR, Eloff JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol. 2004 Aug;93(2–3):207–12.10.1016/j.jep.2004.02.030Search in Google Scholar PubMed
[46] Kraft C, Jenett‐Siems K, Siems K, Jakupovic J, Mavi S, Bienzle U, et al. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother Res: Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2003 Feb;17(2):123–8.10.1002/ptr.1066Search in Google Scholar PubMed
[47] Suh NA, Luyengi L, Fong HH, Kinghorn AD, Pezzuto JM. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model. Anticancer Res. 1995 Mar;15(2):233–9.Search in Google Scholar
[48] Kim AR, Zou YN, Park TH, Shim KH, Kim MS, Kim ND, et al. Active components from Artemisia iwayomogi displaying ONOO− scavenging activity. Phytother Res: An Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2004 Jan;18(1):1–7.10.1002/ptr.1358Search in Google Scholar PubMed
[49] Brožič P, Kocbek P, Sova M, Kristl J, Martens S, Adamski J, et al. Flavonoids and cinnamic acid derivatives as inhibitors of 17β-hydroxysteroid dehydrogenase type 1. Mol Cell Endocrinol. 2009 Mar;301(1–2):229–34.10.1016/j.mce.2008.09.004Search in Google Scholar PubMed
[50] Ma F, Lin Y, Ni Z, Chen T, Wang X. Therapeutic effects of natural polyphenols on colorectal adenomas: Focus on preclinical studies. Oncol Rep. 2023 Jun;49(6):1–22.10.3892/or.2023.8549Search in Google Scholar PubMed PubMed Central
[51] Wang X, Song ZJ, He X, Zhang RQ, Zhang CF, Li F, et al. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APCMin/+ mice. Int Immunopharmacol. 2015 Dec;29(2):701–7.10.1016/j.intimp.2015.09.006Search in Google Scholar PubMed
[52] Androutsopoulos VP, Ruparelia K, Arroo RR, Tsatsakis AM, Spandidos DA. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology. 2009 Oct;264(3):162–70.10.1016/j.tox.2009.07.023Search in Google Scholar PubMed
[53] Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater. 2015 Jan;2015:1.10.1155/2015/216375Search in Google Scholar
[54] Li Y, Hong J, Li H, Qi X, Guo Y, Han M, et al. Genkwanin nanosuspensions: a novel and potential antitumor drug in breast carcinoma therapy. Drug Delivery. 2017 Jan;24(1):1491–500.10.1080/10717544.2017.1384519Search in Google Scholar PubMed PubMed Central
[55] Ao H, Li Y, Li H, Wang Y, Han M, Guo Y, et al. Preparation of hydroxy genkwanin nanosuspensions and their enhanced antitumor efficacy against breast cancer. Drug Delivery. 2020 Jan;27(1):816–24.10.1080/10717544.2020.1770372Search in Google Scholar PubMed PubMed Central
[56] Wei C, Lu J, Zhang Z, Hua F, Chen Y, Shen Z. Genkwanin attenuates lung cancer development by repressing proliferation and invasion via phosphatidylinositol 3-kinase/protein kinase B pathway. Mater Express. 2021 Mar;11(3):319–25.Search in Google Scholar
[57] El-Dakroury WA, Zewail MB, Elsabahy M, Shabana ME, Asaad GF. Famotidine-loaded solid self-nanoemulsifying drug delivery system demonstrates exceptional efficiency in amelioration of peptic ulcer. Int J Pharm. 2022 Jan;611:121303.10.1016/j.ijpharm.2021.121303Search in Google Scholar PubMed
[58] Bouzaiene NN, Chaabane F, Sassi A, Chekir-Ghedira L, Ghedira K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016 Jan;144:80–5.10.1016/j.lfs.2015.11.030Search in Google Scholar PubMed
[59] Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu BM, et al. Emerging role of micro RNA s in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016 Jul;148(3):237–48.10.1111/imm.12608Search in Google Scholar PubMed PubMed Central
[60] Yan W, Li J, Ren F, Sang H. Genkwanin regulates tumor necrosis factor-α-induced hacat cells proliferation and inflammatory cytokines in psoriasis by regulating nuclear factor-kappa B signaling pathway. Curr Top Nutraceutical Res. 2022 May;20(2):346.10.37290/ctnr2641-452X.20:346-351Search in Google Scholar
[61] Leu YL, Wang TH, Wu CC, Huang KY, Jiang YW, Hsu YC, et al. Hydroxygenkwanin suppresses non-small cell lung cancer progression by enhancing EGFR degradation. Molecules. 2020 Feb;25(4):941.10.3390/molecules25040941Search in Google Scholar PubMed PubMed Central
[62] Yin HF, Yin CM, Ouyang T, Sun SD, Chen WG, Yang XL, et al. Self-nanoemulsifying drug delivery system of genkwanin: a novel approach for anti-colitis-associated colorectal cancer. Drug Des Dev Ther. 2021 Feb;15:557–76.10.2147/DDDT.S292417Search in Google Scholar PubMed PubMed Central
[63] Ijaz MU, Ishtiaq A, Tahir A, Alvi MA, Rafique A, Wang P, et al. Antioxidant, anti-inflammatory, and anti-apoptotic effects of genkwanin against aflatoxin B1-induced testicular toxicity. Toxicol Appl Pharmacology. 2023 Dec;481:116750.10.1016/j.taap.2023.116750Search in Google Scholar PubMed
[64] Chen Z, He Y, Hu F, Li M, Yao Y. Genkwanin alleviates mitochondrial dysfunction and oxidative stress in a murine model of experimental colitis: The participation of Sirt1. Ann Clin Labor Sci. 2022 Mar;52(2):301–13.Search in Google Scholar
[65] Zhang HW, Hu JJ, Fu RQ, Liu X, Zhang YH, Li J, et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci Rep. 2018 Jul;8(1):11255.10.1038/s41598-018-29308-7Search in Google Scholar PubMed PubMed Central
[66] Qin G, Yi S. Genkwanin improves inflammatory injury in rats with septic lung injury by regulating NF-κB signaling pathway. Qual Assur Saf Crop Foods. 2022 Apr;14(2):66–73.10.15586/qas.v14i2.991Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models
Articles in the same Issue
- Regular Articles
- Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity
- Biochar from de-oiled Chlorella vulgaris and its adsorption on antibiotics
- Phytochemicals profiling, in vitro and in vivo antidiabetic activity, and in silico studies on Ajuga iva (L.) Schreb.: A comprehensive approach
- Synthesis, characterization, in silico and in vitro studies of novel glycoconjugates as potential antibacterial, antifungal, and antileishmanial agents
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer
- Computational study of ADME-Tox prediction of selected phytochemicals from Punica granatum peels
- Phytochemical analysis, in vitro antioxidant and antifungal activities of extracts and essential oil derived from Artemisia herba-alba Asso
- Two triazole-based coordination polymers: Synthesis and crystal structure characterization
- Phytochemical and physicochemical studies of different apple varieties grown in Morocco
- Synthesis of multi-template molecularly imprinted polymers (MT-MIPs) for isolating ethyl para-methoxycinnamate and ethyl cinnamate from Kaempferia galanga L., extract with methacrylic acid as functional monomer
- Nutraceutical potential of Mesembryanthemum forsskaolii Hochst. ex Bioss.: Insights into its nutritional composition, phytochemical contents, and antioxidant activity
- Evaluation of influence of Butea monosperma floral extract on inflammatory biomarkers
- Cannabis sativa L. essential oil: Chemical composition, anti-oxidant, anti-microbial properties, and acute toxicity: In vitro, in vivo, and in silico study
- The effect of gamma radiation on 5-hydroxymethylfurfural conversion in water and dimethyl sulfoxide
- Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix
- Determination of essential oil and chemical composition of St. John’s Wort
- Computational design and in vitro assay of lantadene-based novel inhibitors of NS3 protease of dengue virus
- Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica
- Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types
- Correlation between in vitro anti-urease activity and in silico molecular modeling approach of novel imidazopyridine–oxadiazole hybrids derivatives
- Spatial mapping of indoor air quality in a light metro system using the geographic information system method
- Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model
- Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri
- Cytotoxic and phytochemical screening of Solanum lycopersicum–Daucus carota hydro-ethanolic extract and in silico evaluation of its lycopene content as anticancer agent
- Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity
- pH-based colorimetric detection of monofunctional aldehydes in liquid and gas phases
- Investigating the effect of resveratrol on apoptosis and regulation of gene expression of Caco-2 cells: Unravelling potential implications for colorectal cancer treatment
- Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets
- Effect of silver nanoparticles formulated by Silybum marianum on menopausal urinary incontinence in ovariectomized rats
- Synthesis of new analogs of N-substituted(benzoylamino)-1,2,3,6-tetrahydropyridines
- Response of yield and quality of Japonica rice to different gradients of moisture deficit at grain-filling stage in cold regions
- Preparation of an inclusion complex of nickel-based β-cyclodextrin: Characterization and accelerating the osteoarthritis articular cartilage repair
- Empagliflozin-loaded nanomicelles responsive to reactive oxygen species for renal ischemia/reperfusion injury protection
- Preparation and pharmacodynamic evaluation of sodium aescinate solid lipid nanoparticles
- Assessment of potentially toxic elements and health risks of agricultural soil in Southwest Riyadh, Saudi Arabia
- Theoretical investigation of hydrogen-rich fuel production through ammonia decomposition
- Biosynthesis and screening of cobalt nanoparticles using citrus species for antimicrobial activity
- Investigating the interplay of genetic variations, MCP-1 polymorphism, and docking with phytochemical inhibitors for combatting dengue virus pathogenicity through in silico analysis
- Ultrasound induced biosynthesis of silver nanoparticles embedded into chitosan polymers: Investigation of its anti-cutaneous squamous cell carcinoma effects
- Copper oxide nanoparticles-mediated Heliotropium bacciferum leaf extract: Antifungal activity and molecular docking assays against strawberry pathogens
- Sprouted wheat flour for improving physical, chemical, rheological, microbial load, and quality properties of fino bread
- Comparative toxicity assessment of fisetin-aided artificial intelligence-assisted drug design targeting epibulbar dermoid through phytochemicals
- Acute toxicity and anti-inflammatory activity of bis-thiourea derivatives
- Anti-diabetic activity-guided isolation of α-amylase and α-glucosidase inhibitory terpenes from Capsella bursa-pastoris Linn.
- GC–MS analysis of Lactobacillus plantarum YW11 metabolites and its computational analysis on familial pulmonary fibrosis hub genes
- Green formulation of copper nanoparticles by Pistacia khinjuk leaf aqueous extract: Introducing a novel chemotherapeutic drug for the treatment of prostate cancer
- Improved photocatalytic properties of WO3 nanoparticles for Malachite green dye degradation under visible light irradiation: An effect of La doping
- One-pot synthesis of a network of Mn2O3–MnO2–poly(m-methylaniline) composite nanorods on a polypyrrole film presents a promising and efficient optoelectronic and solar cell device
- Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia
- A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts
- Processing of alcohol pomelo beverage (Citrus grandis (L.) Osbeck) using saccharomyces yeast: Optimization, physicochemical quality, and sensory characteristics
- Specialized compounds of four Cameroonian spices: Isolation, characterization, and in silico evaluation as prospective SARS-CoV-2 inhibitors
- Identification of a novel drug target in Porphyromonas gingivalis by a computational genome analysis approach
- Physico-chemical properties and durability of a fly-ash-based geopolymer
- FMS-like tyrosine kinase 3 inhibitory potentials of some phytochemicals from anti-leukemic plants using computational chemical methodologies
- Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils
- 3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer
- Deciphering the influenza neuraminidase inhibitory potential of naturally occurring biflavonoids: An in silico approach
- Determination of heavy elements in agricultural regions, Saudi Arabia
- Synthesis and characterization of antioxidant-enriched Moringa oil-based edible oleogel
- Ameliorative effects of thistle and thyme honeys on cyclophosphamide-induced toxicity in mice
- Study of phytochemical compound and antipyretic activity of Chenopodium ambrosioides L. fractions
- Investigating the adsorption mechanism of zinc chloride-modified porous carbon for sulfadiazine removal from water
- Performance repair of building materials using alumina and silica composite nanomaterials with electrodynamic properties
- Effects of nanoparticles on the activity and resistance genes of anaerobic digestion enzymes in livestock and poultry manure containing the antibiotic tetracycline
- Effect of copper nanoparticles green-synthesized using Ocimum basilicum against Pseudomonas aeruginosa in mice lung infection model
- Cardioprotective effects of nanoparticles green formulated by Spinacia oleracea extract on isoproterenol-induced myocardial infarction in mice by the determination of PPAR-γ/NF-κB pathway
- Anti-OTC antibody-conjugated fluorescent magnetic/silica and fluorescent hybrid silica nanoparticles for oxytetracycline detection
- Curcumin conjugated zinc nanoparticles for the treatment of myocardial infarction
- Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery
- Exploring the phytochemical profile and antioxidant evaluation: Molecular docking and ADMET analysis of main compounds from three Solanum species in Saudi Arabia
- Unveiling the molecular composition and biological properties of essential oil derived from the leaves of wild Mentha aquatica L.: A comprehensive in vitro and in silico exploration
- Analysis of bioactive compounds present in Boerhavia elegans seeds by GC-MS
- Homology modeling and molecular docking study of corticotrophin-releasing hormone: An approach to treat stress-related diseases
- LncRNA MIR17HG alleviates heart failure via targeting MIR17HG/miR-153-3p/SIRT1 axis in in vitro model
- Development and validation of a stability indicating UPLC-DAD method coupled with MS-TQD for ramipril and thymoquinone in bioactive SNEDDS with in silico toxicity analysis of ramipril degradation products
- Biosynthesis of Ag/Cu nanocomposite mediated by Curcuma longa: Evaluation of its antibacterial properties against oral pathogens
- Development of AMBER-compliant transferable force field parameters for polytetrafluoroethylene
- Treatment of gestational diabetes by Acroptilon repens leaf aqueous extract green-formulated iron nanoparticles in rats
- Development and characterization of new ecological adsorbents based on cardoon wastes: Application to brilliant green adsorption
- A fast, sensitive, greener, and stability-indicating HPLC method for the standardization and quantitative determination of chlorhexidine acetate in commercial products
- Assessment of Se, As, Cd, Cr, Hg, and Pb content status in Ankang tea plantations of China
- Effect of transition metal chloride (ZnCl2) on low-temperature pyrolysis of high ash bituminous coal
- Evaluating polyphenol and ascorbic acid contents, tannin removal ability, and physical properties during hydrolysis and convective hot-air drying of cashew apple powder
- Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder
- Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa
- Preparation, characterization, and determination of the therapeutic effects of copper nanoparticles green-formulated by Pistacia atlantica in diabetes-induced cardiac dysfunction in rat
- Antioxidant and antidiabetic potentials of methoxy-substituted Schiff bases using in vitro, in vivo, and molecular simulation approaches
- Anti-melanoma cancer activity and chemical profile of the essential oil of Seseli yunnanense Franch
- Molecular docking analysis of subtilisin-like alkaline serine protease (SLASP) and laccase with natural biopolymers
- Overcoming methicillin resistance by methicillin-resistant Staphylococcus aureus: Computational evaluation of napthyridine and oxadiazoles compounds for potential dual inhibition of PBP-2a and FemA proteins
- Exploring novel antitubercular agents: Innovative design of 2,3-diaryl-quinoxalines targeting DprE1 for effective tuberculosis treatment
- Drimia maritima flowers as a source of biologically potent components: Optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and pharmacological properties
- Estimating molecular properties, drug-likeness, cardiotoxic risk, liability profile, and molecular docking study to characterize binding process of key phyto-compounds against serotonin 5-HT2A receptor
- Fabrication of β-cyclodextrin-based microgels for enhancing solubility of Terbinafine: An in-vitro and in-vivo toxicological evaluation
- Phyto-mediated synthesis of ZnO nanoparticles and their sunlight-driven photocatalytic degradation of cationic and anionic dyes
- Monosodium glutamate induces hypothalamic–pituitary–adrenal axis hyperactivation, glucocorticoid receptors down-regulation, and systemic inflammatory response in young male rats: Impact on miR-155 and miR-218
- Quality control analyses of selected honey samples from Serbia based on their mineral and flavonoid profiles, and the invertase activity
- Eco-friendly synthesis of silver nanoparticles using Phyllanthus niruri leaf extract: Assessment of antimicrobial activity, effectiveness on tropical neglected mosquito vector control, and biocompatibility using a fibroblast cell line model
- Green synthesis of silver nanoparticles containing Cichorium intybus to treat the sepsis-induced DNA damage in the liver of Wistar albino rats
- Quality changes of durian pulp (Durio ziberhinus Murr.) in cold storage
- Study on recrystallization process of nitroguanidine by directly adding cold water to control temperature
- Determination of heavy metals and health risk assessment in drinking water in Bukayriyah City, Saudi Arabia
- Larvicidal properties of essential oils of three Artemisia species against the chemically insecticide-resistant Nile fever vector Culex pipiens (L.) (Diptera: Culicidae): In vitro and in silico studies
- Design, synthesis, characterization, and theoretical calculations, along with in silico and in vitro antimicrobial proprieties of new isoxazole-amide conjugates
- The impact of drying and extraction methods on total lipid, fatty acid profile, and cytotoxicity of Tenebrio molitor larvae
- A zinc oxide–tin oxide–nerolidol hybrid nanomaterial: Efficacy against esophageal squamous cell carcinoma
- Research on technological process for production of muskmelon juice (Cucumis melo L.)
- Physicochemical components, antioxidant activity, and predictive models for quality of soursop tea (Annona muricata L.) during heat pump drying
- Characterization and application of Fe1−xCoxFe2O4 nanoparticles in Direct Red 79 adsorption
- Torilis arvensis ethanolic extract: Phytochemical analysis, antifungal efficacy, and cytotoxicity properties
- Magnetite–poly-1H pyrrole dendritic nanocomposite seeded on poly-1H pyrrole: A promising photocathode for green hydrogen generation from sanitation water without using external sacrificing agent
- HPLC and GC–MS analyses of phytochemical compounds in Haloxylon salicornicum extract: Antibacterial and antifungal activity assessment of phytopathogens
- Efficient and stable to coking catalysts of ethanol steam reforming comprised of Ni + Ru loaded on MgAl2O4 + LnFe0.7Ni0.3O3 (Ln = La, Pr) nanocomposites prepared via cost-effective procedure with Pluronic P123 copolymer
- Nitrogen and boron co-doped carbon dots probe for selectively detecting Hg2+ in water samples and the detection mechanism
- Heavy metals in road dust from typical old industrial areas of Wuhan: Seasonal distribution and bioaccessibility-based health risk assessment
- Phytochemical profiling and bioactivity evaluation of CBD- and THC-enriched Cannabis sativa extracts: In vitro and in silico investigation of antioxidant and anti-inflammatory effects
- Investigating dye adsorption: The role of surface-modified montmorillonite nanoclay in kinetics, isotherms, and thermodynamics
- Antimicrobial activity, induction of ROS generation in HepG2 liver cancer cells, and chemical composition of Pterospermum heterophyllum
- Study on the performance of nanoparticle-modified PVDF membrane in delaying membrane aging
- Impact of cholesterol in encapsulated vitamin E acetate within cocoliposomes
- Review Articles
- Structural aspects of Pt(η3-X1N1X2)(PL) (X1,2 = O, C, or Se) and Pt(η3-N1N2X1)(PL) (X1 = C, S, or Se) derivatives
- Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview
- Stimulus-responsive MOF–hydrogel composites: Classification, preparation, characterization, and their advancement in medical treatments
- Electrochemical dissolution of titanium under alternating current polarization to obtain its dioxide
- Special Issue on Recent Trends in Green Chemistry
- Phytochemical screening and antioxidant activity of Vitex agnus-castus L.
- Phytochemical study, antioxidant activity, and dermoprotective activity of Chenopodium ambrosioides (L.)
- Exploitation of mangliculous marine fungi, Amarenographium solium, for the green synthesis of silver nanoparticles and their activity against multiple drug-resistant bacteria
- Study of the phytotoxicity of margines on Pistia stratiotes L.
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part III
- Impact of biogenic zinc oxide nanoparticles on growth, development, and antioxidant system of high protein content crop (Lablab purpureus L.) sweet
- Green synthesis, characterization, and application of iron and molybdenum nanoparticles and their composites for enhancing the growth of Solanum lycopersicum
- Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes
- Photocatalytic treatment of organic dyes using metal oxides and nanocomposites: A quantitative study
- Antifungal, antioxidant, and photocatalytic activities of greenly synthesized iron oxide nanoparticles
- Special Issue on Phytochemical and Pharmacological Scrutinization of Medicinal Plants
- Hepatoprotective effects of safranal on acetaminophen-induced hepatotoxicity in rats
- Chemical composition and biological properties of Thymus capitatus plants from Algerian high plains: A comparative and analytical study
- Chemical composition and bioactivities of the methanol root extracts of Saussurea costus
- In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract
- Insights about the deleterious impact of a carbamate pesticide on some metabolic immune and antioxidant functions and a focus on the protective ability of a Saharan shrub and its anti-edematous property
- A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas
- A study to investigate the anticancer potential of carvacrol via targeting Notch signaling in breast cancer
- Assessment of anti-diabetic properties of Ziziphus oenopolia (L.) wild edible fruit extract: In vitro and in silico investigations through molecular docking analysis
- Optimization of polyphenol extraction, phenolic profile by LC-ESI-MS/MS, antioxidant, anti-enzymatic, and cytotoxic activities of Physalis acutifolia
- Phytochemical screening, antioxidant properties, and photo-protective activities of Salvia balansae de Noé ex Coss
- Antihyperglycemic, antiglycation, anti-hypercholesteremic, and toxicity evaluation with gas chromatography mass spectrometry profiling for Aloe armatissima leaves
- Phyto-fabrication and characterization of gold nanoparticles by using Timur (Zanthoxylum armatum DC) and their effect on wound healing
- Does Erodium trifolium (Cav.) Guitt exhibit medicinal properties? Response elements from phytochemical profiling, enzyme-inhibiting, and antioxidant and antimicrobial activities
- Integrative in silico evaluation of the antiviral potential of terpenoids and its metal complexes derived from Homalomena aromatica based on main protease of SARS-CoV-2
- 6-Methoxyflavone improves anxiety, depression, and memory by increasing monoamines in mice brain: HPLC analysis and in silico studies
- Simultaneous extraction and quantification of hydrophilic and lipophilic antioxidants in Solanum lycopersicum L. varieties marketed in Saudi Arabia
- Biological evaluation of CH3OH and C2H5OH of Berberis vulgaris for in vivo antileishmanial potential against Leishmania tropica in murine models

