Abstract
C23H25NO5, monoclinic, Cc (no. 9), a = 21.2548(2) Å, b = 20.4713(1) Å, c = 16.7944(1) Å, β = 126.267(1)°, V = 5,891.79(10) Å3, Z = 12, Rgt (F) = 0.0240, wRref (F 2) = 0.0649, T = 293 K.
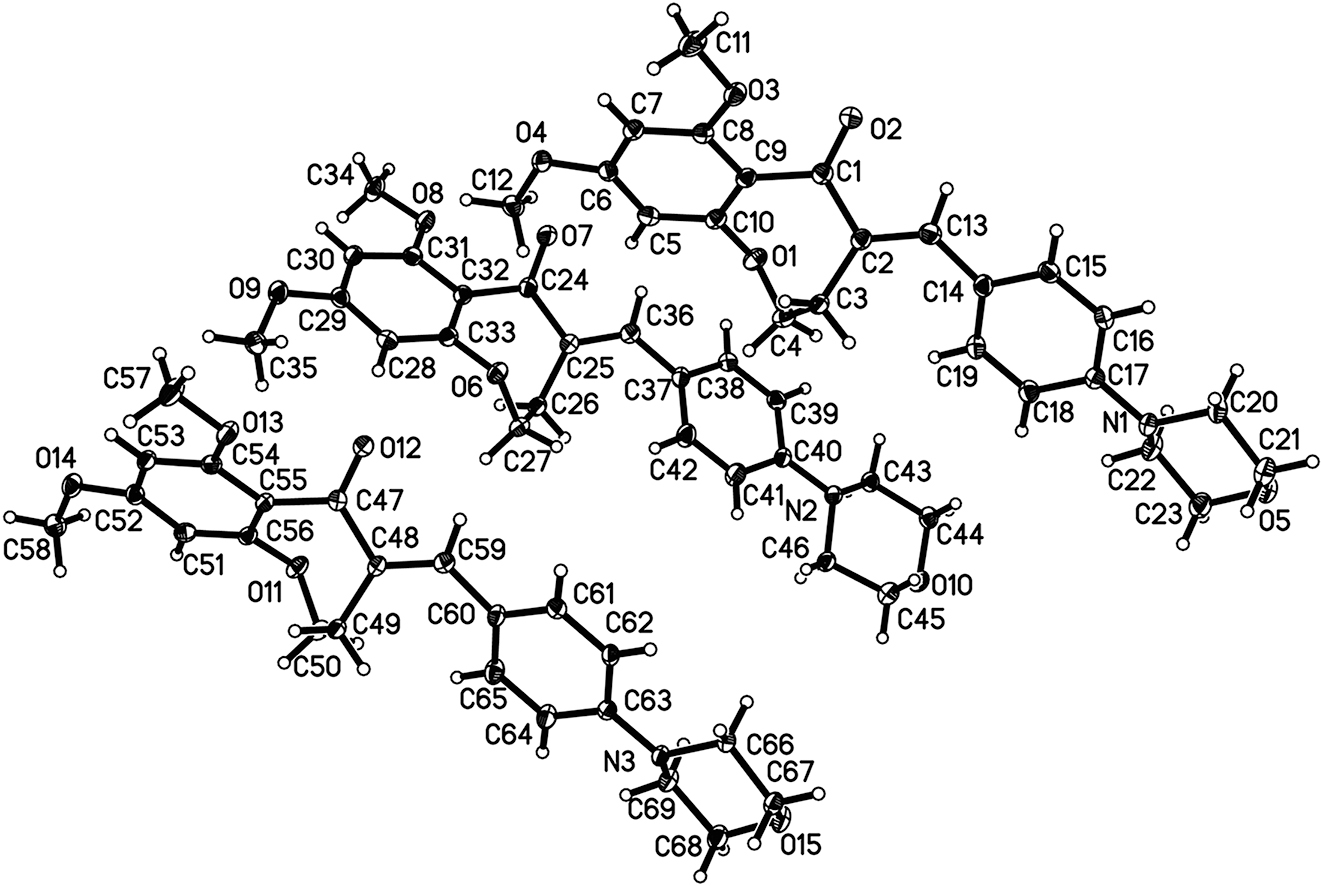
The crystal structure is shown in figure. Displacement ellipsoids are drawn at the 30 % probability level. There are three drug molecules.
Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.16 × 0.13 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.77 mm−1 |
| Diffractometer, scan mode: | Four-circle diffractometer, ω |
| θ max, completeness: | 74.3°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 16,377, 7,213, 0.013 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 7,174 |
| N(param)refined: | 791 |
| Programs: | CrysAlisPRO, 1 SHELX 2 , 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.54398 (9) | 0.35949 (7) | 0.17591 (12) | 0.0172 (3) |
| C2 | 0.61248 (9) | 0.37213 (7) | 0.28030 (11) | 0.0167 (3) |
| C3 | 0.59643 (9) | 0.37333 (7) | 0.35678 (11) | 0.0169 (3) |
| H3A | 0.552902 | 0.402441 | 0.334431 | 0.020* |
| H3B | 0.641730 | 0.390861 | 0.417946 | 0.020* |
| C4 | 0.57761 (10) | 0.30619 (8) | 0.37705 (11) | 0.0202 (3) |
| H4A | 0.625927 | 0.282273 | 0.420708 | 0.024* |
| H4B | 0.553649 | 0.311751 | 0.410996 | 0.024* |
| C5 | 0.38819 (10) | 0.27750 (8) | 0.18550 (11) | 0.0173 (3) |
| H5 | 0.385349 | 0.245559 | 0.222654 | 0.021* |
| C6 | 0.32083 (9) | 0.30455 (8) | 0.10286 (11) | 0.0162 (3) |
| C7 | 0.32489 (9) | 0.35432 (7) | 0.04865 (11) | 0.0162 (3) |
| H7 | 0.279526 | 0.373488 | −0.004755 | 0.019* |
| C8 | 0.39724 (9) | 0.37462 (7) | 0.07556 (11) | 0.0159 (3) |
| C9 | 0.46663 (9) | 0.34612 (7) | 0.15657 (11) | 0.0157 (3) |
| C10 | 0.45983 (9) | 0.29939 (7) | 0.21119 (11) | 0.0163 (3) |
| C11 | 0.33859 (10) | 0.45167 (9) | −0.05637 (12) | 0.0265 (4) |
| H11A | 0.307544 | 0.470036 | −0.037633 | 0.040* |
| H11B | 0.308902 | 0.418934 | −0.106124 | 0.040* |
| H11C | 0.353232 | 0.485501 | −0.082071 | 0.040* |
| C12 | 0.23896 (10) | 0.24172 (8) | 0.12622 (12) | 0.0212 (3) |
| H12A | 0.258069 | 0.199541 | 0.124895 | 0.032* |
| H12B | 0.184952 | 0.238316 | 0.100369 | 0.032* |
| H12C | 0.268513 | 0.257338 | 0.193016 | 0.032* |
| C13 | 0.68063 (9) | 0.38524 (8) | 0.29453 (12) | 0.0189 (3) |
| H13 | 0.677337 | 0.387661 | 0.236869 | 0.023* |
| C14 | 0.75851 (9) | 0.39625 (8) | 0.38481 (12) | 0.0179 (3) |
| C15 | 0.81178 (10) | 0.43401 (8) | 0.38076 (12) | 0.0188 (3) |
| H15 | 0.794998 | 0.454044 | 0.321544 | 0.023* |
| C16 | 0.88841 (9) | 0.44233 (8) | 0.46206 (12) | 0.0180 (3) |
| H16 | 0.921835 | 0.468016 | 0.456703 | 0.022* |
| C17 | 0.91622 (9) | 0.41241 (7) | 0.55248 (11) | 0.0156 (3) |
| C18 | 0.86251 (9) | 0.37606 (8) | 0.55776 (12) | 0.0183 (3) |
| H18 | 0.878749 | 0.357238 | 0.617511 | 0.022* |
| C19 | 0.78640 (10) | 0.36780 (8) | 0.47622 (12) | 0.0187 (3) |
| H19 | 0.752710 | 0.342773 | 0.481900 | 0.022* |
| C20 | 1.04693 (10) | 0.45739 (8) | 0.62867 (12) | 0.0207 (3) |
| H20A | 1.023136 | 0.499309 | 0.599205 | 0.025* |
| H20B | 1.057404 | 0.435442 | 0.586458 | 0.025* |
| C21 | 1.12287 (10) | 0.46767 (8) | 0.73070 (13) | 0.0245 (4) |
| H21A | 1.158562 | 0.492693 | 0.724576 | 0.029* |
| H21B | 1.112460 | 0.492745 | 0.770744 | 0.029* |
| C22 | 1.03072 (9) | 0.35719 (8) | 0.69108 (12) | 0.0217 (3) |
| H22A | 1.039939 | 0.329113 | 0.652418 | 0.026* |
| H22B | 0.995968 | 0.334443 | 0.701253 | 0.026* |
| C23 | 1.10725 (10) | 0.37099 (9) | 0.78990 (13) | 0.0260 (4) |
| H23A | 1.097306 | 0.395375 | 0.830924 | 0.031* |
| H23B | 1.131749 | 0.329998 | 0.822889 | 0.031* |
| C24 | 0.30430 (9) | 0.10507 (7) | 0.27271 (11) | 0.0171 (3) |
| C25 | 0.37854 (9) | 0.10832 (8) | 0.37533 (11) | 0.0167 (3) |
| C26 | 0.37005 (9) | 0.11826 (7) | 0.45764 (11) | 0.0172 (3) |
| H26A | 0.418239 | 0.105499 | 0.519723 | 0.021* |
| H26B | 0.328873 | 0.090040 | 0.446490 | 0.021* |
| C27 | 0.35121 (9) | 0.18851 (8) | 0.46562 (11) | 0.0197 (3) |
| H27A | 0.327716 | 0.189488 | 0.500570 | 0.024* |
| H27B | 0.399180 | 0.213598 | 0.503629 | 0.024* |
| C28 | 0.16168 (10) | 0.21327 (7) | 0.28023 (11) | 0.0167 (3) |
| H28 | 0.163417 | 0.249245 | 0.315305 | 0.020* |
| C29 | 0.09116 (9) | 0.18700 (8) | 0.20363 (11) | 0.0168 (3) |
| C30 | 0.08817 (9) | 0.13104 (8) | 0.15340 (11) | 0.0174 (3) |
| H30 | 0.040305 | 0.113844 | 0.102019 | 0.021* |
| C31 | 0.15708 (9) | 0.10142 (7) | 0.18080 (11) | 0.0167 (3) |
| C32 | 0.23070 (9) | 0.12890 (7) | 0.25507 (11) | 0.0161 (3) |
| C33 | 0.23001 (9) | 0.18456 (7) | 0.30355 (11) | 0.0158 (3) |
| C34 | 0.08455 (10) | 0.01270 (9) | 0.07284 (13) | 0.0258 (4) |
| H34A | 0.060411 | 0.003914 | 0.105226 | 0.039* |
| H34B | 0.092615 | −0.027624 | 0.050878 | 0.039* |
| H34C | 0.051311 | 0.040663 | 0.017047 | 0.039* |
| C35 | 0.01753 (10) | 0.26150 (8) | 0.22797 (12) | 0.0228 (3) |
| H35A | 0.042466 | 0.245701 | 0.294070 | 0.034* |
| H35B | −0.035776 | 0.272331 | 0.199639 | 0.034* |
| H35C | 0.044332 | 0.299706 | 0.229313 | 0.034* |
| C36 | 0.44537 (9) | 0.09920 (7) | 0.38498 (12) | 0.0184 (3) |
| H36 | 0.439314 | 0.091482 | 0.326226 | 0.022* |
| C37 | 0.52585 (9) | 0.09955 (8) | 0.47324 (12) | 0.0180 (3) |
| C38 | 0.58103 (10) | 0.05956 (8) | 0.47722 (12) | 0.0188 (3) |
| H38 | 0.564955 | 0.032979 | 0.423379 | 0.023* |
| C39 | 0.65846 (10) | 0.05801 (8) | 0.55801 (12) | 0.0182 (3) |
| H39 | 0.692794 | 0.029938 | 0.557866 | 0.022* |
| C40 | 0.68625 (9) | 0.09836 (7) | 0.64075 (11) | 0.0160 (3) |
| C41 | 0.63068 (10) | 0.13844 (8) | 0.63726 (13) | 0.0227 (4) |
| H41 | 0.646427 | 0.164982 | 0.691016 | 0.027* |
| C42 | 0.55377 (10) | 0.13920 (8) | 0.55626 (13) | 0.0224 (3) |
| H42 | 0.519124 | 0.166846 | 0.556425 | 0.027* |
| C43 | 0.81545 (9) | 0.04824 (8) | 0.73068 (12) | 0.0177 (3) |
| H43A | 0.806508 | 0.040821 | 0.667670 | 0.021* |
| H43B | 0.802393 | 0.008376 | 0.749143 | 0.021* |
| C44 | 0.90069 (9) | 0.06415 (8) | 0.80789 (12) | 0.0199 (3) |
| H44A | 0.932090 | 0.026551 | 0.816953 | 0.024* |
| H44B | 0.915531 | 0.100057 | 0.784479 | 0.024* |
| C45 | 0.87093 (10) | 0.13710 (8) | 0.88768 (12) | 0.0209 (3) |
| H45A | 0.884166 | 0.173433 | 0.863070 | 0.025* |
| H45B | 0.882885 | 0.149701 | 0.950955 | 0.025* |
| C46 | 0.78463 (9) | 0.12274 (8) | 0.81608 (11) | 0.0182 (3) |
| H46A | 0.770381 | 0.089016 | 0.843290 | 0.022* |
| H46B | 0.755063 | 0.161767 | 0.806675 | 0.022* |
| C47 | 0.16961 (9) | 0.37269 (8) | 0.46108 (12) | 0.0192 (3) |
| C48 | 0.23909 (9) | 0.38163 (8) | 0.56663 (11) | 0.0164 (3) |
| C49 | 0.22389 (9) | 0.37692 (7) | 0.64359 (11) | 0.0154 (3) |
| H49A | 0.178586 | 0.403183 | 0.622839 | 0.019* |
| H49B | 0.268253 | 0.394731 | 0.705361 | 0.019* |
| C50 | 0.20997 (9) | 0.30699 (8) | 0.66056 (11) | 0.0183 (3) |
| H50A | 0.259718 | 0.284667 | 0.702138 | 0.022* |
| H50B | 0.186202 | 0.307595 | 0.695170 | 0.022* |
| C51 | 0.02246 (10) | 0.27728 (7) | 0.47469 (11) | 0.0169 (3) |
| H51 | 0.022896 | 0.243855 | 0.512507 | 0.020* |
| C52 | −0.04731 (9) | 0.30327 (7) | 0.39494 (11) | 0.0159 (3) |
| C53 | −0.04799 (9) | 0.35487 (7) | 0.33958 (11) | 0.0157 (3) |
| H53 | −0.095119 | 0.372471 | 0.287162 | 0.019* |
| C54 | 0.02190 (9) | 0.37952 (8) | 0.36337 (11) | 0.0158 (3) |
| C55 | 0.09430 (9) | 0.35283 (8) | 0.44247 (11) | 0.0166 (3) |
| C56 | 0.09162 (9) | 0.30264 (7) | 0.49639 (11) | 0.0156 (3) |
| C57 | −0.04716 (10) | 0.45801 (9) | 0.23444 (12) | 0.0254 (4) |
| H57A | −0.075782 | 0.425675 | 0.183677 | 0.038* |
| H57B | −0.037447 | 0.495120 | 0.208190 | 0.038* |
| H57C | −0.077013 | 0.471412 | 0.257613 | 0.038* |
| C58 | −0.12318 (10) | 0.23662 (8) | 0.42511 (13) | 0.0210 (3) |
| H58A | −0.097688 | 0.196715 | 0.429220 | 0.032* |
| H58B | −0.176777 | 0.227791 | 0.397674 | 0.032* |
| H58C | −0.097578 | 0.254916 | 0.489968 | 0.032* |
| C59 | 0.30757 (9) | 0.39553 (8) | 0.58245 (11) | 0.0183 (3) |
| H59 | 0.305050 | 0.400264 | 0.525548 | 0.022* |
| C60 | 0.38516 (9) | 0.40436 (8) | 0.67481 (11) | 0.0171 (3) |
| C61 | 0.44016 (9) | 0.44181 (8) | 0.67419 (11) | 0.0176 (3) |
| H61 | 0.425046 | 0.462172 | 0.615834 | 0.021* |
| C62 | 0.51608 (9) | 0.44948 (8) | 0.75738 (11) | 0.0158 (3) |
| H62 | 0.550225 | 0.475732 | 0.754158 | 0.019* |
| C63 | 0.54251 (9) | 0.41832 (7) | 0.84660 (11) | 0.0144 (3) |
| C64 | 0.48718 (9) | 0.38152 (8) | 0.84801 (12) | 0.0198 (3) |
| H64 | 0.502012 | 0.361544 | 0.906492 | 0.024* |
| C65 | 0.41160 (10) | 0.37442 (8) | 0.76481 (12) | 0.0206 (3) |
| H65 | 0.377058 | 0.349028 | 0.768377 | 0.025* |
| C66 | 0.67605 (9) | 0.45531 (7) | 0.92134 (11) | 0.0162 (3) |
| H66A | 0.654804 | 0.496325 | 0.886285 | 0.019* |
| H66B | 0.686704 | 0.427738 | 0.883629 | 0.019* |
| C67 | 0.75132 (9) | 0.46800 (8) | 1.02316 (11) | 0.0181 (3) |
| H67A | 0.788915 | 0.488663 | 1.016244 | 0.022* |
| H67B | 0.740980 | 0.497617 | 1.059263 | 0.022* |
| C68 | 0.72923 (9) | 0.38139 (9) | 1.09220 (12) | 0.0227 (3) |
| H68A | 0.720476 | 0.412274 | 1.128522 | 0.027* |
| H68B | 0.751147 | 0.341938 | 1.131477 | 0.027* |
| C69 | 0.65217 (9) | 0.36539 (8) | 0.99507 (11) | 0.0185 (3) |
| H69A | 0.659883 | 0.330958 | 0.961956 | 0.022* |
| H69B | 0.615695 | 0.349637 | 1.007532 | 0.022* |
| N1 | 0.99378 (8) | 0.41800 (6) | 0.63684 (10) | 0.0163 (3) |
| N2 | 0.76499 (8) | 0.10101 (6) | 0.72043 (9) | 0.0162 (3) |
| N3 | 0.61937 (7) | 0.42330 (6) | 0.93090 (9) | 0.0148 (3) |
| O1 | 0.52608 (7) | 0.26799 (5) | 0.28881 (8) | 0.0197 (2) |
| O2 | 0.54943 (7) | 0.35885 (6) | 0.10744 (8) | 0.0226 (2) |
| O3 | 0.40724 (7) | 0.42268 (6) | 0.02809 (8) | 0.0198 (2) |
| O4 | 0.24695 (7) | 0.28638 (5) | 0.06716 (8) | 0.0190 (2) |
| O5 | 1.15881 (7) | 0.40724 (6) | 0.77905 (9) | 0.0237 (3) |
| O6 | 0.29864 (6) | 0.21822 (5) | 0.36984 (8) | 0.0187 (2) |
| O7 | 0.30364 (7) | 0.08440 (6) | 0.20379 (8) | 0.0250 (3) |
| O8 | 0.15808 (6) | 0.04423 (6) | 0.14049 (8) | 0.0215 (2) |
| O9 | 0.01990 (7) | 0.21180 (6) | 0.16954 (9) | 0.0216 (2) |
| O10 | 0.91687 (7) | 0.08155 (5) | 0.90072 (8) | 0.0207 (2) |
| O11 | 0.16029 (7) | 0.27136 (5) | 0.56949 (8) | 0.0192 (2) |
| O12 | 0.17361 (7) | 0.37980 (8) | 0.39210 (9) | 0.0327 (3) |
| O13 | 0.02569 (6) | 0.43088 (6) | 0.31485 (8) | 0.0199 (2) |
| O14 | −0.11947 (7) | 0.28203 (6) | 0.36316 (8) | 0.0198 (2) |
| O15 | 0.78328 (7) | 0.40850 (6) | 1.07735 (9) | 0.0211 (2) |
1 Source of material
According to the literature synthetic strategies, 4 , 5 morpholine (13.94 g, 0.16 mol) and potassium carbonate (27.64 g, 0.20 mol) have been stirred overnight at 313 K with N,N-dimethylformamide (10 mL) as solvent. Then p-fluorobenzaldehyde (2.48 g, 0.02 mol) was added to the mixture and the temperature was raised to 393 K for 5 h reflux reaction. Thin-layer chromatography (TLC, dichloromethane: methanol = 15:1, v:v) was used to observe the reaction process. After the reaction, the system was purified by extraction, vacuum distillation and silica gel column (dichloromethane: methanol = 20:1, v:v) to obtain the intermediate. Using 25 % sodium hydroxide solution (10 mL) as a catalyst, intermediate (0.96 g, 5.00 mmol) and 6,8-dimethoxy-3,4-dihydro-1-benzoxepin-5(2H)-one (1.11 g, 5.00 mmol) were dissolved in methanol (30 mL) for stirring about 5 h at 298 K. Then the mixture was filtered, and the residue was washed with 50 % methanol. The precipitate was collected and recrystallized from a dichloromethane and methanol solution (1:1, v:v) at room temperature to gain clear light yellow block crystals, (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one.
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.96 Å (methyl), U iso(H) = 1.5U eq(C), and d(C–H) = 0.97 Å (methylene), U iso(H) = 1.2U eq(C), and d(C–H) = 0.93 Å (aromatic), U iso(H) = 1.2U eq(C).
3 Comment
In existing studies, 3,4-dihydro-1-benzoxepin-5(2H)-one derivatives have shown excellent anti-inflammatory activities. 4 , 5 It has been demonstrated that cytotoxicity can be decreased with the aid of introducing another pharmacophore, α,β-unsaturated ketone. 6 , 7 Based on these structural characteristics, a series of compounds obtained by the condensation reaction of aromatic aldehydes with 3,4-dihydro-1-benzoxepin-5(2H)-one have been reported. 8 Research shows that the better activity of the compound can be obtained by introducing the nitrogen-containing heterocycles. 9 So we introduced morpholine substituents at the C(17) position. In this study, intermediate was prepared by morpholine and p-fluorobenzaldehyde. Followed by a Claisen–Schmidt condensation reaction with 6,8-dimethoxy-3,4-dihydro-1-benzoxepin-5(2H)-one to afford the target compound, (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one.
Crystals were obtained by evaporation from an equal volume ratio solution of dichloromethane and methanol solution. The single-crystal structure analysis reveals that the title compound crystallizes in the monoclinic space group Cc. There are three molecules in the asymmetric unit (cf. figure). The pharmacophore of the compound is 3,4-dihydro-1-benzoxepin-5(2H)-one. In the C(2) position, it reacts with 4-morpholinobenzaldehyde to obtain α,β-unsaturated ketone. In first molecule, the bond lengths of O(2)=C(1) and C(2)=C(13) are respectively 1.222(2) and 1.348(2) Å, which are within the normal range of olefinic bonds. The torsion angle of O(2)=C(1)–C(2)=C(13) is about 2.3(2)°. The dihedral angle between the 3,4-dihydro-1-benzoxepin-5(2H)-one and the benzene ring is about 21.08(3)°. In second molecule, the bond lengths of O(7)=C(24) and C(25)=C(36) are 1.225(2) and 1.344(2) Å, respectively. The torsion angle of O(7)=C(24)–C(25)=C(36) is about 11.9(2)°. The dihedral angle between the 3,4-dihydro-1-benzoxepin-5(2H)-one and the benzene ring is about 17.66(3)°. In third molecule, the bond lengths of O(12)=C(47) and C(48)=C(59) are 1.220(2) and 1.346(2) Å, respectively. The torsion angle of O(12)=C(47)–C(48)=C(59) is about −2.5(2)°. The dihedral angle between the 3,4-dihydro-1-benzoxepin-5(2H)-one and the benzene ring is about 22.60(3)°. Moreover, the morpholine ring connected to the benzene ring displays “chair” conformation and the whole molecule has a linear structure. 10 , 11 In first molecule, the torsion angle of N(1)–C(20)–C(21)–O(5) and N(1)–C(22)–C(23)–O(5) are 57.09(18)° and −56.19(19)°, respectively. In second molecule, the torsion angle of N(2)–C(43)–C(44)–O(10) and N(2)–C(46)–C(45)–O(10) are 53.66(17)° and −56.84(17)°, respectively. In third molecule, the torsion angle of N(3)–C(66)–C(67)–O(15) and N(3)–C(69)–C(68)–O(15) are 58.28(17)° and −55.58(18)°, respectively.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Shandong Laboratory Program (No. SYS202205), Shandong Provincial Natural Science Foundation (Nos. ZR2022MH159 and ZR2023MH190) and Shandong Province Science and Technology-based Small and Medium-sized Enterprises Innovation Capacity Enhancement Project (No. 2023TSGC0870).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122, https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Gao, C. L.; Hou, G. G.; Liu, J.; Ru, T.; Xu, Y. Z.; Zhao, S. Y.; Ye, H.; Zhang, L. Y.; Chen, K. X.; Guo, Y. W.; Pang, T.; Li, X. W. Synthesis and Target Identification of Benzoxepane Derivatives as Potential Anti-neuroinflammatory Agents for Ischemic Stroke. Angew. Chem., Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Search in Google Scholar PubMed
5. Yang, Y.; Gao, Z. F.; Hou, G. G.; Meng, Q. G.; Hou, Y. Discovery of Anti-neuroinflammatory Agents from 1,4,5,6-Tetrahydrobenzo[2,3]oxepino[4,5-d]pyrimidin-2-Amine Derivatives by Regulating Microglia Polarization. Eur. J. Med. Chem. 2023, 259, 115688; https://doi.org/10.1016/j.ejmech.2023.115688.Search in Google Scholar PubMed
6. Li, W. X.; Yu, L.; Chi, J. B.; Wang, J. P.; Liu, Y. J.; Wang, C. H.; Zhang, M.; Hou, G. G. Discovery of Anti-inflammatory Agents from 3, 4-Dihydronaphthalene-1(2H)-One Derivatives by Inhibiting NLRP3 Inflammasome Activation. Eur. J. Med. Chem. 2024, 268, 116284; https://doi.org/10.1016/j.ejmech.2024.116284.Search in Google Scholar PubMed
7. Li, Y. L.; Meng, Q. G.; Hou, G. G.; Geng, Z. K. Crystal Structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-One, C19H16F4O3. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 1157–1159; https://doi.org/10.1515/ncrs-2023-0373.Search in Google Scholar
8. Li, N.; Yao, B.; Wang, C.; Meng, Q.; Hou, G. Synthesis, Crystal Structure and Activity Evaluation of Novel 3,4-dihydro-1-Benzoxepin-5(2H)-One Derivatives as Protein-Tyrosine Kinase (PTK) Inhibitors. Acta Crystallogr. 2017, C73, 1003–1009; https://doi.org/10.1107/s2053229617015145.Search in Google Scholar PubMed
9. Marshall, C. M.; Federice, J. G.; Bell, C. N.; Cox, P. B.; Njardarson, J. T. An Update on the Nitrogen Heterocycle Compositions and Properties of U. S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655; https://doi.org/10.1021/acs.jmedchem.4c01122.Search in Google Scholar PubMed
10. Yuan, X. Q.; Zhao, L. H.; Zhang, J. J.; Hou, G. G. Crystal Structure of (E)-7-methoxy-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H23NO3. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 363–365; https://doi.org/10.1515/ncrs-2022-0578.Search in Google Scholar
11. Luo, H. L.; Li, W. X.; Bai, X. Y.; Meng, Q. G.; Hou, Y. Crystal Structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 495–497; https://doi.org/10.1515/ncrs-2023-0053.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

