Abstract
The title compound has been synthesized and characterized by IR, 1H NMR, elemental analysis as well as single crystal X-ray diffraction techniques. Crystal structure analysis reveals that the boron atom is tetrahedrally bonding with one carbon atom, one nitrogen atom, and two oxygen atoms. The bond between nitrogen atom and boron atom is a coordination bond.
Source of material
All commercially available starting materials were used without additional purification. [3-[(2-Hydroxyl-5-nitrophenyl)-methylene]amino]phenyl boronic acid (Hnapb) was obtained following the general procedure for the synthesis of Schiff bases [1]. 2-Hydroxy-5-nitrobenzaldehyde (10 mmol) and 3-aminophenylboronic acid (10 mmol) were dissolved in methanol (30 ml) and the resulting solution was stirred 12 h under room temperature. The resulting yellow solid was then washed with ethanol to afford product, yield 87.5%, m.p. >300 °C. IR (KBr, cm−1) 3429, 3067, 1626, 1561, 1514, 1478, 1341, 702. 1H NMR (DMSO-d6): 7.12 (1 H, d, ArH), 7.46–7.57 (2 H, m, ArH), 7.78 (1 H, d, ArH), 7.87 (1 H, s, ArH), 8.23 (2 H, s, BOH), 8.25–8.28 (1 H, t, ArH), 8.70 (1 H, d, ArH), 9.21 (1 H, s, CH=N), 14.80 (1 H, s, OH).
Data collection and handling.
| Crystal: | Yellow, block, size 0.35×0.35×0.41 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.10 cm−1 |
| Diffractometer, scan mode: | Bruker SMART CCD area-detector, φ and ω scans |
| 2θmax: | 52.12° |
| N(hkl)measured, N(hkl)unique: | 10882, 3487 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2952 |
| N(param)refined: | 274 |
| Programs: | SHELXS-97 [2] |
Atomic coordinates and displacement parameters (in Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(1) | 4a | 0.3206 | −0.2802 | 0.1145 | 0.048 |
| H(9) | 4a | 0.3225 | 0.1499 | 0.2116 | 0.032 |
| H(7) | 4a | 0.1542 | −0.2022 | 0.093 | 0.029 |
| H(11) | 4a | 0.1942 | 0.6409 | 0.315 | 0.032 |
| H(14A) | 4a | −0.0701 | 0.4991 | 0.1829 | 0.036 |
| H(14B) | 4a | −0.0617 | 0.6399 | 0.256 | 0.036 |
| H(13) | 4a | 0.1203 | 0.1111 | 0.1747 | 0.026 |
| H(3) | 4a | 0.294 | −1.0081 | −0.0655 | 0.034 |
| H(2A) | 4a | 0.3674 | −0.7896 | 0.0083 | 0.032 |
| H(5) | 4a | 0.1374 | −0.5414 | 0.0027 | 0.03 |
| H(10) | 4a | 0.3035 | 0.4911 | 0.2937 | 0.036 |
| H(17A) | 4a | 0.0115 | 0.2082 | 0.4088 | 0.046 |
| H(17B) | 4a | −0.0391 | 0.4291 | 0.3774 | 0.046 |
| H(15A) | 4a | −0.0791 | 0.1844 | 0.2842 | 0.043 |
| H(15B) | 4a | −0.0312 | 0.0811 | 0.2237 | 0.043 |
| H(16A) | 4a | 0.0434 | 0.707 | 0.4214 | 0.044 |
| H(16B) | 4a | 0.1022 | 0.4977 | 0.4022 | 0.044 |
| H(2WB) | 4a | 0.427(2) | 0.753(8) | 0.056(2) | 0.06(1) |
| H(2WA) | 4a | 0.490(3) | 0.66(1) | 0.047(3) | 0.08(2) |
| H(1WB) | 4a | 0.011(2) | 0.850(8) | 0.065(2) | 0.06(1) |
| H(1WA) | 4a | 0.042(2) | 0.671(8) | 0.114(2) | 0.05(1) |
| H(1A) | 4a | 0.043(2) | 0.083(7) | 0.306(2) | 0.04(1) |
Atomic coordinates and displacement parameters (in Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| O(2W) | 4a | 0.4688(2) | 0.8180(6) | 0.0496(1) | 0.036(2) | 0.043(2) | 0.051(2) | −0.008(1) | 0.003(1) | −0.002(1) |
| O(1W) | 4a | 0.0379(1) | 0.6918(5) | 0.0702(1) | 0.030(2) | 0.048(2) | 0.037(2) | 0.005(1) | 0.005(1) | 0.010(1) |
| O(4) | 4a | 0.0265(1) | 0.5737(4) | 0.2081(1) | 0.022(1) | 0.028(1) | 0.026(1) | −0.0021(9) | −0.003(1) | 0.0019(9) |
| N(2) | 4a | 0.2406(1) | −0.1091(5) | 0.1364(1) | 0.025(2) | 0.024(1) | 0.022(1) | −0.003(1) | −0.001(1) | −0.002(1) |
| O(5) | 4a | 0.0672(1) | 0.7078(4) | 0.3218(1) | 0.035(1) | 0.021(1) | 0.023(1) | 0.0077(9) | 0.002(1) | −0.0013(9) |
| O(1) | 4a | 0.3416(1) | −0.3903(5) | 0.0904(1) | 0.023(1) | 0.040(1) | 0.034(1) | 0.005(1) | 0.002(1) | −0.008(1) |
| C(4) | 4a | 0.2088(2) | −0.7904(6) | −0.0383(2) | 0.031(2) | 0.022(2) | 0.025(2) | 0.000(1) | −0.001(2) | −0.002(1) |
| C(1) | 4a | 0.2998(2) | −0.5173(6) | 0.0510(2) | 0.022(2) | 0.028(2) | 0.022(2) | −0.002(1) | 0.000(1) | 0.003(1) |
| O(2) | 4a | 0.1850(1) | −1.1257(5) | −0.1192(1) | 0.062(2) | 0.037(1) | 0.043(2) | 0.008(1) | −0.003(1) | −0.024(1) |
| C(9) | 4a | 0.2783(2) | 0.2110(6) | 0.2208(2) | 0.019(2) | 0.027(2) | 0.033(2) | 0.001(1) | 0.003(2) | −0.003(1) |
| C(6) | 4a | 0.2277(2) | −0.4498(6) | 0.0493(2) | 0.021(2) | 0.022(2) | 0.022(2) | −0.002(1) | 0.002(1) | 0.002(1) |
| N(3) | 4a | 0.0222(1) | 0.2509(5) | 0.3038(1) | 0.031(2) | 0.017(1) | 0.036(2) | 0.008(1) | 0.008(1) | 0.002(1) |
| C(7) | 4a | 0.2007(2) | −0.2425(6) | 0.0941(2) | 0.022(2) | 0.028(2) | 0.024(2) | 0.002(1) | 0.003(1) | 0.006(1) |
| N(1) | 4a | 0.1629(2) | −0.9324(5) | −0.0855(1) | 0.043(2) | 0.027(2) | 0.030(2) | 0.002(1) | −0.005(1) | −0.003(1) |
| O(3) | 4a | 0.1038(1) | −0.8491(5) | −0.0907(2) | 0.043(2) | 0.048(2) | 0.071(2) | 0.009(1) | −0.021(2) | −0.027(1) |
| C(11) | 4a | 0.2012(2) | 0.5033(6) | 0.2822(2) | 0.030(2) | 0.023(2) | 0.026(2) | −0.000(1) | −0.001(1) | −0.006(1) |
| C(14) | 4a | −0.0423(2) | 0.5058(6) | 0.2244(2) | 0.024(2) | 0.028(2) | 0.038(2) | 0.001(1) | −0.002(2) | −0.001(1) |
| C(13) | 4a | 0.1568(2) | 0.1885(6) | 0.1987(2) | 0.021(2) | 0.024(2) | 0.022(2) | −0.004(1) | −0.002(1) | −0.001(1) |
| C(3) | 4a | 0.2781(2) | −0.8680(6) | −0.0368(2) | 0.036(2) | 0.022(2) | 0.027(2) | 0.008(1) | 0.009(2) | −0.001(1) |
| C(2) | 4a | 0.3218(2) | −0.7355(6) | 0.0071(2) | 0.023(2) | 0.028(2) | 0.029(2) | 0.008(1) | 0.007(2) | 0.004(1) |
| C(12) | 4a | 0.1446(2) | 0.3945(5) | 0.2477(1) | 0.025(2) | 0.017(1) | 0.021(2) | −0.001(1) | 0.002(1) | 0.005(1) |
| C(5) | 4a | 0.1835(2) | −0.5873(6) | 0.0041(2) | 0.024(2) | 0.025(2) | 0.026(2) | −0.001(1) | −0.000(1) | 0.001(1) |
| C(10) | 4a | 0.2670(2) | 0.4146(6) | 0.2696(2) | 0.021(2) | 0.034(2) | 0.035(2) | −0.007(1) | −0.007(2) | −0.008(1) |
| C(8) | 4a | 0.2229(2) | 0.0992(6) | 0.1859(1) | 0.024(2) | 0.020(2) | 0.021(2) | −0.001(1) | 0.002(1) | 0.000(1) |
| C(17) | 4a | 0.0069(2) | 0.3546(6) | 0.3749(2) | 0.049(2) | 0.031(2) | 0.036(2) | 0.017(2) | 0.020(2) | 0.006(2) |
| C(15) | 4a | −0.0381(2) | 0.2247(6) | 0.2579(2) | 0.027(2) | 0.029(2) | 0.053(2) | −0.002(1) | 0.007(2) | −0.007(2) |
| B(1) | 4a | 0.0690(2) | 0.4988(6) | 0.2672(2) | 0.027(2) | 0.016(2) | 0.024(2) | 0.001(1) | 0.002(2) | 0.000(1) |
| C(16) | 4a | 0.0592(2) | 0.5767(7) | 0.3868(2) | 0.051(2) | 0.034(2) | 0.026(2) | 0.015(2) | −0.003(2) | 0.001(1) |
The title compound was prepared from Hnapb and diethanolamine. To CH3CN (25 mL) containing Hnapb (0.5 mmol), diethanolamine (0.5 mmol) in CH2Cl2 (25 mL) were added sequentially. The reaction mixture was stirred under reflux for 12 h, then allowed to cool to r.t.. The resulting yellow amorphous solid was filtered, washed with absolute ethanol and dried. Yield 89.4%, m.p. 222.5–222.7 °C. IR (KBr, cm−1) 3435, 2872, 1617, 1593, 1560, 1409, 1485, 1340, 700. 1H NMR (DMSO-d6): 2.89–2.90 (2 H, m, CH2), 3.12–3.17 (2 H, m, CH2), 3.82–3.85 (2 H, m, CH2), 3.90–3.93 (2 H, m, CH2), 7.02 (1 H, d, ArH), 7.04 (1 H, s, ArH), 7.33–7.37 (1 H, m, ArH), 7.47 (1 H, d, ArH), 7.53 (1 H, s, ArH), 8.22–8.24 (1 H, q, ArH), 8.72 (1 H, d, ArH), 9.26 (1 H, s, CH=N), 15.25 (1 H, s, ArOH). Anal. Calcd. for C17H22BN3O7: C, 52.20; H, 5.67; N, 10.74. Found: C, 52.19; H, 5.69; N, 10.74.
Crystals suitable for X-ray analysis were obtained by recrystallization of the product in acetonitrile.
Experimental details
Data were corrected for absorption using the SADABS program [3]. H atoms were treated by a mixture of independent and constrained refinement. All H atoms, except hydrogen atom of water, were positioned geometrically and refined using riding model, with C(sp2)-H = 0.93 Å, C(sp3)-H = 0.97 Å, O—H = 0.82 Å and with Uiso(H) = 1.2 Ueq(C) and 1.5 Ueq(O), respectively. All the calculations were performed using the WINGX System, Ver 1.70.01 [4].
Discussion
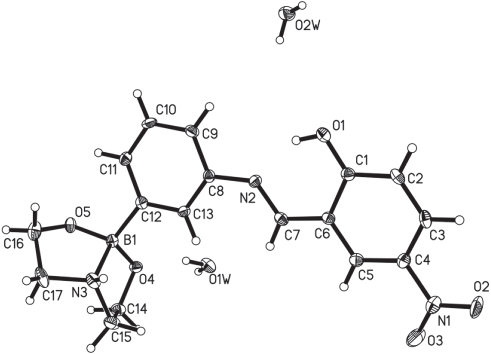
The significant contemporary interest in boronic acids for covalent organic assembly reflects a desire for exploitation of new materials with a wider range of physical properties. Boronic acids have being utilized as building blocks for the construction of molecular nanostructures and polymeric materials [5]. Condensation reactions of boronic acids can give covalent organic frameworks (COFs), which were linked by boroxine or boronate ester units, exhibiting high apparent surface areas and good thermal stabilities [6–8]. Synthetic strategies for the formation of boron-based macrocycles, cages, dendrimers, rotaxanes by multicomponent self-assemblies have also been described [9–11]. For organic boron compounds, the boron center is in a planar sp2 hybridization with vacant p orbital. It can accept lone pair electrons of a Lewis base such as pyridine or other nitrogen-containing ligands to form Lewis acid-base adducts. Schiff base derivatives of 3-aminophenylboronic acid and substituted salicylaldehyde are used to built polymeric structures through donor- acceptor intermolecular interactions. However, Barba et al. found that electron-withdrawing substituents in 3,5-positions of the salicylaldehyde would reduce the ability of nitrogen and oxygen atoms to coordinate to the boron atom [12]. The reason is suggested that the groups with negative inductive effects would reduce the donor character of the nitrogen and oxygen atoms through the π conjugated system. The molecular structure of the title compound can be divided into two parts. Part one is the [(2-hydroxyl-5-nitrophenyl)methylene]amino]phenyl group and the part two is the diethanolamine adduct of boronic acid. The boron atom is in a tetrahedral environment. B—O bond lengths are 1.459(4) and 1.463(4) Å. The dative B—N bond is 1.668(4) Å indicating its coordinating nature. Part one is nearly planar. The dihedral angle between the two phenyl rings is 5.65°. The intermolecular interaction occurs through π−π stacking of part one. The planes are parallel to each other with a distance of 3.38 Å. The hydroxyl and the methylene amino group form an intramolecular hydrogen bond [O1⋯N2 = 2.559 Å, O1—H1⋯N2 = 149°]. Each molecule of title compound contacts to others by an intermolecular hydrogen bond [N3⋯O5 (symmetry code: x, −1+y, z) = 2.2.797 Å, N3—H1A⋯O5 = 167°]. The above mensioned intermolecular π−π stacking contacts and intermolecular hydrogen bond link molucules to form chains. Water molecules are further linked to these chains to form two dimensional layer [O2W⋯O1 = 2.798 Å, O2W—H2WB⋯O1 = 168°; O2W⋯O1W (symmetry code: 1/2+x, 1−y, z) = 2.843 Å, O2W—H2WA⋯O1W = 164°; O1W⋯O2W (symmetry code: −1/2+x, 2−y, z) = 2.762 Å, O1W—H1WB⋯O2W = 173°; O1W⋯O4 (symmetry code: x, 1−y, z) = 2.736 Å, O1W—H1WA⋯O4 = 164°] (Figure 1).
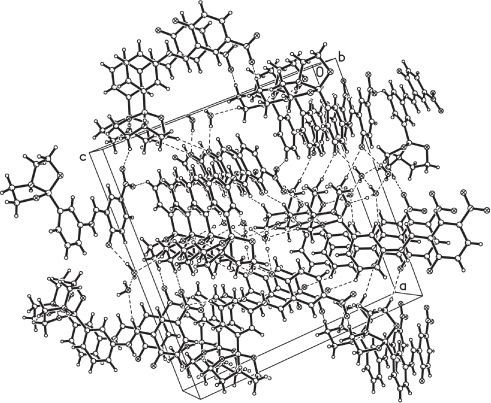
The packing diagram of the title compound, showing supramolecular network.
Acknowledgements:
The authors wish to thank the National Natural Science Foundation of China (No. 21171081/B0103 and No. 20971062/B010303), Natural Science Foundation of Liaoning Province (2013020085 and 201202093) and Shenyang Science and Technology Plan Project (F13–289–1–00), China, for funding.
References
1. Hernández-Molina, R., Mederos, A.: Acyclic and macrocyclic Schiff base ligands, Comprehensive Coordination Chemistry II 1 (2003) 411–446.10.1016/B0-08-043748-6/01070-7Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. Sect. A 64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Bruker, SADABS (Version 2007/4). Bruker AXS Inc., Madison, Wisconsin, USA, 2008.Search in Google Scholar
4. Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 32 (1999) 837–838.10.1107/S0021889899006020Search in Google Scholar
5. Severin, K.: Boronic acids as building blocks for molecular nanostructures and polymeric materials. Dalton Trans. (2009) 5254–5264.10.1039/b902849hSearch in Google Scholar
6. Côté, A. P., Benin, A. I., Ockwig, N. W., O'Keeffe, M., Matzger, A. J., Yaghi, O. M.: Porous, crystalline, covalent organic frameworks. Science 310 (2005) 1166–1170.10.1126/science.1120411Search in Google Scholar
7. El-Kaderi, H. M., Hunt, J. R., Mendoza-Cortés, J. L., Côté, A. P., Taylor, R. E., O'Keeffe, M., Yaghi, O. M.: Designed synthesis of 3D covalent organic frameworks. Science 316 (2007) 268–272.10.1126/science.1139915Search in Google Scholar
8. Wan, S., Guo, J., Kim, J., Ihee, H., Jiang, D.: A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem., Int. Ed. 48 (2009) 5439–5442.10.1002/anie.200900881Search in Google Scholar
9. Christinat, N., Scopelliti, R., Severin, K.: Multicomponent Assembly of Boronic Acid Based Macrocycles and Cages. Angew. Chem., Int. Ed. 47 (2008) 1848–1852.10.1002/anie.200705272Search in Google Scholar
10. Christinat, N., Scopelliti, R., Severin, K.: Boron-based rotaxanes by multicomponent self-assembly. Chem. Commun. (2008) 3660–3662.10.1039/b805437aSearch in Google Scholar
11. Christinat, N., Scopelliti, R., Severin, K.: Multicomponent assembly of boron-based dendritic nanostructures. J. Org. Chem. 72 (2007) 2192–2200.10.1021/jo062607pSearch in Google Scholar
12. Barba, V., Hernández, R., Höfl, H., Santillan, R., Farfán, N.: 3-Aminophenylboronic acid as building block for the construction of calix- and cage-shaped boron complexes. J. Organomet. Chem. 694 (2009) 2127–2133.10.1016/j.jorganchem.2009.02.025Search in Google Scholar
©2016 Chunhua Ge et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2

