Abstract
C29H22F6OS2, monoclinic, P21/c, a = 6.4504(2) Å, b = 16.9752(4) Å, c = 22.8762(6) Å, β = 91.7790(10)°, V = 2503.66(12) Å3, Z = 4, Rgt(F) = 0.0377, wRref(F2) = 0.1156, T = 100(2) K.
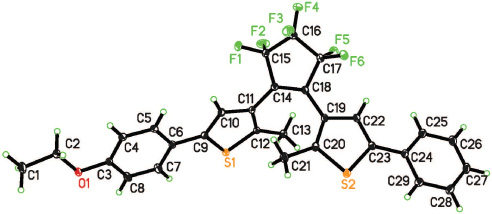
Molecular view of the title compound. Ellipsoids are drawn at 30% probability level.
The crystal structure is shown in the figure, Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless, block, size 0.26×0.28×0.42 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.80 cm−1 |
| Diffractometer, scan mode: | CCD area detector, φ and ω scans |
| 2θmax: | 55° |
| N(hkl)measured, N(hkl)unique: | 21976, 5746 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4806 |
| N(param)refined: | 346 |
| Programs: | SHELX [16] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(1A) | 4e | 1.6534 | 0.6156 | 0.8291 | 0.053 |
| H(1B) | 4e | 1.8022 | 0.6837 | 0.8107 | 0.053 |
| H(1C) | 4e | 1.8097 | 0.6019 | 0.7789 | 0.053 |
| H(2A) | 4e | 1.4777 | 0.7100 | 0.7688 | 0.029 |
| H(2B) | 4e | 1.6262 | 0.6880 | 0.7180 | 0.029 |
| H(4) | 4e | 1.3122 | 0.7311 | 0.6799 | 0.024 |
| H(5) | 4e | 1.0231 | 0.7456 | 0.6194 | 0.024 |
| H(7) | 4e | 0.8637 | 0.5213 | 0.6486 | 0.027 |
| H(8) | 4e | 1.1506 | 0.5065 | 0.7102 | 0.027 |
| H(10) | 4e | 0.7488 | 0.7602 | 0.5593 | 0.024 |
| H(13A) | 4e | 0.1031 | 0.6437 | 0.4942 | 0.031 |
| H(13B) | 4e | 0.1429 | 0.5619 | 0.5244 | 0.031 |
| H(13C) | 4e | 0.2291 | 0.5784 | 0.4623 | 0.031 |
| H(21A) | 4e | 0.7537 | 0.6964 | 0.4256 | 0.038 |
| H(21B) | 4e | 0.8107 | 0.6250 | 0.3858 | 0.038 |
| H(21C) | 4e | 0.6874 | 0.6109 | 0.4428 | 0.038 |
| H(22) | 4e | 0.0708 | 0.7317 | 0.3362 | 0.022 |
| H(25) | 4e | −0.1483 | 0.6842 | 0.2633 | 0.026 |
| H(26) | 4e | −0.3442 | 0.6486 | 0.1809 | 0.031 |
| H(27) | 4e | −0.2082 | 0.5630 | 0.1131 | 0.035 |
| H(28) | 4e | 0.1229 | 0.5142 | 0.1275 | 0.035 |
| H(29) | 4e | 0.3203 | 0.5497 | 0.2094 | 0.029 |
Atomic displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| C(1) | 4e | 1.7209(3) | 0.6397(1) | 0.7968(1) | 0.030(1) | 0.029(1) | 0.045(1) | −0.0018(9) | −0.0200(9) | 0.0005(9) |
| C(2) | 4e | 1.5597(3) | 0.6680(1) | 0.75242(8) | 0.0218(9) | 0.0212(9) | 0.0278(9) | 0.0013(7) | −0.0082(7) | −0.0043(7) |
| C(3) | 4e | 1.2625(3) | 0.6171(1) | 0.70114(7) | 0.0143(8) | 0.0209(8) | 0.0147(8) | 0.0032(6) | −0.0004(6) | −0.0012(6) |
| C(4) | 4e | 1.2223(3) | 0.6889(1) | 0.67380(8) | 0.0210(9) | 0.0155(8) | 0.0243(9) | −0.0007(7) | −0.0029(7) | −0.0023(7) |
| C(5) | 4e | 1.0483(3) | 0.6973(1) | 0.63743(8) | 0.0234(9) | 0.0149(8) | 0.0205(8) | 0.0021(7) | −0.0027(7) | 0.0012(6) |
| C(6) | 4e | 0.9098(3) | 0.6356(1) | 0.62697(7) | 0.0171(8) | 0.0186(8) | 0.0145(8) | 0.0018(7) | 0.0005(6) | 0.0007(6) |
| C(7) | 4e | 0.9529(3) | 0.5637(1) | 0.65490(8) | 0.0197(9) | 0.0207(9) | 0.0277(9) | −0.0042(7) | −0.0050(7) | 0.0070(7) |
| C(8) | 4e | 1.1258(3) | 0.5546(1) | 0.69172(8) | 0.0201(9) | 0.0208(9) | 0.0271(9) | −0.0006(7) | −0.0032(7) | 0.0088(7) |
| C(9) | 4e | 0.7273(3) | 0.6473(1) | 0.58768(7) | 0.0185(8) | 0.0170(8) | 0.0156(8) | −0.0006(7) | −0.0007(6) | −0.0006(6) |
| C(10) | 4e | 0.6724(3) | 0.7138(1) | 0.55731(7) | 0.0238(9) | 0.0178(8) | 0.0176(8) | −0.0019(7) | −0.0049(7) | 0.0005(6) |
| C(11) | 4e | 0.4860(3) | 0.7053(1) | 0.52219(7) | 0.0221(9) | 0.0174(8) | 0.0147(8) | 0.0022(7) | −0.0044(6) | −0.0018(6) |
| C(12) | 4e | 0.3972(3) | 0.6323(1) | 0.52718(7) | 0.0175(8) | 0.0182(8) | 0.0143(8) | 0.0036(6) | −0.0010(6) | −0.0016(6) |
| C(13) | 4e | 0.2004(3) | 0.6013(1) | 0.49955(8) | 0.0189(9) | 0.0219(9) | 0.0205(9) | 0.0005(7) | −0.0019(7) | −0.0011(7) |
| C(14) | 4e | 0.3992(3) | 0.7692(1) | 0.48575(8) | 0.0241(9) | 0.0158(8) | 0.0201(8) | −0.0002(7) | −0.0054(7) | −0.0010(7) |
| C(15) | 4e | 0.3562(4) | 0.8480(1) | 0.51352(8) | 0.045(1) | 0.0185(9) | 0.0205(9) | 0.0039(8) | −0.0116(8) | −0.0023(7) |
| C(16) | 4e | 0.3013(3) | 0.9027(1) | 0.46203(8) | 0.036(1) | 0.0169(9) | 0.025(1) | 0.0044(8) | −0.0066(8) | −0.0003(7) |
| C(17) | 4e | 0.2230(3) | 0.8453(1) | 0.41457(8) | 0.028(1) | 0.0216(9) | 0.0194(9) | 0.0050(8) | −0.0061(7) | 0.0008(7) |
| C(18) | 4e | 0.3323(3) | 0.7685(1) | 0.42914(8) | 0.0201(8) | 0.0166(8) | 0.0197(8) | −0.0002(7) | −0.0047(7) | 0.0012(7) |
| C(19) | 4e | 0.3463(3) | 0.7082(1) | 0.38379(7) | 0.0201(8) | 0.0158(8) | 0.0161(8) | −0.0020(7) | −0.0032(6) | 0.0018(6) |
| C(20) | 4e | 0.5082(3) | 0.6564(1) | 0.37674(7) | 0.0178(8) | 0.0209(9) | 0.0152(8) | −0.0030(7) | −0.0001(6) | 0.0015(6) |
| C(21) | 4e | 0.7078(3) | 0.6463(1) | 0.41078(8) | 0.0175(9) | 0.037(1) | 0.0221(9) | 0.0026(8) | −0.0015(7) | −0.0012(8) |
| C(22) | 4e | 0.1895(3) | 0.7006(1) | 0.33824(7) | 0.0183(8) | 0.0185(8) | 0.0185(8) | 0.0007(7) | −0.0032(7) | 0.0012(6) |
| C(23) | 4e | 0.2302(3) | 0.6435(1) | 0.29816(7) | 0.0172(8) | 0.0165(8) | 0.0165(8) | −0.0022(6) | −0.0004(6) | 0.0040(6) |
| C(24) | 4e | 0.1068(3) | 0.6209(1) | 0.24539(7) | 0.0238(9) | 0.0148(8) | 0.0150(8) | −0.0057(7) | −0.0017(6) | 0.0024(6) |
| C(25) | 4e | −0.0929(3) | 0.6500(1) | 0.23613(8) | 0.0230(9) | 0.0217(9) | 0.0196(8) | −0.0029(7) | −0.0021(7) | −0.0006(7) |
| C(26) | 4e | −0.2108(3) | 0.6286(1) | 0.18672(8) | 0.026(1) | 0.025(1) | 0.025(1) | −0.0030(8) | −0.0078(8) | 0.0027(7) |
| C(27) | 4e | −0.1294(3) | 0.5776(1) | 0.14615(8) | 0.042(1) | 0.023(1) | 0.0201(9) | −0.0070(9) | −0.0115(8) | 0.0007(7) |
| C(28) | 4e | 0.0683(3) | 0.5484(1) | 0.15478(8) | 0.042(1) | 0.024(1) | 0.0200(9) | −0.0004(9) | −0.0036(8) | −0.0055(7) |
| C(29) | 4e | 0.1868(3) | 0.5697(1) | 0.20398(8) | 0.028(1) | 0.0209(9) | 0.0227(9) | 0.0001(8) | −0.0020(7) | −0.0017(7) |
| F(1) | 4e | 0.5140(3) | 0.87658(8) | 0.54642(7) | 0.087(1) | 0.0205(6) | 0.0556(9) | 0.0058(7) | −0.0519(8) | −0.0095(6) |
| F(2) | 4e | 0.1909(3) | 0.84331(8) | 0.54884(6) | 0.085(1) | 0.0314(7) | 0.0271(7) | 0.0181(7) | 0.0163(7) | 0.0015(5) |
| F(3) | 4e | 0.4734(2) | 0.93884(7) | 0.44376(6) | 0.0466(8) | 0.0244(6) | 0.0406(7) | −0.0071(6) | −0.0054(6) | −0.0008(5) |
| F(4) | 4e | 0.1643(2) | 0.95866(7) | 0.47495(5) | 0.0546(8) | 0.0234(6) | 0.0281(6) | 0.0155(6) | −0.0060(5) | −0.0036(5) |
| F(5) | 4e | 0.0124(2) | 0.83709(7) | 0.41781(5) | 0.0269(6) | 0.0312(6) | 0.0341(6) | 0.0089(5) | −0.0091(5) | −0.0060(5) |
| F(6) | 4e | 0.2567(2) | 0.87300(7) | 0.36040(5) | 0.0511(8) | 0.0240(6) | 0.0208(6) | 0.0062(5) | −0.0045(5) | 0.0055(4) |
| O(1) | 4e | 1.4302(2) | 0.60236(7) | 0.73724(5) | 0.0176(6) | 0.0200(6) | 0.0232(6) | 0.0009(5) | −0.0061(5) | −0.0002(5) |
| S(1) | 4e | 0.54413(7) | 0.57411(2) | 0.57437(2) | 0.0179(2) | 0.0155(2) | 0.0176(2) | 0.0007(2) | −0.0014(2) | 0.0012(2) |
| S(2) | 4e | 0.46363(7) | 0.59855(3) | 0.31584(2) | 0.0181(2) | 0.0236(2) | 0.0174(2) | 0.0014(2) | 0.0007(2) | −0.0017(2) |
Source of material
The title compound was prepared according to the literature method [1, 2] in 35.45% yield. Elemental analysis for C29H22F6OS2: calcd: C 61.69, H 3.93%; found: C 61.71, H 3.95%. The title compound crystallized from hexane at room temperature.
Upon irradiation with 297 nm light, the colorless single-crystals of the title structure turn blue quickly. When the blue crystals were dissolved in hexane, the colorless solution also showed a blue color, with an absorption maximum at 575 nm, accompanied with the formation of the closed-ring isomer. Upon irradiation with visible light (≥510 nm), blue crystals return to their colorless state. The absorption spectrum of a hexane solution of such colorless crystals is the same as that of the open-ring form, with an absorption maximum at 292 nm.
Experimental details
The hydrogen atoms were located by geometrically calculations, and their positions and thermal parameters were fixed during the structure refinement.
Discussion
Photochromism is defined as the phenomenon that a compound can be switched between two distinct states with its corresponding isomers by external light-triggered reversible reaction with chemical bond rearrangement which induces electronic as well as geometrical structure changes of the molecules (see the Scheme). Photochormic compounds have been attracted interest due to their promising wide applications in photonic devices, such as optical memory media, full-color display and various photoswitching devices [3]. Among various photochromic compounds, diarylethenes are promising candidates for such applications due to their excellent thermal stability of both isomers, fatigue-resistant property, high sensitivity, rapid response, and reactivity in solid state [4]. On one hand, the photochromic properties of diarylethene is strongly influenced by the substituents at the terminal groups at the aryl moieties [4]. Recently we have reported the photochromic diarylethene bearing different group, such as fluorine, chlorine, methoxy and methyl [5–7]. On the other hand, the application of the light responsive compounds need the solid state – especial crystal-based state – for their easily using [8]. In general, photochromic reactions rarely occur in crystals, [9, 10] because the photochromic reactions require large geometrical structural changes to form the closed-ring isomer. In our previous crystallographic studies of photochromic diarylethene, we have found that the diarylethenes can exhibite the photochromism in crystal states when they are bearing some special heterocyclic groups [11–13]. On the basis of these considerations, we have designed an assymmetrical photochromic diarylethene bearing an ethoxy group.
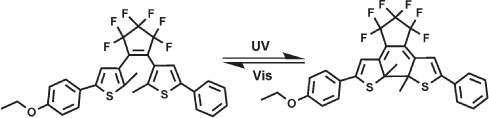
Left: ground state as found in the crystal structure, right: excited state as found in the structure.
The title molecule adopts a photo-active antiparallel conformation (see the Figure). In the cyclopentene ring, the two thiophene rings are linked by the C14=C18 double bond 1.352(2) Å. The two methyl groups are located on different sides of the C14=C18 double bond and this configuration is crucial to allow the compound to exhibit photochromic and photoinduced properties [4]. This geometry allows the breaking of the central hexafluorocyclopentene C=C double bond to form a new C—C single bond and the molecule thus can undergo a phototransition. The dihedral angles between the least-square planes of the cyclopentene (C14, C15, C17, C18) and the adjacent phenyl thienyl ring, ethoxylphenyl thienyl ring are 32.3(5)° and 126.9(6), respectively. The dihedral angle between the least-square planes of phenyl, ethoxylphenyl and their adjacent thienyl rings are 32.3(5)° and 1.9(7)°, respectively. The angle between the thienyl rings is 118.6(4)°.
The intramolecular distance between the two reactive C atoms (C20—C12) is 3.560(2) Å. This distance indicates that the crystal can be expected to undergo photochromism to form the closed ring isomer because photochromic reactivity usually appears when the distance between the reactive C atoms is less than 4.2 Å [14, 15].
Acknowledgements:
This work was supported by the National Natural Science Foundation of China (21363009, 21362013), and the Project of Natural Science Foundation of Jiangxi Province (20132BAB203005, 20142BAB203005) and the Program for the Top Young Innovative Talents in University (2013QNBJRC002).
References
1. Fan, C.-B.; Pu, S.-Z.; Liu, G.; Xu, J.-K.; Zheng, C.-H.: Substituent effect on oxidative electrochemical switching for photochromic asymmetrical dithienylcyclopentene. J. Mater. Sci. & Eng. 25 (2007) 943–949.Search in Google Scholar
2. Fan, C.-B.; Cui, S.-Q.; Pu, S.-Z.; Huang, Q.-L.; Liu, G.: Synthesis, crystal structure and photochromic properties of a new unsymmetrical diarylethene. J. Chem. Res. 37 (2013) 574–578.10.3184/174751913X13756095995841Search in Google Scholar
3. Higashiguchi, K.; Matsuda, K.; Tanifuji, N.; Irie, M.: Full-color photochromism of a fused dithienylethene trimer. J. Am. Chem. Soc. 127 (2005) 8922–8923.10.1021/ja051467iSearch in Google Scholar
4. Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S.: Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114 (2014) 12174–12277.10.1021/cr500249pSearch in Google Scholar
5. Pu, S.-Z.; Yang, T.-S.; Li, G.-Z.; Xu, J.-K.; Chen, B.: Substituent position effect on the optoelectronic properties of photochromic diarylethenes. Tetrahedron Lett. 47 (2006) 3167–3171.10.1016/j.tetlet.2006.02.124Search in Google Scholar
6. Fan, C.-B.; Pu, S.-Z.; Liu, G.; Yang, T.-S.: Substituent position effect on the properties of isomeric photochromic diarylethenes bearing chlorine atoms. J. Photochem. Photobiol. A: Chem. 194 (2008) 333–343.10.1016/j.jphotochem.2007.08.032Search in Google Scholar
7. Pu, S.-Z.; Yang, T.-S.; Xu, J.-K.; Chen, B.: Syntheses and properties of new photochromic diarylethene derivatives having a pyrazole unit. Tetrahedron Lett. 47 (2006) 6473–6477.10.1016/j.tetlet.2006.06.073Search in Google Scholar
8. Kobatake, S.; Hasegawa, H.; Miyamura, K.: High-convertible photochromism of a diarylethene single crystal accompanying the crystal shape deformation. Cryst. Growth Des. 11 (2011) 1223–1229.10.1021/cg101448mSearch in Google Scholar
9. Morimoto, M.; Irie, M.: Photochromism of diarylethene single crystals:crystal structures and photochromic performance. Chem. Commun. 31 (2005) 3895–3905.10.1039/b505256dSearch in Google Scholar
10. Yamada, T.; Kobatake, S.; Muto, K.; Irie, M.: X-ray crystallographic study on single-crystalline photochromism of bis(2,5-dimethyl-3-thienyl)perfluorocyclopentene. J. Am. Chem. Soc. 122 (2000) 1589–1592.10.1021/ja993289xSearch in Google Scholar
11. Fan, C.-B.; Pu, S.-Z.; Liu, G.: Effects of solvents on the growth of an asymmetrical photochromic diarylethene crystal. Dyes Pigments 113 (2015) 61–69.10.1016/j.dyepig.2014.07.023Search in Google Scholar
12. Cui, S.-Q.; Li, H.; Fan, C.-B.: Crystal structure of 1-[5-(3,4-difluorophenyl)-2-methylthiophen-3-yl]-2-(2-cyano-1,5-dimethyl-1H-pyrrol-4-yl)-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C23H14F8N2S. Z. Kristallogr. NCS 228 (2013) 290–292.10.1524/ncrs.2013.0122Search in Google Scholar
13. He, S.-H.; Fan, C.-B.: Crystal structure of 1-[2-n-butyl-5-formyl-3-thienyl]-2-[2-cyano-1,5-dimethyl-4-pyrryl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C21H18F6N2OS. Z. Kristallogr. NCS 228 (2013) 219–220.10.1524/ncrs.2013.0108Search in Google Scholar
14. Woodward, R. B.; Hoffmann, R.: (1970) The conservation of orbital symmetry. Weinheim: Verlag Chemie GmbH.10.1016/B978-1-4832-3290-4.50006-4Search in Google Scholar
15. Kobatake, S.; Irie, M.: Singe-crystalline photochromism of diarylethenes. Bull. Chem. Soc. Jpn. 77 (2004) 195–210.10.1246/bcsj.77.195Search in Google Scholar
16. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2016 Congbin Fan et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2


