Abstract
[C16H34N2Na2O7S4]n, triclinic, P1̄ (no. 2), a = 5.3734(1) Å, b = 11.0473(2) Å, c = 22.2264(5) Å, α = 102.075(2)°, β = 96.202(2)°, γ = 97.383(2)°, V = 1267.06(5) Å3, Z = 2, Rgt(F) = 0.0335, wRref(F2) = 0.0941, T = 100(2) K.
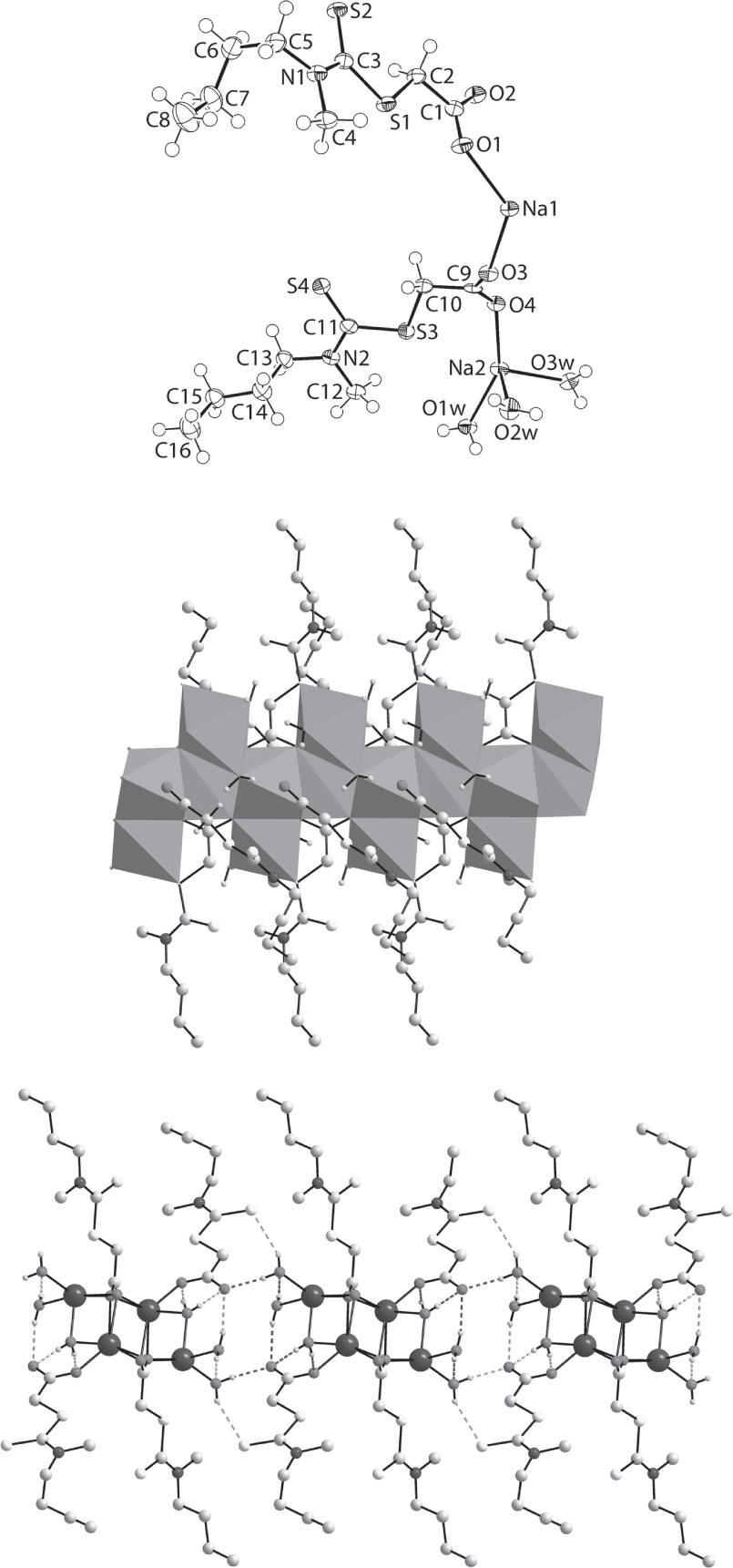
The constituents of the asymmetric unit are shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.17 × 0.04 × 0.02 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 4.12 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θmax, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 30669, 4523, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4210 |
| N(param)refined: | 302 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 1.14745(10) | 0.13521(5) | 0.31092(3) | 0.01677(13) |
| S2 | 1.20238(10) | −0.12848(5) | 0.24774(3) | 0.01810(14) |
| S3 | −0.09303(11) | 0.55782(5) | 0.30283(3) | 0.01951(14) |
| S4 | 0.07497(11) | 0.40808(5) | 0.18787(3) | 0.01860(14) |
| O1 | 0.8756(3) | 0.23449(14) | 0.40388(8) | 0.0185(3) |
| O2 | 0.6459(3) | 0.06077(15) | 0.41707(8) | 0.0195(3) |
| O3 | 0.4094(3) | 0.51778(14) | 0.42949(7) | 0.0163(3) |
| O4 | 0.0078(3) | 0.54681(14) | 0.43245(7) | 0.0158(3) |
| N1 | 1.4657(4) | 0.08311(18) | 0.23274(9) | 0.0171(4) |
| N2 | −0.2638(4) | 0.56527(18) | 0.19006(9) | 0.0175(4) |
| C1 | 0.8092(4) | 0.1187(2) | 0.39188(11) | 0.0158(4) |
| C2 | 0.9367(4) | 0.0355(2) | 0.34436(11) | 0.0173(5) |
| H2A | 0.8070 | −0.0189 | 0.3114 | 0.021* |
| H2B | 1.0333 | −0.0188 | 0.3649 | 0.021* |
| C3 | 1.2883(4) | 0.0276(2) | 0.26040(10) | 0.0156(4) |
| C4 | 1.5381(4) | 0.2203(2) | 0.24791(12) | 0.0197(5) |
| H4A | 1.3916 | 0.2592 | 0.2363 | 0.030* |
| H4B | 1.6758 | 0.2432 | 0.2249 | 0.030* |
| H4C | 1.5950 | 0.2497 | 0.2926 | 0.030* |
| C5 | 1.5901(4) | 0.0086(2) | 0.18504(11) | 0.0204(5) |
| H5A | 1.6369 | −0.0653 | 0.1995 | 0.024* |
| H5B | 1.7481 | 0.0599 | 0.1799 | 0.024* |
| C6 | 1.4227(5) | −0.0357(2) | 0.12220(12) | 0.0250(5) |
| H6A | 1.2690 | −0.0904 | 0.1273 | 0.030* |
| H6B | 1.5155 | −0.0871 | 0.0930 | 0.030* |
| C7 | 1.3407(5) | 0.0692(3) | 0.09316(12) | 0.0287(6) |
| H7A | 1.2108 | 0.0320 | 0.0566 | 0.034* |
| H7B | 1.2616 | 0.1255 | 0.1237 | 0.034* |
| C8 | 1.5590(6) | 0.1466(3) | 0.07287(14) | 0.0369(7) |
| H8A | 1.6828 | 0.1887 | 0.1093 | 0.055* |
| H8B | 1.4935 | 0.2095 | 0.0530 | 0.055* |
| H8C | 1.6409 | 0.0914 | 0.0433 | 0.055* |
| C9 | 0.1897(4) | 0.51760(19) | 0.40392(10) | 0.0131(4) |
| C10 | 0.1442(4) | 0.4765(2) | 0.33315(11) | 0.0163(4) |
| H10A | 0.0865 | 0.3849 | 0.3204 | 0.020* |
| H10B | 0.3040 | 0.4956 | 0.3162 | 0.020* |
| C11 | −0.1018(4) | 0.5105(2) | 0.22109(11) | 0.0166(5) |
| C12 | −0.4020(4) | 0.6599(2) | 0.22157(11) | 0.0194(5) |
| H12A | −0.5205 | 0.6213 | 0.2452 | 0.029* |
| H12B | −0.4964 | 0.6946 | 0.1907 | 0.029* |
| H12C | −0.2818 | 0.7273 | 0.2499 | 0.029* |
| C13 | −0.3042(4) | 0.5392(2) | 0.12204(11) | 0.0197(5) |
| H13A | −0.4853 | 0.5384 | 0.1075 | 0.024* |
| H13B | −0.2621 | 0.4552 | 0.1052 | 0.024* |
| C14 | −0.1418(5) | 0.6370(2) | 0.09735(11) | 0.0223(5) |
| H14A | −0.1658 | 0.7218 | 0.1189 | 0.027* |
| H14B | 0.0391 | 0.6295 | 0.1070 | 0.027* |
| C15 | −0.2070(5) | 0.6224(2) | 0.02784(11) | 0.0229(5) |
| H15A | −0.3840 | 0.6364 | 0.0186 | 0.028* |
| H15B | −0.1959 | 0.5355 | 0.0065 | 0.028* |
| C16 | −0.0323(5) | 0.7133(3) | 0.00220(13) | 0.0309(6) |
| H16A | −0.0551 | 0.7994 | 0.0200 | 0.046* |
| H16B | −0.0737 | 0.6956 | −0.0431 | 0.046* |
| H16C | 0.1442 | 0.7032 | 0.0132 | 0.046* |
| Na1 | 0.70753(15) | 0.40096(8) | 0.46389(4) | 0.01489(19) |
| Na2 | −0.26699(16) | 0.69607(8) | 0.43202(4) | 0.0169(2) |
| O1W | −0.5177(3) | 0.81183(15) | 0.38060(8) | 0.0201(3) |
| H1W | −0.587(5) | 0.800(3) | 0.3438(6) | 0.030* |
| H2W | −0.450(5) | 0.8867(13) | 0.3939(12) | 0.030* |
| O2W | 0.0821(3) | 0.85602(17) | 0.45241(8) | 0.0247(4) |
| H3W | 0.135(5) | 0.891(3) | 0.4898(6) | 0.037* |
| H4W | 0.206(4) | 0.839(3) | 0.4337(12) | 0.037* |
| O3W | −0.3720(3) | 0.75892(15) | 0.53094(8) | 0.0184(3) |
| H5W | −0.247(4) | 0.755(3) | 0.5561(10) | 0.028* |
| H6W | −0.447(5) | 0.817(2) | 0.5469(12) | 0.028* |
Source of material
All chemicals and solvents were used as purchased without purification. The melting point was determined using a Mel-temp II digital melting point apparatus and was uncorrected. The solid-state IR spectrum was obtained on a Bruker Vertex 70v FTIR Spectrometer from 4000 to 400 cm−1. The 1H and 13C{1H} NMR spectra were recorded at room temperature in CDCl3 solution on a Bruker Ascend 400 MHz NMR spectrometer with chemical shifts relative to tetramethylsilane.
The dithiocarbamate ligand was prepared in situ (acetone) from the reaction of CS2 (Merck, 0.25 mmol) with n-butylmethylamine (Merck, 0.25 mmol) and NaOH (0.02 mL; 50% w/v); CS2 was added dropwise into the methanol solution (10 mL). The resulting mixture solution was kept at 273 K for 1 h. Sodium chloroacetate (Merck, 0.03 g, 0.25 mmol) was added into the solution. The filtrate was evaporated slowly until a white precipitate was formed. The precipitate was washed with n-hexane and recrystallized from a methanol-acetone solution. Colourless crystals of the title salt were obtained from the slow evaporation of the solvent. Yield: 0.027 g (20.0%). M.pt: >623 K. IR (cm−1) 1570 (s) ν(C—O), 1486 (s) ν(C—N), 1382 (s) ν(C—N), 1117 (m) ν(C—O), 1012 (m) ν(C—S), 980 (m) ν(C—S). 1H NMR (CDCl3, p.p.m.): δ 0.93 (s, 3H, CH3), 1.20–1.44 (m, 4H, CH2CH2), 2.80–2.82 (m, 2H, CH2), 2.87–2.90 (m, 6H, water-OH), 3.44 (s, 3H, NCH3), 4.00–4.04 (m, 4H, NCH2). 13C{1H} NMR (CDCl3, p.p.m.): 13.9 (CH3), 20.0, 28.5 (CH2CH2), 40.0 (SCH2), 43.6 (NCH3), 57.0 (NCH2), 175.2 (CO), 197.2 (CS2).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.98–0.99 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The O-bound H-atoms were located in a difference Fourier map but were refined with a distance restraint O—H = 0.84 ± 0.01 Å, and with Uiso(H) set to 1.5Ueq(O). A number of reflections were omitted from the final cycles of refinement owing to poor agreement; details are given in the CIF.
Comment
The title sodium salt of a hybrid dithiocarbamate ester of a carboxylic acid, isolated as a trihydrate, was investigated as a part of on-going studies of the structural chemistry of these molecules [5] and their organotin derivatives [6]. The motivation for these studies is to ultimately investigate the biological potential of the organotin derivatives, as both organotin carboxylates [7] and organotin dithiocarbamates [8] are known to exhibit a range of pharmaceutical potential. Indeed, a very recent publication highlighted the potential anti-tumour activity of several organotin species containing these molecules [9]. The structure of the substituted tribenzyl tin complex comprising the same dithiocarbamate anion reported in this publication has also been reported in the literature [10].
The constituents comprising the asymmetric unit of the title salt hydrate are shown in the upper view of the figure (70% displacement ellipsoids) and comprise two independent sodium cations, two n-Bu(Me)NC(=S)SCH2CO2 anions and three water molecules of crystallization. The pattern in C—S bond lengths, with C3—S3 [1.682(2) Å] being significantly shorter than C3—S1 [1.762(2) Å] and C2—S1 [1.804(2) Å] match those involving the S3 and S4 atoms [1.675(2), 1.776(2) and 1.802(2) Å, respectively. Further, they follow the pattern established in another dithiocarbamate ester, i.e. recently determined MeSC(=S)N(Me)Ph [11], with equivalent values being 1.6590(18), 1.7662(17) and 1.789(2) Å. Further, the pattern in the angles about the C3 atom, with the wider angles subtended by the S2 atom [S1—C3—S2 = 122.81(13) Å and S2—C3—N1 = 124.12(17) Å cf. S1—C3—N1 = 113.06(16) Å] is consistent with the presence of a C3=S2 and C11=S4 thione bonds, as the equivalent angles about the C11 atom follow the same trends [S3—C11—S4 = 122.93(14)°, S4—C11—N2 = 124.66(18)° and S3—C11—N2 = 112.41(17)°]. Further, in a recently authenticated dithiocarbamate anion, −S2CN(CH2CH2)2NPh [12], the angles about the quaternary-carbon atom spanned a very narrow range 119.45(8) to 120.57(10)°, consistent with significant delocalization of π-electron density over the CS2 chromophore, clearly absent in the anions of the title compound. The above descriptors are consistent with the presence of carboxylate groups in the anions. However, the C—O bond lengths are not equivalent [C1—O1, O2 = 1.247(3) and 1.267(3) Å; C9—O3, O4 = 1.253(3) and 1.261(3) Å]. As discussed below, these variations are related to the different interactions the carboxylate-O atoms have with the sodium cations and in the supramolecular assembly. There is a significant difference in the conformations of the carboxylate ligands. While the CO2 and CS2 residues are close to co-planar in the O1-carboxylate anion, with the CO2/CS2 dihedral angle being 4.5(5)°, these are inclined in the O3-carboxylate anion with the CO2/CS2 dihedral angle being 32.55(16)°. This conformational difference arises from variable twists about the O1—C1—C2—S1 [−5.0(3)°] and O3—C9—C10—S3 torsion angles [−148.67(16)°]. The other conformational difference in the carboxylate anions relates to the n-butyl groups. Thus, in the O1-carboxylate anion, the N1—C5—C6—C7 [60.2(3)°] and C5—C6—C7—C8 [68.2(3)°] torsion angles are indicative of + syn-clinal conformation whereas the equivalent N2—C13—C14—C15 [−172.0(2)°] and C13—C14—C15—C16 [−175.6(2)°] torsion angles indicate an – anti-periplanar conformation.
The sodium cations have quite distinct donor sets and coordination geometries. The Na1 cation is coordinated by six oxygen atoms, five of which are carboxylate-O and the sixth being a water-O atom. The Na1—O bond lengths range from 2.3443(18) Å, for Na1—O3, to 2.5370(18) Å for Na1—O4i [symmetry operation (i) 1 – x, 1 – y, 1 – z]. The O6 donor set defines a distorted trigonal prismatic geometry. By contrast, the Na2 cation is coordinated within a O5S donor set defined by two carboxylate-O, three water-O and thioester-S atoms. The Na2—O bonds range from 2.3161(19) Å, for Na2—O3w, to 2.4410(18) Å, for Na2—O3ii [(ii) −1 + x, y, z] and define a square-pyramidal geometry. The S3 atom occupies the sixth site [Na2—S3 = 3.2502(11) Å] leading to a distorted octahedral geometry. As seen in the middle view of the figure, the aforementioned connections give rise to a one-dimensional chain along the a axis with edge-shared coordination polyhedra. The inner polyhedra encompass the Na1-cations and the outer polyhedra contain the Na2-cations. Additional stability to the one-dimensional coordination polymer arises from hydrogen bonding interactions of the type water-O—H⋯O(carboxylate, water) [O2w—H3w⋯O2i: H3w⋯O2i = 2.192(16) Å, O2w⋯O2i = 3.003(2) Å with angle at H3w = 162(3)°; O2w—H4w⋯O1wiii: H4w⋯O1wiii = 2.01(2) Å, O2w⋯O1wiii =2.845(2) Å, with angle at H4w = 172(3)°; O3w—H5w⋯O1i: H5w⋯O1i = 2.07(2) Å, O3w⋯O1i = 2.879(2) Å with angle at H5w = 161(2)° and O3w—H6w⋯O2iv: H6w⋯O2iv = 1.93(3) Å, O3w⋯O2iv = 2.762(2) Å with angle at H6w = 175(3)° for (iii) 1 + x, y, z and (iv) −x, 1 − y, 1 − z]. The connections between the chains along the b axis to form a supramolecular layer are of the type water-O—H⋯S(thione) and water-O—H⋯O(carboxylate) O1w—H1w⋯S2v: H1w⋯S2v = 2.62(2) Å, O1w⋯S2v = 3.3952(18) Å with angle at H1w = 154(3)° and O1w—H2w⋯O2vi: H2w⋯O2vi = 1.872(17) Å, O1w⋯O2vi = 2.699(2) Å with angle at H2w = 170(3)° for (v) −2 + x, 1 + y, z and (vi) −1 + x, 1 + y, z]. The layers stack along the c axis direction without directional interactions between them.
Further analysis of the molecular packing was performed using Crystal Explorer 17 [13] to calculate the Hirshfeld surfaces (including the full and delineated two-dimensional fingerprint plots) for the specified asymmetric unit (see figure), following standard procedures [14]. Reflecting, to a large extent, the hydrophobic contacts along the c axis, H⋯H contacts make the greatest contribution to the overall Hirshfeld surface, at 52.5%. The next most significant contribution to the surface contacts are S⋯H/H⋯S at 17.1% followed closely by O⋯H/H⋯H [14.6%], then Na⋯O/O⋯Na [7.7%] and C⋯H/H⋯C [3.9%] contacts. An accompanying structural report of a closely related sodium salt, Na[S2CN(Me)n-Bu]⋅H2O [15], also adopts a layer structure with the layers separated by hydrophobic interactions. The percentage contribution by H⋯H contacts to the Hirshfeld surface also computes to 52.5% in this crystal [15].
Acknowledgements
Sunway University Sdn Bhd is thanked for financial support of this work through Grant No. STR-RCTR-RCCM-001–2019.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO. Rigaku Corporation, Oxford, UK (2018).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
5. Lee, S. M.; Azizan, A. H. S.; Lo, K. M.; Tan, S. L.; Tiekink, E. R. T.: 2-{[bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid. Molbank 2019 (2019) M1082 (seven pages).10.3390/M1082Suche in Google Scholar
6. Lee, S. M.; Lo, K. M.; Tiekink, E. R. T.: Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio)acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio) acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4. Z. Kristallogr. NCS 234 (2019) 943–946.10.1515/ncrs-2019-0161Suche in Google Scholar
7. Gielen, M.; Tiekink, E. R. T.: Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. (Eds. Gielen, M. and Tiekink, E. R. T.), p. 421–439. John Wiley & Sons Ltd., Chichester, England (2005).10.1002/0470864052Suche in Google Scholar
8. Tiekink, E. R. T.: Tin dithiocarbamates: applications and structures. Appl. Organomet. Chem. 22 (2008) 533–550.10.1002/aoc.1441Suche in Google Scholar
9. Anasamy, T.; Thy, C. K.; Lo, K. M.; Chee, C. F.; Yeap, S. K.; Kamalidehghan, B.; Chung, L. Y.: Tribenzyltin carboxylates as anticancer drug candidates: effect on the cytotoxicity, motility and invasiveness of breast cancer cell lines. Eur. J. Med. Chem. 125 (2017) 770–783.10.1016/j.ejmech.2016.09.061Suche in Google Scholar PubMed
10. Keng, T. C.; Lo, K. M.; Ng, S. W.: {[(N-Butyl-N-methylcarbamothioyl)sulfanyl]acetato-κO}tris(2-chlorobenzyl) tin(IV). Acta Crystallogr. E66 (2010) m307.10.1107/S1600536810005830Suche in Google Scholar PubMed PubMed Central
11. Lo, K. M.; Lee, S. M.; Tiekink, E. R. T.: Crystal structure of N-methyl-N-phenyl(methylsulfanyl) carbothioamide, C9H11NS2. Z. Kristallogr. NCS 234 (2019) 1325–1327.10.1515/ncrs-2019-0511Suche in Google Scholar
12. Lo, K. M.; Lee, S. M.; Tiekink, E. R. T.: Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]. Z. Kristallogr. NCS 234 (2019) 1329–1331.10.1515/ncrs-2019-0512Suche in Google Scholar
13. Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Crystal Explorer v17. The University of Western Australia, Australia (2017).Suche in Google Scholar
14. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T.: Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E75 (2019) 308–318.10.1107/S2056989019001129Suche in Google Scholar PubMed PubMed Central
15. Lo, K. M.; Lee, S. M.; Zaldi, N. B.; Hussen, R. S. D.; Tiekink, E. R. T.: Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n. Z. Kristallogr. NCS 234 (2019) 1333–1335.10.1515/ncrs-2019-0514Suche in Google Scholar
©2019 Kong Mun Lo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Artikel in diesem Heft
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n

