Abstract
C63H39Cl12Eu2N6O8, triclinic, P1̄ (no. 2), a = 10.720(5) Å, b = 12.232(5) Å, c = 14.267(5) Å, α = 65.288(32)°, β = 71.325(5)°, γ = 88.067(5)°, V = 1599.1(11) Å3, Z = 1, Rgt(F) = 0.0358, wRref(F2) = 0.0785, T = 293(2) K.
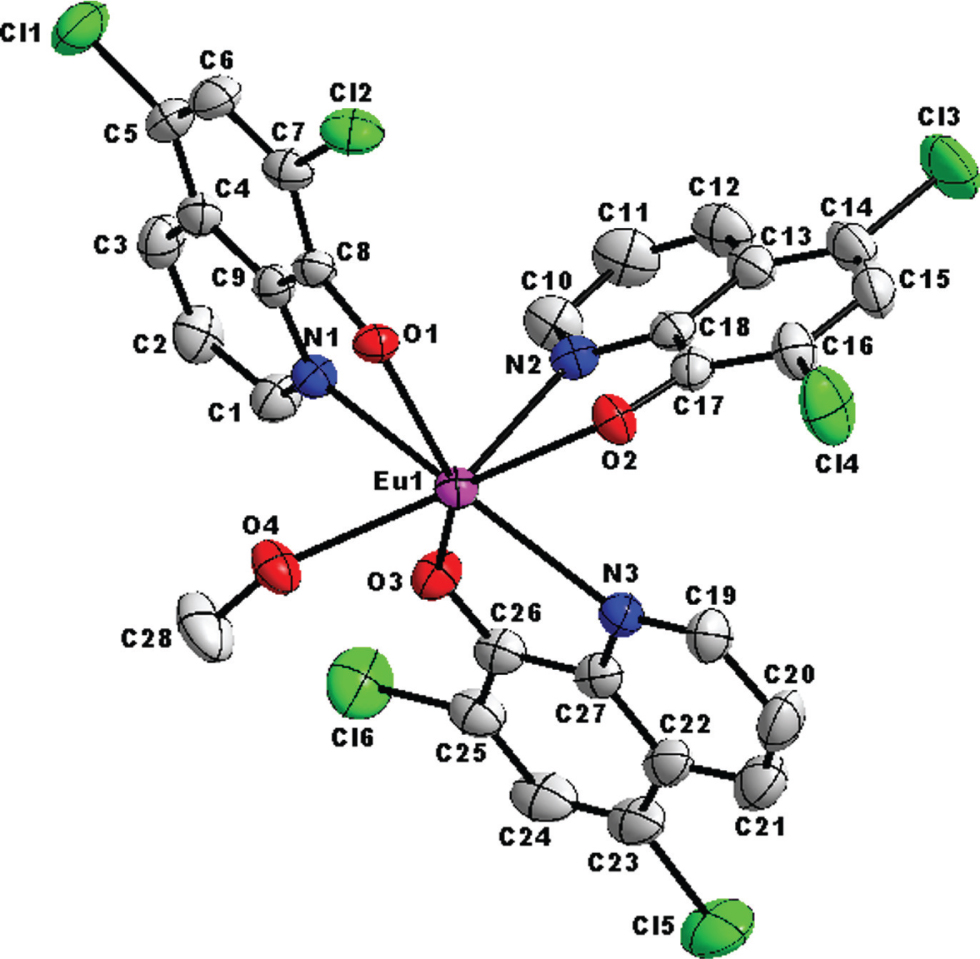
The asymmetric unit of the dinuclear title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.22 × 0.19 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.51 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 28°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 34241, 7633, 0.077 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6291 |
| N(param)refined: | 48 |
| Programs: | Bruker programs [1], SIR97 [2], OLEX2 [3], SHELX [4], DIAMOND [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Eu1 | 0.54445(2) | 0.54657(2) | 0.34215(2) | 0.02670(6) |
| O1 | 0.3651(2) | 0.4942(2) | 0.51137(16) | 0.0282(5) |
| O2 | 0.5502(2) | 0.7324(2) | 0.35541(16) | 0.0329(6) |
| O3 | 0.6046(2) | 0.5072(2) | 0.19228(17) | 0.0368(6) |
| O4 | 0.5610(3) | 0.3291(2) | 0.42868(19) | 0.0416(7) |
| H4 | 0.524(4) | 0.304(4) | 0.491(3) | 0.062* |
| N1 | 0.3384(3) | 0.4253(2) | 0.3613(2) | 0.0307(7) |
| N2 | 0.4262(3) | 0.7128(3) | 0.2262(2) | 0.0318(7) |
| N3 | 0.7478(3) | 0.6933(3) | 0.1754(2) | 0.0338(7) |
| Cl1 | −0.15406(11) | 0.26395(11) | 0.58949(9) | 0.0602(3) |
| Cl2 | 0.13815(10) | 0.52058(10) | 0.68640(7) | 0.0450(2) |
| Cl3 | 0.36730(15) | 1.17002(11) | 0.07931(10) | 0.0748(4) |
| Cl4 | 0.64713(14) | 0.96320(10) | 0.34746(10) | 0.0686(4) |
| Cl5 | 1.09450(13) | 0.72247(13) | −0.18544(9) | 0.0800(4) |
| Cl6 | 0.65696(14) | 0.40191(12) | 0.03112(9) | 0.0680(4) |
| C1 | 0.3285(4) | 0.3817(3) | 0.2935(3) | 0.0394(9) |
| H1 | 0.4022 | 0.3955 | 0.2319 | 0.047* |
| C2 | 0.2139(4) | 0.3156(3) | 0.3083(3) | 0.0431(10) |
| H2 | 0.2135 | 0.2838 | 0.2595 | 0.052* |
| C3 | 0.1024(4) | 0.2988(3) | 0.3961(3) | 0.0418(9) |
| H3 | 0.0242 | 0.2579 | 0.4060 | 0.050* |
| C4 | 0.1076(4) | 0.3439(3) | 0.4708(3) | 0.0346(8) |
| C5 | −0.0011(4) | 0.3351(3) | 0.5631(3) | 0.0384(9) |
| C6 | 0.0103(4) | 0.3869(4) | 0.6278(3) | 0.0425(10) |
| H6 | −0.0634 | 0.3833 | 0.6859 | 0.051* |
| C7 | 0.1316(4) | 0.4455(3) | 0.6079(3) | 0.0346(8) |
| C8 | 0.2464(3) | 0.4491(3) | 0.5259(2) | 0.0279(7) |
| C9 | 0.2289(3) | 0.4040(3) | 0.4528(2) | 0.0299(8) |
| C10 | 0.3607(4) | 0.7026(4) | 0.1661(3) | 0.0443(10) |
| H10 | 0.3495 | 0.6274 | 0.1664 | 0.053* |
| C11 | 0.3064(5) | 0.8003(4) | 0.1011(3) | 0.0531(11) |
| H11 | 0.2582 | 0.7879 | 0.0619 | 0.064* |
| C12 | 0.3248(5) | 0.9111(4) | 0.0960(3) | 0.0521(12) |
| H12 | 0.2897 | 0.9757 | 0.0530 | 0.063* |
| C14 | 0.4269(5) | 1.0404(4) | 0.1564(3) | 0.0498(12) |
| C15 | 0.5023(5) | 1.0501(4) | 0.2126(3) | 0.0494(11) |
| H15 | 0.5241 | 1.1254 | 0.2083 | 0.059* |
| C16 | 0.5484(4) | 0.9464(3) | 0.2780(3) | 0.0422(10) |
| C17 | 0.5171(4) | 0.8321(3) | 0.2890(3) | 0.0319(8) |
| C18 | 0.4457(4) | 0.8247(3) | 0.2225(2) | 0.0321(8) |
| C13 | 0.3973(4) | 0.9286(3) | 0.1561(3) | 0.0398(10) |
| C19 | 0.8195(4) | 0.7810(3) | 0.1714(3) | 0.0434(10) |
| H19 | 0.7894 | 0.8071 | 0.2266 | 0.052* |
| C20 | 0.9408(4) | 0.8372(4) | 0.0861(4) | 0.0570(12) |
| H20 | 0.9899 | 0.8984 | 0.0861 | 0.068* |
| C21 | 0.9855(4) | 0.8016(4) | 0.0040(3) | 0.0546(12) |
| H21 | 1.0652 | 0.8389 | −0.0524 | 0.066* |
| C22 | 0.9123(4) | 0.7089(4) | 0.0038(3) | 0.0404(9) |
| C23 | 0.9473(4) | 0.6618(4) | −0.0747(3) | 0.0486(12) |
| C24 | 0.8709(4) | 0.5702(4) | −0.0654(3) | 0.0509(12) |
| H24 | 0.8968 | 0.5410 | −0.1186 | 0.061* |
| C25 | 0.7539(4) | 0.5186(4) | 0.0227(3) | 0.0416(10) |
| C26 | 0.7110(4) | 0.5569(3) | 0.1060(2) | 0.0337(8) |
| C27 | 0.7909(4) | 0.6556(3) | 0.0940(2) | 0.0331(8) |
| C28 | 0.5864(6) | 0.2385(4) | 0.3909(4) | 0.0694(16) |
| H28A | 0.6223 | 0.2756 | 0.3123 | 0.104* |
| H28B | 0.5053 | 0.1883 | 0.4146 | 0.104* |
| H28C | 0.6490 | 0.1899 | 0.4201 | 0.104* |
| C29a | −0.034(2) | 0.0017(17) | 0.7046(13) | 0.173(8) |
| H29Aa | −0.1262 | −0.0185 | 0.7500 | 0.259* |
| H29Ba | −0.0058 | 0.0834 | 0.6874 | 0.259* |
| H29Ca | 0.0177 | −0.0526 | 0.7430 | 0.259* |
| C30 | −0.0176(8) | −0.0096(6) | 0.6003(6) | 0.1002(17) |
| C31 | 0.1062(8) | −0.0082(6) | 0.5351(6) | 0.1118(17) |
| H31 | 0.1763 | −0.0136 | 0.5614 | 0.134* |
| C32 | 0.1315(8) | 0.0009(6) | 0.4321(6) | 0.1178(19) |
| H32 | 0.2163 | 0.0015 | 0.3867 | 0.141* |
aOccupancy: 0.5.
Source of materials
In this two-step synthesis, [tris-(5,7-dichloro-8-hydroxyquinoline)(H2O)2Eu(III)] was prepared first by dissolving 5,7-dichloro-8-hydroxyquinoline (0.707 g, 3.33 mmol) in 15 mL of absolute ethanol and then reacting with EuCl3 ⋅ 6H2O (0.308 g, 0.84 mmol) in 20 mL of distilled water. NaOH (0.266 g, 6.5 mmol) in 10 mL of distilled water was added after one hour. Yellow-orange cubic crystals were obtained from ethanol solvent after one week. Yield: 0.4747 g, 68%. UV-Vis (nm; L mol−1cm−1): λmax = 408, ϵ = 5 E-3.
In the second reaction step [(5,7-dichloro-8-hydroxyquinoline)(H2O)2Eu(III)] (1 mg, 1.21 mmol) in 20 mL of toluene was refluxed with 2,2′-bipyridine (1.9 mg) for six hours with vigorous stirring using a Dean Stark setup to remove excess water. The colour of the solution turned yellow-orange after a while. The reaction solution was filtered and layered with methanol and then left to crystallize. Yellowish crystals precipitated within a few hours.
Experimental details
The aromatic H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.95 and 0.98 Å and Uiso(H) = 1.5 Ueq(C) and 1.2 Ueq(C), respectively. The placement of the H atoms of the methyl group was that of an idealised methyl group according to the electron-density map (HFIX 137). The highest peak is 0.87 e. Å−3 and deepest hole is −0.69 e. Å−3.
Comment
There are various uses of lanthanides for an array of applications across many scientific spheres. The broader spectrum of applications for this series encapsulates magnetic studies [6], [7], nuclear chemistry [8], catalysis [9], [10], radio-therapy [11], [12], ceramic’s [13], [14] and many others. Europium ions are extensively utilised in photoluminescence research, particularly as a potential photo-emissive layer in the edifice of optoelectronic devices [15], [16], [17]. Owing to its luminescent nature [18], the europium ion conjugated to bulky multi-dentate organic matrices, which are also used as bio-imaging agents in biomedical sciences [19], [20]. The use of organic matrices coordinated to the europium ion is primarily to induce photo-sensitization since the metal ion itself exhibits slow emission rates and low absorption coefficients [21]. The whole phenomenon of using organic chromophores to sensitize the europium ion is affectionately known as the antenna effect [22], [23]. This has certainly become a trend of coordinating ligands on the immediate outer shell of the metal ion to access its metallic effect and/or characteristics with respect to various scientific researches undertaken [24], [25].
The dinuclear crystal structure was solved in the centrosymmetric space group, P1̄, with only half a complex in the asymmetric unit. It has an inversion centre which propagates right between the two europium metal centres justifying the dimeric nature of the two joint isostructural monomeric halves. This dimeric behaviour was also observed by Y.-C. Liu et al. with zinc [26]. The crystal structure is stabilized by inter- and intra-molecular interactions. The intra-molecular hydrogen interactions occur between the para-substituted chlor substituents (Cl1, Cl3 and Cl5) with the nearby hydrogen atoms riding on C3, C12 and C21 respectively. There are three sets of bifurcation networks observed in this crystal structure. The first bifurcation occurs on oxygen O2 with hydrogen atoms riding on oxygen O4 of the coordinated methanol solvent and carbon C19 of the pyridyl ring of the ligand respectively making an angle of 128.88°. Furthermore, Cl1 and Cl5 undergo 90° bifurcation with hydrogen atoms riding on C3, C29 (H29B) and C21, C29 (H29C) respectively. There is half a molecule of the toluene solvent trapped in the asymmetric unit cell, also affected by the inversion centre. Subsequently, the para-substituted carbon of this solvent is disordered (50:50%) equally over two positions.
Acknowledgements
This work is based on the research supported in part by the National Research Foundation of South Africa (Gran UID: 99139) The authors would also like to thank the University of the Free State for financial support.
References
1. Bruker, SAINT-Plus (Version 7.12), Bruker AXS Inc., Madison, WI, USA (2004).Search in Google Scholar
2. Altomare, A.; Burla, M. C.; Camalli, M.; Cascarano, G. L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A. G. G.; Polidori, G.; Spagna, R.: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 32 (1999) 115–119.10.1107/S0021889898007717Search in Google Scholar
3. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst., 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
4. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. Sect. A. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
5. Brandenbug, K.; Putz, H.; DIAMOND. Visual crystal structure information system; Version 3.0c Crystal Impact GbR, Bonn, Germany (2005).Search in Google Scholar
6. Akkus, T.; Uğurlu, M.; Demir, L.: Variation of coherent to Compton scattering differential cross-section ratios of some lanthanides with the external magneticfield at 59.54 keV. Result Phys. 13 (2019) 1–4.10.1016/j.rinp.2019.102265Search in Google Scholar
7. Soek, R. N.; Ferreira, C. M.; Santana, F. S.; Hughes, D. L.; Poneti, G.; Ribeiro, R. R.; Nunes, F. S.: Structure and magnetic properties of two new lanthanide complexes with the 1-((E)-2-pyridinylmethylidene)semicarbazone ligand. J. Mol. Struct. 1184 (2019) 254–261.10.1016/j.molstruc.2019.02.036Search in Google Scholar
8. Bunzli, J. C. G.: Lanthanide probes in life, Chemical and earth sciences. Elsevier, Netherlands (1989).Search in Google Scholar
9. Shibasaki, M.; Yoshikawa, N.: Lanthanide complexes in multifunctional asymmetric catalysis. Chem. Rev. 102 (6) (2002) 2187–2210.10.1021/cr010297zSearch in Google Scholar
10. Mikami, K.; Terada, M.; Matsuzawa, H.: Asymmetric catalysis by lanthanide complexes. Angew. Chem., Int. Ed. 41 (2002) 3554–3571.10.1002/1521-3773(20021004)41:19<3554::AID-ANIE3554>3.0.CO;2-PSearch in Google Scholar
11. Fricker, S. P.: The therapeutic application of lanthanides. Chem. Soc. Rev. 35 (2006) 524–533.10.1039/b509608cSearch in Google Scholar
12. Kostova, I.: Lanthanides as anticancer agents. Curr. Med. Chem. – Anti-Cancer Agents. 5 (2005) 591–602.10.2174/156801105774574694Search in Google Scholar
13. Bardez-Giboire, I.; Kidari, A.; Magnin, M.; Dussossoy, J.; Peuget, S.; Caraballo, R.; Tribet, M.; Doreau, F.; Jegou, C.: Americium and trivalent Lanthanides incorporation in high-level waste glass-ceramics. J. Nucl. Mater. 492 (2017) 231–238.10.1016/j.jnucmat.2017.05.045Search in Google Scholar
14. Crum, J. V.; Turo, L.; Riley, B.; Tang, M.; Kossoy, A.: Multi–phase glass–ceramics as a waste form for combined fission products: Alkalis, alkaline earths, lanthanides, and transition metals. J. Am. Ceram. Soc. 95 (4) (2012) 1297–1303.10.1111/j.1551-2916.2012.05089.xSearch in Google Scholar
15. Eliseeva, S. V.; Bunzli, J. C.: Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 39 (1) (2010) 189–227.10.1039/B905604CSearch in Google Scholar
16. Gallardo, H.; Braga, H. C.; Tuzimoto, P.; Bortoluzzi, A.; Salla, C. A. M.; Bechtold, I. H.; Martins, J. S.; Legnani, C.; Quirino, W. G.: Synthesis, structure and OLED application of a new europium(III)complex:{tris (thenoyltrifluoroacetonate)[1,2,5]selenadiazolo[3,4f][1,10]phenanthroline} europium(III). Inorgica Chim. Acta. 473 (2018) 75–82.10.1016/j.ica.2017.12.034Search in Google Scholar
17. Biju, S.; Raj, D. B. A.; Reddy, M. L. P.; Kariuki, B. M.: Synthesis, crystal structure, and luminescent properties of novel Eu3+ heterocyclic β-diketonate complexes with bidentate nitrogen donors. Inorg. Chem. 45 (2006) 10651–10660.10.1021/ic061425aSearch in Google Scholar PubMed
18. Werts, M. H. V.: Making sense of lanthanide luminescence. Sci. Progress. 88 (2) (2005) 101–131.10.3184/003685005783238435Search in Google Scholar PubMed
19. Amoroso, A. J.; Pope, S. J.: Using lanthanide ions in molecular bio-imaging. Chem. Soc. Rev. 44 (14) (2015) 4723–4742.10.1039/C4CS00293HSearch in Google Scholar PubMed
20. Bunzli, J. C.; Piguet, C.: Taking advantage of luminescent lanthanide ions. Chem. Soc. rev. 34 (12) (2005) 1048–1077.10.1039/b406082mSearch in Google Scholar PubMed
21. Im, S. Y.; Go, D. H.; Ryu, J. G.; Kim, Y. S.: New Narrow-Band Luminescence Using Lanthanide Coordination Compounds for Light-Emitting Diodes. IEICE Trans. Electronics. E100.C (11) (2017) 1021–1025.10.1587/transele.E100.C.1021Search in Google Scholar
22. Bünzli, J. C. G.: On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 293–294 (2015) 19–47.10.1016/j.ccr.2014.10.013Search in Google Scholar
23. Alpha, B.; Ballardini, R.; Balzani, V.; Lehn, J. M.; Perathoner, S.; Sabbatini, N.: Antenna effect in luminescent lanthanide cryptates: a photophysical study. Photochem. Photobiol. 52 (2) (1990) 299–306.10.1111/j.1751-1097.1990.tb04185.xSearch in Google Scholar
24. Suli, L. M.; Ibrahim, W. H. W.; Aziz, B. A.; Deraman, M. R.; Ismail, N. A.: A review of rare earth mineral processing technology, Chem. Eng. Res. Bull. 19 (2013) 20–35.10.3329/cerb.v19i0.33773Search in Google Scholar
25. Bünzli, J. C. G.; Eliseeva, S. V.: Photophysics of lanthanoid coordination compounds. Elsevier (2013) 339–398.10.1016/B978-0-08-097774-4.00803-2Search in Google Scholar
26. Liu, Y.-C.; Wei, J.-H.; Chen, Z.-F.; Liu, M.; Gu, Y.-Q.; Huang, K.-B.; Li, Z.-Q.; Liang, H.: The antitumor activity of zinc(II) and copper(II) complexes with 5,7-dihalo-substituted-8-quinolinoline. Eur. J. Med. Chem. 69 (2013) 554–563.10.1016/j.ejmech.2013.08.033Search in Google Scholar PubMed
© 2019 Orbett T. Alexander et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n


