Abstract
C19H24ClNS2Sn, triclinic, P1̄ (no. 2), a = 10.1894(1) Å, b = 14.0236(2) Å, c = 14.5114(2) Å, α = 91.070(1)°, β = 96.997(1)°, γ = 98.222(1)°, V = 2035.59(5) Å3, Z = 4, Rgt(F) = 0.0211, wRref(F2) = 0.0556, T = 100(2) K.
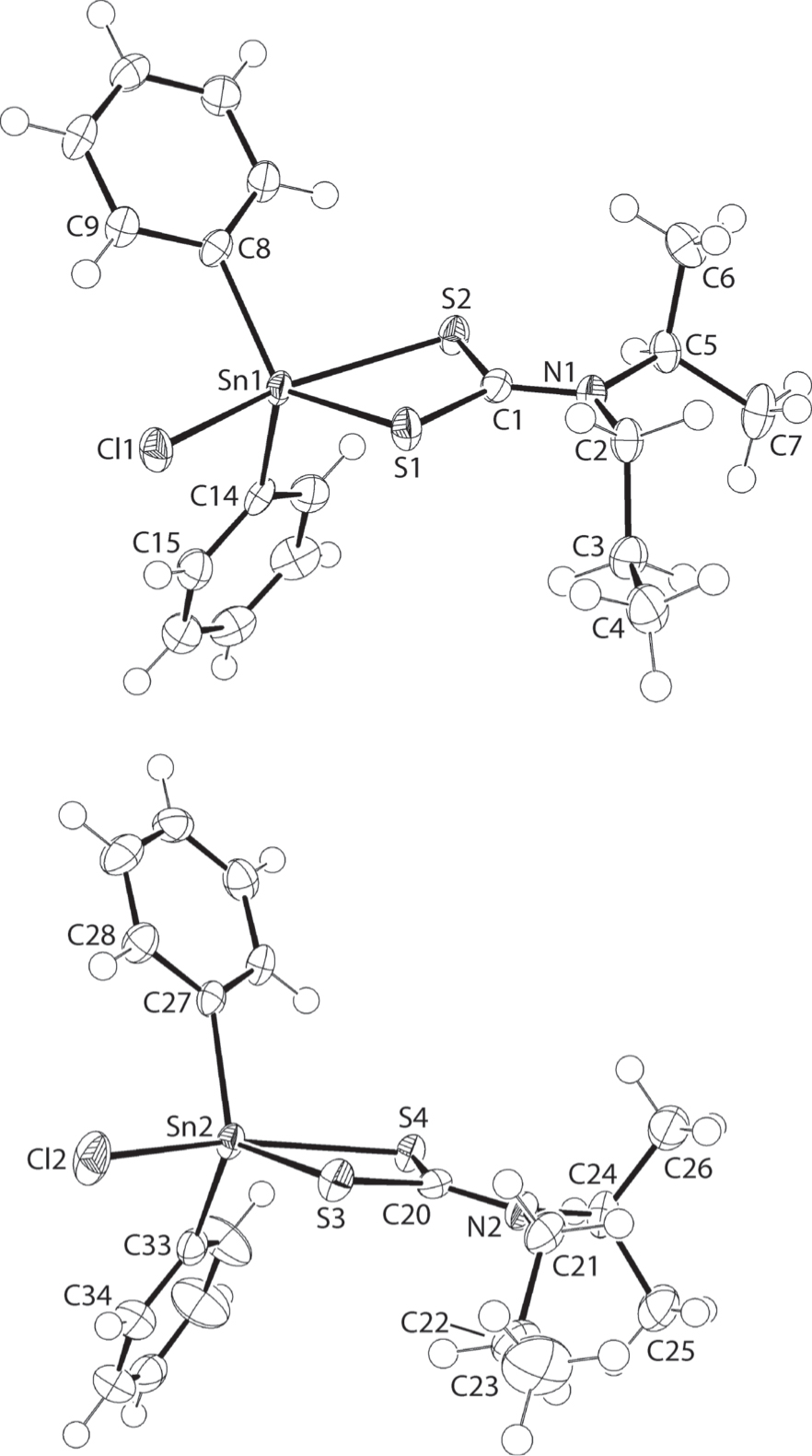
The molecular structures are shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.16 × 0.09 × 0.06 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 13.1 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θmax, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 48998, 7265, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7034 |
| N(param)refined: | 439 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.29681(2) | 0.50913(2) | 0.20104(2) | 0.01305(5) |
| Cl1 | 0.20862(5) | 0.58071(4) | 0.05728(3) | 0.01851(11) |
| S1 | 0.51360(5) | 0.54532(4) | 0.14276(4) | 0.01681(11) |
| S2 | 0.48866(5) | 0.44108(4) | 0.31606(4) | 0.01597(11) |
| N1 | 0.72222(16) | 0.49820(12) | 0.25025(12) | 0.0135(3) |
| C1 | 0.59125(19) | 0.49484(14) | 0.24007(15) | 0.0137(4) |
| C2 | 0.80146(19) | 0.54385(15) | 0.17977(15) | 0.0158(4) |
| H2A | 0.751369 | 0.528051 | 0.117246 | 0.019* |
| H2B | 0.886377 | 0.516830 | 0.182353 | 0.019* |
| C3 | 0.8326(2) | 0.65321(16) | 0.19414(16) | 0.0186(4) |
| H3A | 0.749904 | 0.679685 | 0.203196 | 0.022* |
| H3B | 0.897730 | 0.669757 | 0.250525 | 0.022* |
| C4 | 0.8902(2) | 0.69760(17) | 0.11006(17) | 0.0232(5) |
| H4A | 0.967137 | 0.666869 | 0.097754 | 0.035* |
| H4B | 0.918710 | 0.766856 | 0.122525 | 0.035* |
| H4C | 0.821735 | 0.687643 | 0.055785 | 0.035* |
| C5 | 0.7909(2) | 0.44837(15) | 0.32799(15) | 0.0164(4) |
| H5 | 0.735014 | 0.445690 | 0.380232 | 0.020* |
| C6 | 0.7961(2) | 0.34522(17) | 0.29588(17) | 0.0227(5) |
| H6A | 0.704979 | 0.312152 | 0.277237 | 0.034* |
| H6B | 0.839271 | 0.311320 | 0.346844 | 0.034* |
| H6C | 0.847242 | 0.345735 | 0.242888 | 0.034* |
| C7 | 0.9288(2) | 0.50159(19) | 0.36431(17) | 0.0257(5) |
| H7A | 0.988322 | 0.499751 | 0.316327 | 0.039* |
| H7B | 0.964951 | 0.470538 | 0.419793 | 0.039* |
| H7C | 0.921900 | 0.568770 | 0.380212 | 0.039* |
| C8 | 0.16850(19) | 0.37313(15) | 0.18152(15) | 0.0146(4) |
| C9 | 0.0589(2) | 0.36164(16) | 0.11294(15) | 0.0173(4) |
| H9 | 0.043024 | 0.413287 | 0.073630 | 0.021* |
| C10 | −0.0277(2) | 0.27468(17) | 0.10167(16) | 0.0209(5) |
| H10 | −0.101443 | 0.266933 | 0.053980 | 0.025* |
| C11 | −0.0069(2) | 0.19963(16) | 0.15952(16) | 0.0203(5) |
| H11 | −0.066588 | 0.140654 | 0.152002 | 0.024* |
| C12 | 0.1018(2) | 0.21081(16) | 0.22874(17) | 0.0212(5) |
| H12 | 0.116048 | 0.159600 | 0.268970 | 0.025* |
| C13 | 0.1896(2) | 0.29704(16) | 0.23909(16) | 0.0191(4) |
| H13 | 0.264544 | 0.304041 | 0.285818 | 0.023* |
| C14 | 0.25981(19) | 0.61706(15) | 0.29714(15) | 0.0157(4) |
| C15 | 0.1999(2) | 0.69557(16) | 0.26487(17) | 0.0213(5) |
| H15 | 0.178060 | 0.702168 | 0.199991 | 0.026* |
| C16 | 0.1719(2) | 0.76427(17) | 0.32719(18) | 0.0252(5) |
| H16 | 0.129483 | 0.816824 | 0.304647 | 0.030* |
| C17 | 0.2054(2) | 0.75657(17) | 0.42163(18) | 0.0249(5) |
| H17 | 0.186454 | 0.803622 | 0.464100 | 0.030* |
| C18 | 0.2669(2) | 0.67945(18) | 0.45377(17) | 0.0265(5) |
| H18 | 0.291197 | 0.674272 | 0.518589 | 0.032* |
| C19 | 0.2932(2) | 0.60985(16) | 0.39238(16) | 0.0211(5) |
| H19 | 0.334301 | 0.556901 | 0.415427 | 0.025* |
| Sn2 | 0.29554(2) | 0.07307(2) | 0.68663(2) | 0.01405(5) |
| Cl2 | 0.09550(5) | −0.04057(4) | 0.62779(4) | 0.02780(13) |
| S3 | 0.21280(5) | 0.05799(4) | 0.83896(4) | 0.01713(11) |
| S4 | 0.47280(5) | 0.17403(4) | 0.81987(3) | 0.01521(10) |
| N2 | 0.37993(17) | 0.13865(13) | 0.98338(12) | 0.0157(4) |
| C20 | 0.36089(19) | 0.12676(14) | 0.89191(15) | 0.0142(4) |
| C21 | 0.2754(2) | 0.10127(16) | 1.04139(15) | 0.0188(4) |
| H21A | 0.187042 | 0.110172 | 1.008808 | 0.023* |
| H21B | 0.289763 | 0.139921 | 1.100485 | 0.023* |
| C22 | 0.2729(2) | −0.00537(18) | 1.06352(17) | 0.0261(5) |
| H22A | 0.262791 | −0.044303 | 1.004799 | 0.031* |
| H22B | 0.358837 | −0.014245 | 1.099730 | 0.031* |
| C23 | 0.1592(3) | −0.0408(2) | 1.1184(2) | 0.0409(7) |
| H23A | 0.169768 | −0.003012 | 1.177081 | 0.061* |
| H23B | 0.160405 | −0.108958 | 1.131519 | 0.061* |
| H23C | 0.073857 | −0.033189 | 1.082226 | 0.061* |
| C24 | 0.5044(2) | 0.19648(17) | 1.03192(16) | 0.0204(5) |
| H24 | 0.571350 | 0.205541 | 0.986660 | 0.025* |
| C25 | 0.5638(2) | 0.14378(19) | 1.11376(18) | 0.0295(5) |
| H25A | 0.503758 | 0.139210 | 1.161893 | 0.044* |
| H25B | 0.650969 | 0.179305 | 1.139168 | 0.044* |
| H25C | 0.574844 | 0.078838 | 1.093002 | 0.044* |
| C26 | 0.4775(2) | 0.29593(17) | 1.06142(17) | 0.0255(5) |
| H26A | 0.432365 | 0.325542 | 1.008195 | 0.038* |
| H26B | 0.562325 | 0.336504 | 1.083751 | 0.038* |
| H26C | 0.420390 | 0.289737 | 1.111310 | 0.038* |
| C27 | 0.2554(2) | 0.19319(15) | 0.60531(14) | 0.0146(4) |
| C28 | 0.1354(2) | 0.18840(16) | 0.54688(16) | 0.0193(4) |
| H28 | 0.067778 | 0.134397 | 0.548131 | 0.023* |
| C29 | 0.1140(2) | 0.26208(18) | 0.48688(17) | 0.0248(5) |
| H29 | 0.031710 | 0.258643 | 0.447554 | 0.030* |
| C30 | 0.2125(2) | 0.34059(16) | 0.48420(16) | 0.0223(5) |
| H30 | 0.197925 | 0.390870 | 0.442903 | 0.027* |
| C31 | 0.3323(2) | 0.34577(16) | 0.54178(17) | 0.0223(5) |
| H31 | 0.399977 | 0.399531 | 0.539671 | 0.027* |
| C32 | 0.3538(2) | 0.27265(16) | 0.60262(15) | 0.0179(4) |
| H32 | 0.435819 | 0.276851 | 0.642449 | 0.021* |
| C33 | 0.4408(2) | −0.01222(16) | 0.65181(15) | 0.0178(4) |
| C34 | 0.4109(3) | −0.11193(17) | 0.64007(18) | 0.0269(5) |
| H34 | 0.323774 | −0.143426 | 0.646799 | 0.032* |
| C35 | 0.5085(3) | −0.16546(18) | 0.61846(19) | 0.0325(6) |
| H35 | 0.488397 | −0.233714 | 0.612151 | 0.039* |
| C36 | 0.6336(3) | −0.12066(19) | 0.60616(17) | 0.0283(5) |
| H36 | 0.699650 | −0.157813 | 0.591201 | 0.034* |
| C37 | 0.6635(3) | −0.0215(2) | 0.6156(2) | 0.0407(7) |
| H37 | 0.749332 | 0.009961 | 0.605666 | 0.049* |
| C38 | 0.5668(3) | 0.03219(19) | 0.6397(2) | 0.0360(7) |
| H38 | 0.588175 | 0.100249 | 0.647869 | 0.043* |
Source of material
All chemicals and solvents were used as purchased without purification. The melting point was determined on a Mel-temp II digital melting point apparatus and was uncorrected. The IR spectrum was obtained on a Bruker Vertex 70v FTIR Spectrometer from 4000 to 400 cm−1.
The dithiocarbamate ligand was prepared in situ (methanol) from the reaction of CS2 (Merck 0.25 mmol) with isopropyl(n-propyl)amine (Alfa Aesar, 0.25 mmol) and KOH (0.03 mL; 50% w/v); CS2 was added drop-wise into the methanolic solution (15 mL). The resulting mixture was kept at 273 K for 0.5 h. Diphenyltin dichloride (0.25 mmol, 0.09 g) in methanol (10 mL) was added to the prepared potassium isopropyl-n-propyl dithiocarbamate. The resulting mixture was stirred and refluxed for 2 h. The filtrate was evaporated slowly until a colourless precipitate was formed. The precipitate was recrystallised from its acetone-methanol solution. The title compound was a side-product/incomplete reaction product obtained from the slow evaporation of the solvent. Yield: 0.02 g (17%). M.pt: 381–383 K. IR (cm−1) 550 (w) ν(Sn—S), 1477 (s) ν(C—N), 1187 (m) ν(C—S), 1048 (s) ν(C—N).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.95–1.00 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C).
Comment
Molecules/complexes of the general formula R2Sn(S2CNR′R′′)Cl have been the subject of structure determinations by both X-ray crystallography and computational chemistry for nearly 20 years [5], [6]. These studies showed that tin-ligand parameters were sometimes influenced significantly by the crystalline/solid state environment, often leading to non-systematic variations that were not evident in their calculated gas-phase structures. Interest in this area continues [7], [8] and indeed, there are now over 70 mononuclear species of this type in the crystallographic literature [9]. Herein, a new derivative, Ph2Sn[S2CN(i-Pr)n-Pr]Cl, (I), is described, which was synthesised in the context of evaluating the biological potential of organotin dithiocarbamates [10].
The molecular structures of the two independent molecules comprising the asymmetric unit of (I) are shown in the figure (70% displacement ellipsoids). The tin atom in each molecule is penta-coordinated by two ipso-carbon atoms of the phenyl groups, a chloride and two sulphur atoms derived from an asymmetrically chelating dithiocarbamate ligand. The asymmetry in the Sn—S bonds [Sn1—S1, S2 = 2.4501(5), 2.7049(5) Å and Sn2—S3, S4 = 2.4622(5), 2.7058(5) Å] is reflected in the magnitude of Δ(Sn—S) = (Sn—Slong – Sn—Sshort) = 0.25 and 0.26 Å, respectively. There is a small disparity in the Sn1—Cl1 [2.4591(5) Å] and Sn2—Cl2 [2.4534(5) Å] bond lengths. The asymmetric mode of coordination of the dithiocarbamate ligand influences the C—S bond lengths with the C—S bonds associated with the more tightly bound sulphur atoms [C1—S1 = 1.745(2) Å and C20—S3 = 1.752(2) Å] being longer than the C—S bonds involving the less tightly bound sulphur atoms [C1—S2 = 1.722(2) Å and C20—S4 = 1.712(2) Å]. Typically for dithiocarbamate ligands [11], the relatively short C1—N1 [1.319(3)] and C20—N2 [1.322(3) Å] bond lengths are indicative of significant contributions of the dithiolate, −S2C=N+(i-Pr)n-Pr, canonical form to the electronic structure of the ligand.
The resulting C2ClS2 donor set is highly distorted and this is seen in a quantitative measure of a five-coordinate geometry, i.e. τ [12]. For an ideal square-pyramidal geometry, τ = 0.0 whereas for an ideal trigonal-bipyramidal geometry, τ = 1.0. In the present study, the values of τ compute to 0.49 and 0.58 for the Sn1 and Sn2 atoms, respectively. The distortions arise partly to the acute chelate angles: S1—Sn1—S2 [69.663(16)°] and S3—Sn2—S4 [69.447(16)°] as reflected, for example, in the trans Cl1—Sn1—S2 [154.209(16)°] and Cl2—Sn2—S4 [154.996(18)°] angles. The molecular structures described herein conform to the motif always seen for mononuclear molecules/complexes of the general formula R2Sn(S2CNR′R′′)Cl [10].
The molecular packing is largely devoid of directional points of contact between atoms/residues [13], with the exception of methyl- and phenyl-C—H⋯π(phenyl) interactions [C6—H6c⋯Cg(C8—C13)i: H6c⋯Cg(C8—C13)i = 2.93 Å, C6⋯Cg(C8—C13)i = 3.784(2) Å with angle at H6c = 146° and C36—H36⋯Cg(C27—C32)ii: H36⋯Cg(C27—C32)ii = 2.68 Å, C36⋯Cg(C27—C32)ii = 3.489(3) Å with angle at H36 = 143° for symmetry operations (i) 1 + x, y, z and (ii) 1 − x, −y, 1 − z]. Each of these interactions occurs between like-molecules. In the case of the Sn1-molecule, the result of these contacts is the formation of a linear supramolecular chain along the a-axis direction. For the Sn2-molecule, the specified interaction leads to a centrosymmetric dimer. The dimers stack in columns parallel to the chains. Globally, the crystal comprises alternating layers of chains and dimers stacked along the b-axis.
A further analysis of the intermolecular connectivity was performed by evaluating the calculated Hirshfeld surfaces using Crystal Explorer 17 [14] following literature procedures [15]; the full and decomposed two-dimensional fingerprint plots were also calculated. There are four dominant contacts to the Hirshfeld surface for the entire asymmetric unit in (I), namely H⋯H [55.5%], H⋯C/C⋯H [23.9%], Cl⋯H/H⋯Cl [9.2%] and S⋯H/H⋯S [8.7%] but, generally beyond the sum of the respective van der Waals radii. A recent study [16] emphasised how different molecules/complexes comprising the asymmetric unit could present different percentage profiles depending on the intermolecular interactions they form. Accordingly, each of the Sn1- and Sn2-molecules/complexes in (I) were analysed separately. Not surprisingly, the same major contributions are evident but, in different percentages, i.e. H⋯H [59.1% for the Sn1-molecule and 52.8% for the Sn2-molecule/complex], H⋯C/C⋯H [23.5 and 24.6%], Cl⋯H/H⋯Cl [9.0 and 8.3%] and S⋯H/H⋯S [7.1 and 10.6%]. Thus, small differences between the independent molecules are seen in the percentage contributions for the H⋯H and S⋯H/H⋯S contacts. Further, for the Sn2-molecule, C⋯C contacts amounts to 2.3% of the Hirshfeld surface contacts compared with 0.2% for the Sn1-molecule complex.
Acknowledgements
Sunway University Sdn Bhd is thanked for financial support of this work through Grant No. STR-RCTR-RCCM-001–2019.
References
1. Rigaku Oxford Diffraction: CrysAlisPRO. Rigaku Corporation, Oxford, UK (2018).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
5. Buntine, M. A.; Hall, V. J.; Kosovel, F. J.; Tiekink, E. R. T.: Influence of crystal packing on molecular geometry: a crystallographic and theoretical investigation of selected diorganotin systems. J. Phys. Chem. A 102 (1998) 2472–2482.10.1021/jp9728722Search in Google Scholar
6. Tiekink, E. R. T.; Hall, V. J.; Buntine, M. A.: An examination of the influence of crystal structure on molecular structure. The crystal and molecular structures of some diorganotinchloro-(N,N-dialkyldithiocarbamate)s, R2Sn(S2CNR′2)Cl, R = Me, tBu, Ph, Cy; R′2 = (Et)2, (Et, Cy) and (Cy)2: a comparison between solid state and theoretical structures. Z. Kristallogr. − Cryst. Mat. 214 (1999) 124–134.10.1524/zkri.1999.214.2.124Search in Google Scholar
7. Lo, K. M.; Lee, S. M.; Tiekink, E. R. T.: Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-S,S)tin(IV), C13H19ClN2S2Sn. Z. Kristallogr. NCS 234 (2019) 1309–1311.10.1515/ncrs-2019-0501Search in Google Scholar
8. Lee, S. M.; Lo, K. M.; Tiekink, E. R. T.: Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-S,S)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn. Z. Kristallogr. NCS 234 (2019) 1313–1315.10.1515/ncrs-2019-0502Search in Google Scholar
9. Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C.: The Cambridge Structural Database. Acta Crystallogr. B72 (2016) 171–179.10.1016/B978-0-12-409547-2.02529-4Search in Google Scholar
10. Tiekink, E. R. T.: Tin dithiocarbamates: applications and structures. Appl. Organomet. Chem. 22 (2008) 533–550.10.1002/aoc.1441Search in Google Scholar
11. Lee, S. M.; Lo, K. M.; Tiekink, E. R. T.: Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2. Z. Kristallogr. NCS 234 (2019) 1325–1327.10.1515/ncrs-2019-0511Search in Google Scholar
12. Addison, A. W.; Rao, T. N.; Reedijk, J.; van Rijn, J.; Verschoor, G. C.: Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]-copper(II) perchlorate. J. Chem. Soc. Dalton Trans. (1984) 1349–1356.10.1039/DT9840001349Search in Google Scholar
13. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
14. Turner, M. J.; Mckinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Crystal Explorer v17. The University of Western Australia, Australia (2017).Search in Google Scholar
15. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T.: Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E75 (2019) 308–318.10.1107/S2056989019001129Search in Google Scholar PubMed PubMed Central
16. Jotani, M. M.; Wardell, J. L.; Tiekink, E. R. T.: Supramolecular association in the triclinic (Z′ = 1) and monoclinic (Z′ = 4) polymorphs of 4-(4-acetylphenyl)piperazin-1-ium 2-amino-4-nitrobenzoate. Z. Kristallogr. – CM 234 (2019) 43–57.10.1515/zkri-2018-2101Search in Google Scholar
©2019 See Mun Lee et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n


