Abstract
C52H48Br2Cl6N2O2Sn2, triclinic, P1̄ (no. 2), a = 8.8297(1) Å, b = 12.2632(2) Å, c = 12.6884(2) Å, α = 84.191(1)°, β = 83.940(1)°, γ = 76.086(1)°, V = 1322.04(3) Å3, Z = 1, Rgt(F) = 0.0222, wRref(F2) = 0.0621, T = 100(2) K.
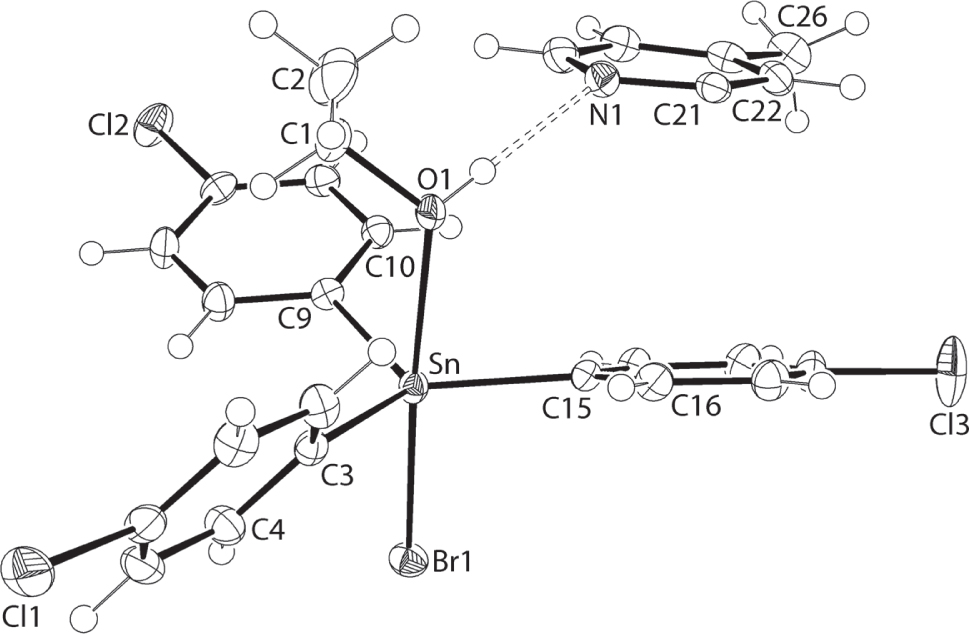
The components of the asymmetric unit are shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.10 × 0.08 × 0.05 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 12.4 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θmax, completeness: | 67.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 31356, 4730, 0.038 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4577 |
| N(param)refined: | 303 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn | 0.73807(2) | 0.34325(2) | 0.32425(2) | 0.01554(6) |
| Br1 | 0.82458(3) | 0.50549(2) | 0.40150(2) | 0.02187(8) |
| Cl1 | 1.32005(6) | 0.22787(6) | −0.05566(5) | 0.03271(14) |
| Cl2 | 0.12395(6) | 0.67606(5) | 0.10294(5) | 0.03057(14) |
| Cl3 | 0.65562(9) | 0.07952(6) | 0.80098(5) | 0.04340(17) |
| O1 | 0.65514(18) | 0.18457(13) | 0.26191(12) | 0.0192(3) |
| H1O | 0.589(3) | 0.160(2) | 0.302(2) | 0.029* |
| N1 | 0.4237(2) | 0.11121(15) | 0.40045(16) | 0.0201(4) |
| C1 | 0.6116(3) | 0.1884(2) | 0.15543(19) | 0.0262(5) |
| H1A | 0.659181 | 0.243684 | 0.109560 | 0.031* |
| H1B | 0.496455 | 0.214307 | 0.155373 | 0.031* |
| C2 | 0.6636(4) | 0.0754(2) | 0.1101(2) | 0.0410(7) |
| H2A | 0.777979 | 0.050722 | 0.107402 | 0.062* |
| H2B | 0.629927 | 0.081345 | 0.038175 | 0.062* |
| H2C | 0.616754 | 0.020336 | 0.155270 | 0.062* |
| C3 | 0.9358(2) | 0.30000(18) | 0.21079(17) | 0.0178(4) |
| C4 | 1.0063(3) | 0.3850(2) | 0.16265(19) | 0.0232(5) |
| H4 | 0.971705 | 0.458935 | 0.185578 | 0.028* |
| C5 | 1.1256(3) | 0.3635(2) | 0.0822(2) | 0.0265(5) |
| H5 | 1.172840 | 0.422038 | 0.050181 | 0.032* |
| C6 | 1.1751(2) | 0.2558(2) | 0.04900(19) | 0.0228(5) |
| C7 | 1.1093(3) | 0.1693(2) | 0.0961(2) | 0.0239(5) |
| H7 | 1.145237 | 0.095355 | 0.073241 | 0.029* |
| C8 | 0.9903(3) | 0.19163(19) | 0.17717(19) | 0.0219(5) |
| H8 | 0.945409 | 0.132342 | 0.210118 | 0.026* |
| C9 | 0.5369(2) | 0.44804(17) | 0.25672(18) | 0.0169(4) |
| C10 | 0.3869(2) | 0.46200(18) | 0.30821(18) | 0.0186(4) |
| H10 | 0.371967 | 0.422596 | 0.375468 | 0.022* |
| C11 | 0.2586(3) | 0.53288(19) | 0.26251(19) | 0.0206(4) |
| H11 | 0.156574 | 0.542364 | 0.298043 | 0.025* |
| C12 | 0.2825(3) | 0.58921(19) | 0.16438(19) | 0.0214(5) |
| C13 | 0.4308(3) | 0.5779(2) | 0.11177(19) | 0.0240(5) |
| H13 | 0.445601 | 0.617902 | 0.044816 | 0.029* |
| C14 | 0.5570(3) | 0.5070(2) | 0.15883(19) | 0.0226(5) |
| H14 | 0.659006 | 0.498686 | 0.123521 | 0.027* |
| C15 | 0.7096(2) | 0.25247(18) | 0.47340(17) | 0.0167(4) |
| C16 | 0.7999(2) | 0.14475(19) | 0.49814(18) | 0.0202(4) |
| H16 | 0.873449 | 0.107795 | 0.445433 | 0.024* |
| C17 | 0.7837(3) | 0.09094(19) | 0.59844(19) | 0.0222(5) |
| H17 | 0.846220 | 0.017788 | 0.615195 | 0.027* |
| C18 | 0.6748(3) | 0.1455(2) | 0.67406(18) | 0.0228(5) |
| C19 | 0.5814(3) | 0.2521(2) | 0.65189(19) | 0.0232(5) |
| H19 | 0.506017 | 0.287884 | 0.704282 | 0.028* |
| C20 | 0.6009(3) | 0.30488(18) | 0.55139(18) | 0.0201(4) |
| H20 | 0.538864 | 0.378333 | 0.535167 | 0.024* |
| C21 | 0.4339(2) | 0.05090(18) | 0.49552(18) | 0.0178(4) |
| C22 | 0.3257(3) | 0.08082(18) | 0.58212(19) | 0.0204(4) |
| H22 | 0.338496 | 0.037974 | 0.648620 | 0.025* |
| C23 | 0.1995(3) | 0.17291(19) | 0.5717(2) | 0.0218(5) |
| C24 | 0.1851(3) | 0.23137(19) | 0.4723(2) | 0.0239(5) |
| H24 | 0.098688 | 0.293174 | 0.460424 | 0.029* |
| C25 | 0.2991(3) | 0.19791(19) | 0.3907(2) | 0.0233(5) |
| H25 | 0.288231 | 0.239204 | 0.323311 | 0.028* |
| C26 | 0.0870(3) | 0.2089(2) | 0.6659(2) | 0.0290(5) |
| H26A | 0.129000 | 0.257287 | 0.706258 | 0.043* |
| H26B | 0.072418 | 0.142143 | 0.711514 | 0.043* |
| H26C | −0.013938 | 0.250655 | 0.641314 | 0.043* |
Source of material
General: All chemicals and solvents were used as purchased without purification. The melting point was determined using a Mel-temp II digital melting point apparatus and was uncorrected. The solid-state IR spectrum was obtained on a Bruker Vertex 70v FTIR Spectrometer from 4000 to 400 cm−1. The 1H and 13C{1H} NMR spectra were recorded at room temperature in CDCl3 solution on a Bruker Ascend 400 MHz NMR spectrometer with chemical shifts relative to tetramethylsilane.
Synthesis: Tetra(4-chlorophenyl)tin was synthesized from the reaction of stannic chloride (Fluka) with 4-chlorophenylmagnesium bromide (Fluka) in a 1:4 molar ratio. Tetra(4-chlorophenyl)tin (0.57 g, 1 mmol) and 4-(dimethylamino)pyridine hydrobromide perbromide (Sigma-Aldrich, 0.36 g, 1 mmol) were dissolved in ethanol (50 mL). The resulting mixture was stirred at room temperature until a colourless solution was obtained. 4,4′-Dimethyl-2,2′-dipyridyl (Sigma-Aldrich, 0.18 g, 1 mmol) in ethanol (5 mL) was added to the mixture which was then refluxed for 2 h. After filtration, the filtrate was evaporated slowly until colourless crystals formed. The crystals were filtered, washed with a minimum amount of hexane and air-dried. Yield: 0.34 g (50.6%). M.pt: 395–397 K. IR (cm−1) 1644 (s) ν(C—N), 1561 (s) ν(C—N), 509 (w) ν(Sn—O). 1H NMR (CDCl3, p.p.m.): δ 1.30 (s, 3H, ethanol-CH3) 2.43 (s, 3H, CH3), 3.23 (s, 2H, OCH2), 7.11 (d, 6H, J = 7.70, Ph—H), 7.59 (d, 1H, J = 6.50 Hz, py—H), 8.11 (d, 1H, J = 6.82, py—H), 8.22 (s, 1H, py—H), 8.53 (d, 6H, J = 7.68, Ph—H); the hydroxy-O—H was not observed. 13C{1H} NMR (CDCl3, p.p.m.): δ 21.2 (CH3), 23.0 (CH3), 40.2 (OCH2), 116.7, 129.4, 135.4, 137.3 (Ph—H), 122.1, 124.6, 139.0, 149.0, 156.2 (py—H).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.95–0.99 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The O-bound H-atom was located in a difference Fourier map but, was refined with a distance restraint of O—H = 0.84 ± 0.01 Å, and with Uiso(H) set to 1.5Ueq(O).
Comment
The title compound became available during recent studies of the binding of bis(substituted-benzyl)tin dihalides by molecules with potentially neutral, bridging ligands, such as 4,4′-bipyridyl-N-oxide [5]. The title compound is formulated as {(4-ClC6H5)3Sn[O(H)CH2CH3]Br}2(4,4′-dimethyl-2,2′-dipyridyl) (I), and was characterized by X-ray crystallography.
The molecular structures comprising the asymmetric unit of (I) are shown in the figure (50% displacement ellipsoids; the full 4,4′-dimethyl-2,2′-dipyridyl molecule is generated by the application of the symmetry operation (i) 1 − x, − y, 1 − z). The tin atom in (I) is penta-coordinated by a bromide atom [2.6050(3) Å], three ipso-carbon atoms of the three 4-chlorophenyl groups [Sn—C3, C9, C15 = 2.141(2), 2.131(2) and 2.119(2) Å] and the oxygen atom [2.4634(15) Å] of the ethanol molecule. The 4-chlorophenyl substituents occupy equatorial positions in a distorted trigonal-bipyramidal geometry. The Br1—Sn—O1 axial angle is 176.63(4)°, and the tin atom lies 0.2174(12) Å out of the plane through the C3, C9 and C15 atoms in the direction of the Br1 atom. There are discrepancies in the angles subtended at the tin atom by the phenyl substituents with the angles subtended by the phenyl-C15 atom [C3—Sn—C15 = 124.36(8)° and C9—Sn—C15 = 119.12(8)°] being systematically wider than the C3—Sn—C9 angle [113.42(8)°]. While the C9—Sn—C15 is close to the ideal, the deviation from 120° of the narrowest angle is correlated with the wide dihedral angle formed between the C3- and C9-rings, i.e. 60.40(7)°, suggesting minimal steric repulsion between them and hence, the narrow angle.
As indicated in the figure, there is an ethanol-O—H⋯N(pyridyl) hydrogen bond [O1—H1o⋯N1: H1o⋯N1 = 1.98(3) Å, O1⋯N1 = 2.807(2) Å with angle at H1o = 176(2)°] between the title complex and the organic component of this co-crystal. Bonding parameters are in the expected ranges [6]. As the 4,4′-dimethyl-2,2′-dipyridyl is disposed about a crystallographic centre of inversion, a three-molecule aggregate ensues. Further stability to these aggregates is provided by π⋯π interactions between the chlorophenyl and pyridyl rings [inter-centroid Cg(C15–C20)⋯Cg(N1,C21–C25) distance = 3.8951(14) Å with angle of inclination = 13.28(11)°]. The aggregates assemble in the crystal via a combination of weak non-covalent interactions. A supramolecular layer parallel to (0 1 1) is formed through the agency of chlorophenyl-C—H⋯π(chlorophenyl) interactions [C19—H19⋯Cg(C9—C14)ii: H19⋯Cg(C9—C14)ii = 2.98 Å, C19⋯Cg(C9–C14)ii = 3.471(3) Å with angle at H19 = 114° for symmetry operation (ii) 1 − x, 1 − y, 1 − z]. The connections between layers to consolidate the three-dimensional architecture are of the type end-on chlorophenyl-C—Cl⋯π(chlorophenyl) [C12—Cl2⋯Cg(C3—C8)iii: Cl2⋯Cg(C3—C8)iii = 3.4361(12) Å with angle at Cl2 = 142.34(8)° for (iii) 1 − x, 1 − y, −z] interactions.
Further insight into the molecular packing was achieved through an analysis of the calculated Hirshfeld surface employing Crystal Explorer 17 [7] and established procedures [8], including the calculation of the full and decomposed two-dimensional fingerprint plots. The four major contributing contacts to the overall Hirshfeld surface (i.e. for both components of the asymmetric unit) are, in descending order H⋯H [33.2%], Cl⋯H/H⋯Cl [25.3%], C⋯H/H⋯C [18.9%] and Br⋯H/H⋯Br [10.7%]. There are several other contacts to the surface but, at distances at or greater than the sum of the respective van der Waals radii, such as Cl⋯C/C⋯Cl [3.4%], Cl⋯Cl [1.9%] and N⋯H/H⋯N [1.6%]. Subsequently, calculations were performed on the tin compound itself as well as upon the entire 4,4′-dimethyl-2,2′-dipyridyl molecule. For the former, the percentage contributions for the four most important contacts are, to a first approximation, the same, H⋯H [34.6%], Cl⋯H/H⋯Cl [26.9%], C⋯H/H⋯C, Br⋯H/H⋯Br [9.2%]. As anticipated from the chemical composition, greater variations are not for the bipyridyl molecule, with significant increases to the surface of the molecule from H⋯H [41.9%] and C⋯H/H⋯C [25.9%] contacts complemented by notable decreases from Cl⋯H/H⋯Cl [8.3%] and Br⋯H/H⋯Br [7.5%] contacts. Also, the relative importance of N⋯H/H⋯N [6.3%] contacts increases.
Acknowledgements
Sunway University Sdn Bhd is thanked for financial support of this work through Grant No. STR-RCTR-RCCM-001–2019.
References
1. Rigaku Oxford Diffraction: CrysAlisPRO. Rigaku Corporation, Oxford, UK (2018).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
5. Lee, S. M., Lo, K. M.; Tiekink, E. R. T.: Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2. Z. Kristallogr. NCS 235 (2019) 143–145.10.1515/ncrs-2019-0542Search in Google Scholar
6. Ng, S. W.; Das, V. G. K.: Outer-sphere coordination of o-phenanthroline in bis[aquachlorotri(p-chlorophenyl) tin⋅o-phenanthroline]. J. Organomet. Chem. 513 (1996) 105–108.10.1016/0022-328X(95)05966-SSearch in Google Scholar
7. Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Crystal Explorer v17. The University of Western Australia, Australia (2017).Search in Google Scholar
8. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T.: Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E75 (2019) 308–318.10.1107/S2056989019001129Search in Google Scholar PubMed PubMed Central
©2019 See Mun Lee et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n


