Abstract
C16H12N2O4, triclinic, P1̄ (no. 2), a = 10.7105(5) Å, b = 11.2685(5) Å, c = 13.0145(6) Å, α = 99.292(4)°, β = 109.607(4)°, γ = 96.771(4)°, V = 1435.01(12) Å3, Z = 4, Rgt(F) = 0.0427, wRref(F2) = 0.1124, T = 295(2) K.
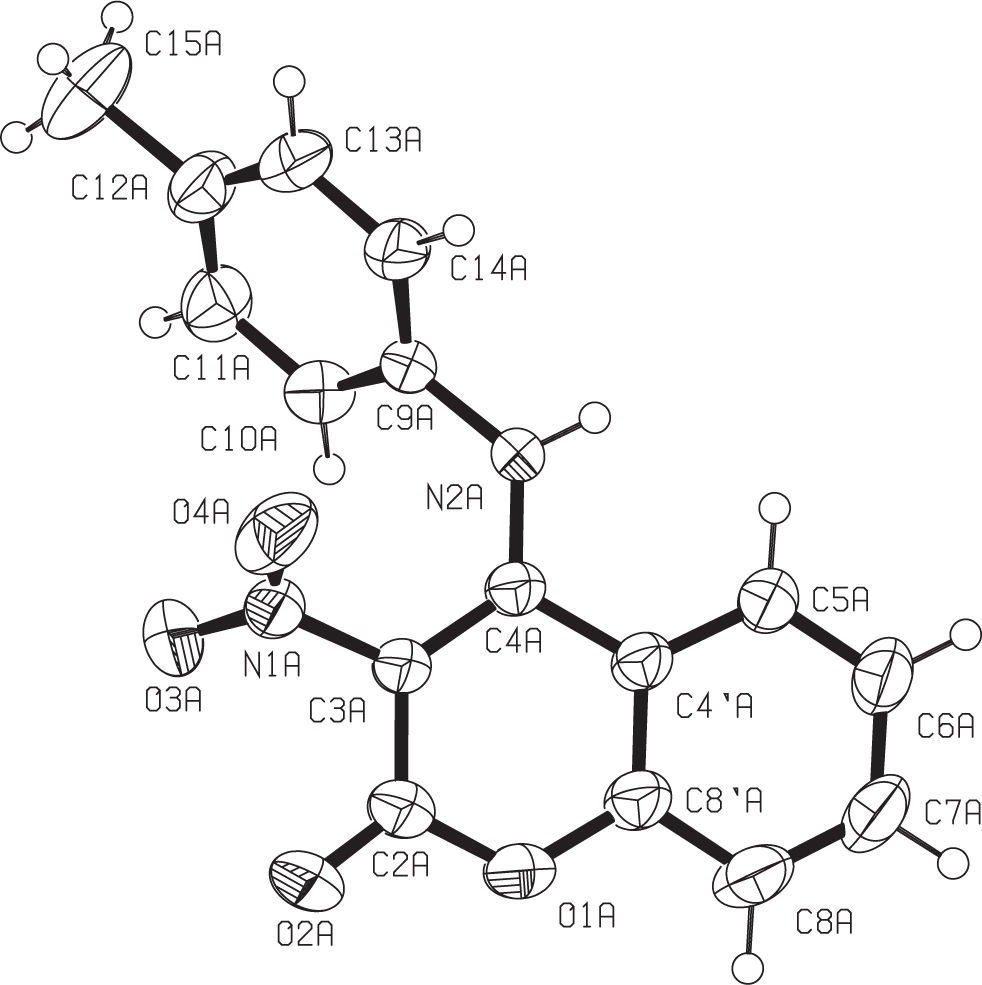
The molecular structure of molecule A is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Trapezoid, yellow |
| Size: | 0.60 × 0.37 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.1 mm−1 |
| Diffractometer, scan mode: | Gemini S, ω-scans |
| θmax, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14917, 5870, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4398 |
| N(param)refined: | 399 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], PLATON [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1B | 1.16119(11) | 0.91869(10) | 0.56562(9) | 0.0507(3) |
| O2B | 1.15133(12) | 1.03279(10) | 0.44271(10) | 0.0519(3) |
| O4B | 0.81452(11) | 0.84478(10) | 0.23184(9) | 0.0479(3) |
| O3B | 1.00662(12) | 0.91945(11) | 0.22776(10) | 0.0558(3) |
| N1B | 0.93786(13) | 0.87025(11) | 0.27307(10) | 0.0362(3) |
| N2B | 0.87600(12) | 0.63433(11) | 0.33237(10) | 0.0387(3) |
| H2B | 0.836251 | 0.583155 | 0.359005 | 0.046* |
| C2B | 1.10733(15) | 0.93653(14) | 0.45939(13) | 0.0388(3) |
| C3B | 1.00335(14) | 0.84102(13) | 0.38018(12) | 0.0340(3) |
| C4B | 0.95786(14) | 0.73335(13) | 0.40659(12) | 0.0332(3) |
| C4′B | 1.00456(15) | 0.72883(14) | 0.52459(12) | 0.0375(3) |
| C5B | 0.95315(18) | 0.63703(15) | 0.56831(14) | 0.0475(4) |
| H5B | 0.885596 | 0.572694 | 0.520475 | 0.057* |
| C6B | 1.0014(2) | 0.64103(18) | 0.68098(15) | 0.0593(5) |
| H6B | 0.965341 | 0.580279 | 0.709059 | 0.071* |
| C7B | 1.1031(2) | 0.73457(19) | 0.75265(15) | 0.0673(6) |
| H7B | 1.136165 | 0.735796 | 0.828719 | 0.081* |
| C8B | 1.1560(2) | 0.82598(18) | 0.71280(15) | 0.0615(5) |
| H8B | 1.224721 | 0.889028 | 0.761301 | 0.074* |
| C8′B | 1.10573(17) | 0.82301(14) | 0.59954(13) | 0.0439(4) |
| C9B | 0.84650(15) | 0.60311(13) | 0.21529(12) | 0.0370(3) |
| C14B | 0.71886(17) | 0.54160(15) | 0.14572(13) | 0.0470(4) |
| H14B | 0.652399 | 0.524001 | 0.175034 | 0.056* |
| C13B | 0.69084(18) | 0.50656(16) | 0.03269(14) | 0.0534(4) |
| H13B | 0.605387 | 0.463541 | −0.013288 | 0.064* |
| C12B | 0.78597(18) | 0.53343(15) | −0.01451(13) | 0.0491(4) |
| C11B | 0.91319(18) | 0.59404(15) | 0.05704(14) | 0.0478(4) |
| H11B | 0.979087 | 0.613224 | 0.027542 | 0.057* |
| C10B | 0.94511(16) | 0.62686(14) | 0.17099(13) | 0.0415(4) |
| H10B | 1.032336 | 0.664676 | 0.217631 | 0.050* |
| C15B | 0.7517(2) | 0.4984(2) | −0.13898(15) | 0.0696(6) |
| H15D | 0.656614 | 0.492578 | −0.176750 | 0.104* |
| H15E | 0.776372 | 0.420753 | −0.157462 | 0.104* |
| H15F | 0.800415 | 0.559467 | −0.161945 | 0.104* |
| O1A | 0.53546(13) | 0.31509(12) | 0.23852(10) | 0.0589(3) |
| O2A | 0.71073(13) | 0.43775(13) | 0.36920(11) | 0.0705(4) |
| O3A | 0.76230(14) | 0.37834(16) | 0.57852(12) | 0.0860(5) |
| O4A | 0.58391(16) | 0.43433(13) | 0.59828(11) | 0.0721(4) |
| N1A | 0.64180(16) | 0.38220(14) | 0.54349(12) | 0.0536(4) |
| N2A | 0.39533(13) | 0.19486(12) | 0.47211(10) | 0.0420(3) |
| H2A | 0.310529 | 0.164988 | 0.448302 | 0.050* |
| C2A | 0.60992(17) | 0.36344(16) | 0.34898(14) | 0.0495(4) |
| C3A | 0.56108(16) | 0.32163(14) | 0.42846(13) | 0.0417(4) |
| C4A | 0.43935(15) | 0.24380(13) | 0.40064(12) | 0.0374(3) |
| C4′A | 0.35433(16) | 0.21171(13) | 0.28208(12) | 0.0405(4) |
| C5A | 0.22108(17) | 0.14846(15) | 0.24021(14) | 0.0512(4) |
| H5A | 0.181416 | 0.124955 | 0.289111 | 0.061* |
| C6A | 0.1483(2) | 0.12077(18) | 0.12750(15) | 0.0641(5) |
| H6A | 0.059217 | 0.080087 | 0.100911 | 0.077* |
| C7A | 0.2058(2) | 0.15255(18) | 0.05325(15) | 0.0678(6) |
| H7A | 0.156086 | 0.131784 | −0.023043 | 0.081* |
| C8A | 0.3359(2) | 0.21456(17) | 0.09166(15) | 0.0621(5) |
| H8A | 0.375516 | 0.235287 | 0.041860 | 0.074* |
| C8′A | 0.40807(18) | 0.24621(14) | 0.20584(13) | 0.0472(4) |
| C9A | 0.47220(15) | 0.18618(14) | 0.58334(12) | 0.0388(3) |
| C10A | 0.59289(17) | 0.14569(15) | 0.60714(14) | 0.0471(4) |
| H10A | 0.626842 | 0.124809 | 0.550974 | 0.056* |
| C11A | 0.66338(19) | 0.13623(17) | 0.71500(16) | 0.0562(5) |
| H11A | 0.746203 | 0.111065 | 0.731199 | 0.067* |
| C12A | 0.6135(2) | 0.16335(17) | 0.79925(15) | 0.0571(5) |
| C13A | 0.4899(2) | 0.19918(19) | 0.77235(15) | 0.0613(5) |
| H13A | 0.453202 | 0.215518 | 0.827419 | 0.074* |
| C14A | 0.41943(17) | 0.21134(17) | 0.66578(14) | 0.0523(4) |
| H14A | 0.336571 | 0.236442 | 0.649507 | 0.063* |
| C15A | 0.6927(3) | 0.1566(3) | 0.91842(18) | 0.0957(8) |
| H15A | 0.684746 | 0.224738 | 0.969055 | 0.144* |
| H15B | 0.785947 | 0.159033 | 0.928055 | 0.144* |
| H15C | 0.657656 | 0.081621 | 0.933552 | 0.144* |
Source of material
The synthesis and characterization of the title compound was previously reported [5]. The product was obtained in the reaction of 4-chloro-3-nitrocoumarin and p-toluidine in ethyl acetate, in the presence of triethylamine. The obtained solid was dissolved in chloroform and allowed to slowly evaporate to give yellow crystals of the title compound.
Experimental details
Hydrogen atoms were introduced in idealized positions and refined using riding model. Their Uiso values are aproximated as Uiso = kUeq of the parent atom (k = 1.2 for sp2 and 1.5 for sp3 atoms).
Comment
Coumarins are a large group of organic compounds of natural or synthetic origin. They are known to possess promising pharmacological activities, such as antimicrobial [6], antioxidant [7], anticancer [8], anti-inflammatory [9]. The coumarin framework is a promising scaffold for the synthesis of analogs, with an aim to study and improve their biological properties. Varying the types of substitution and substituent identity led to different conformations of the molecules that had a profound impact on their biological activity [10]. We have previously synthesized a number of coumarin derivatives with significant antimicrobial and/or antioxidant activities [5], [11], [12], [13].
The asymmetric part of the unit cell contains two molecules (A) and (B) of the 3-nitro-4-(p-tolylamino)-2H-chromen-2-one. Bond lengths within the coumarin core of these molecules are in good agreement with those found in other 3-nitro-4-(arylamino) coumarins [10]. The two molecules have very similar conformations, as RMSD of their fit is 0.2417 Å. For both molecules, the coumarin core of the molecule is approximately planar, while both substituent groups at C3 and C4 are turned out from the aforementioned planes. This is visualized through torsion angle C4—C3—N1—O3 (131.55(17)° for molecule A and −141.34(15)° for molecule B) for nitro group twisting, as well as torsion angle C4—N2—C9—C10 (–46.8(2)° for molecule A and 37.7(2)° for molecule B) for the p-tolylamino group twisting.
Hydrogen bonds N2B—H2B⋯O2A and N2A—H2A⋯O2Bi [symmetry code (i) = x − 1, y − 1, z] connect molecules in ⋯A⋯B⋯A⋯B⋯chains.
Funding source: Ministry of Education, Science and Technological Development of the Republic of Serbia
Award Identifier / Grant number: 172061
Award Identifier / Grant number: 172014
Award Identifier / Grant number: 45022
Funding statement: This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grants Nos. 172061, 172014 and 45022).
References
1. Rigaku Oxford Diffraction: CrysAlisPro Software system. Rigaku Corporation, Oxford, UK (2018).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
5. Dekić, V.; Radulović, N.; Vukićević, R.; Dekić, B.; Skropeta, D.; Palić, R.: Complete assignment of the 1H and 13C NMR spectra of antimicrobial 4-arylamino-3-nitrocoumarin derivatives. Magn. Reson. Chem. 48 (2010) 896–902.10.1002/mrc.2681Search in Google Scholar PubMed
6. Al-Majedy, Y. K.; Kadhum, A. A. H.; Al-Amiery, A. A.; Mohamad, A. B.: Coumarins: The antimicrobial agents. Sys. Rev. Pharm. 8 (2017) 62–70.10.5530/srp.2017.1.11Search in Google Scholar
7. Bubols, G. B.; Vianna, D. R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R. M.; Eifler-Lima, V. L.; Garcia, S. C.: The antioxidant activity of coumarins and flavonoids. Mini-Rev. Med. Chem. 13 (2013) 318–334.10.2174/1389557511313030002Search in Google Scholar
8. Kapoor, S.: The anti-neoplastic effects of coumarin: An emerging concept. Cytotechnology 65 (2013) 787–788.10.1007/s10616-013-9538-6Search in Google Scholar PubMed PubMed Central
9. Bansal, Y.; Sethi, P.; Bansal, G.: Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 22 (2013) 3049–3060.10.1007/s00044-012-0321-6Search in Google Scholar
10. Radulović, N.; Bogdanović, G.; Blagojević, P.; Dekić, V.; Vukićević, R.: Could X-ray analysis explain for the differing antimicrobial and antioxidant activity of two 2-arylamino-3-nitro-coumarins? J. Chem. Crystallogr. 41 (2011) 545–551.10.1007/s10870-010-9918-0Search in Google Scholar
11. Dekić, V.; Radulović, N.; Vukićević, R.; Dekić, B.; Stojanović-Radić, Z.; Palić, R.: Influence of the aryl substituent identity in 4-arylamino-3-nitrocoumarins on their antimicrobial activity. Afr. J. Pharm. Pharmacol. 5 (2011) 371–375.10.5897/AJPP10.408Search in Google Scholar
12. Radulović, N.; Stojanović-Radić, Z.; Stojanović, P.; Stojanović, N.; Dekić, V.; Dekić, B.: A small library of 4-(alkylamino)-3-nitrocoumarin derivatives with potent antimicrobial activity against gastrointestinal pathogens. J. Serb. Chem. Soc. 80 (2015) 315–327.10.2298/JSC140619085RSearch in Google Scholar
13. Ristić, M.; Radulović, N.; Dekić, B.; Dekić, V.; Ristić, N.; Stojanović–Radić, Z.: Synthesis and spectral characterization of asymmetric azines containing a coumarin moiety: The discovery of new antimicrobial and antioxidant agents. Chem. Biodivers. 16 (2019) e1800486.10.1002/cbdv.201800486Search in Google Scholar PubMed
©2019 Vidoslav S. Dekić et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n


