Abstract
The (Electro)-Fenton-like reaction, recognized for its high efficiency in eliminating persistent organic pollutants from aqueous systems, has found extensive applications in industrial wastewater treatment. In recent years, metal-organic frameworks (MOFs) have garnered significant attention across the fields of physics, chemistry, and materials science due to their large specific surface area, flexible compositions, and tunable active sites. However, difficulty to recover, Fe ion leaching, and the demand for electron donors limited the development of current MOFs. This review concluded the classification, modification, and application of recent studies on Fenton-like reactions of MOFs. Initially, the classification of MOFs, including their single-site and bimetal MOFs, are introduced. Then, various modification methods are discussed, such as activation and pyrolysis, amino-functionalization, metal nanoparticle doping and co-catalysts loading. And those methods could significantly improve the efficiency by adding active sites, enhancing electron densities, and facilitating charge transfer. Finally, the advantages and limitations of MOFs as Fenton catalysts are evaluated, such as poor stability, and future perspectives are proposed. This review aims to provide a comprehensive overview of the application and modification strategies of MOFs in Fenton-like systems.
1 Introduction
Fenton reaction is developed by utilizing the redox reaction of Fe2+ or other transition metal ions with H2O2 to generate ˙OH with strong oxidation, for the degradation of refractory organic pollutants [1]. However, the narrow range of pH, large amount of iron sludge, and low utilization efficiency of H2O2 significantly restrict its application [2]. To address the mentioned issues, Fenton-like reaction has been developed. Among various catalysts, metal-organic frameworks (MOFs) have gained significant attention for their porous structure and high specific surface area [3].
The transformation between Fe(ii) and Fe(iii) of traditional catalysts usually become sluggish and decremental after few cycles, which prohibited the utilization of H2O2 [4]. Furthermore, the hydrolysis of Fe species to insoluble substances occurs at neutral and alkaline conditions. Consequently, homogeneous Fenton reactions are only feasible in acidic conditions [5]. Compared to traditional Fe-based catalysts, MOFs offer a greater range of metal ions and organic ligands, which can effectively address the issue of the traditional Fenton catalysts’ declining efficiency in utilizing hydrogen peroxide over time. MOFs possess several key attributes that make them ideal for use as Fenton-like catalysts: (i) a highly tunable porous structure [6], (ii) intrinsic organic-inorganic hybrid composition [7], (iii) multiple metal centers and active sites, and (iv) the potential for performance optimization from various angles [8]. Following the publication of the initial study on the catalytic potential of MOFs, these materials rapidly became a prominent research focus in materials science.
To highlight the significance of MOFs in Fenton-like catalysis, we conducted a search using the terms “metal-organic framework” and “Fenton.” As shown in Figure 1(a), the number of related publications surged from 6 in 2013 to 233 in 2022, while the corresponding citations increased from 26 to 11,536, marking a 443-fold rise (Figure 1b).
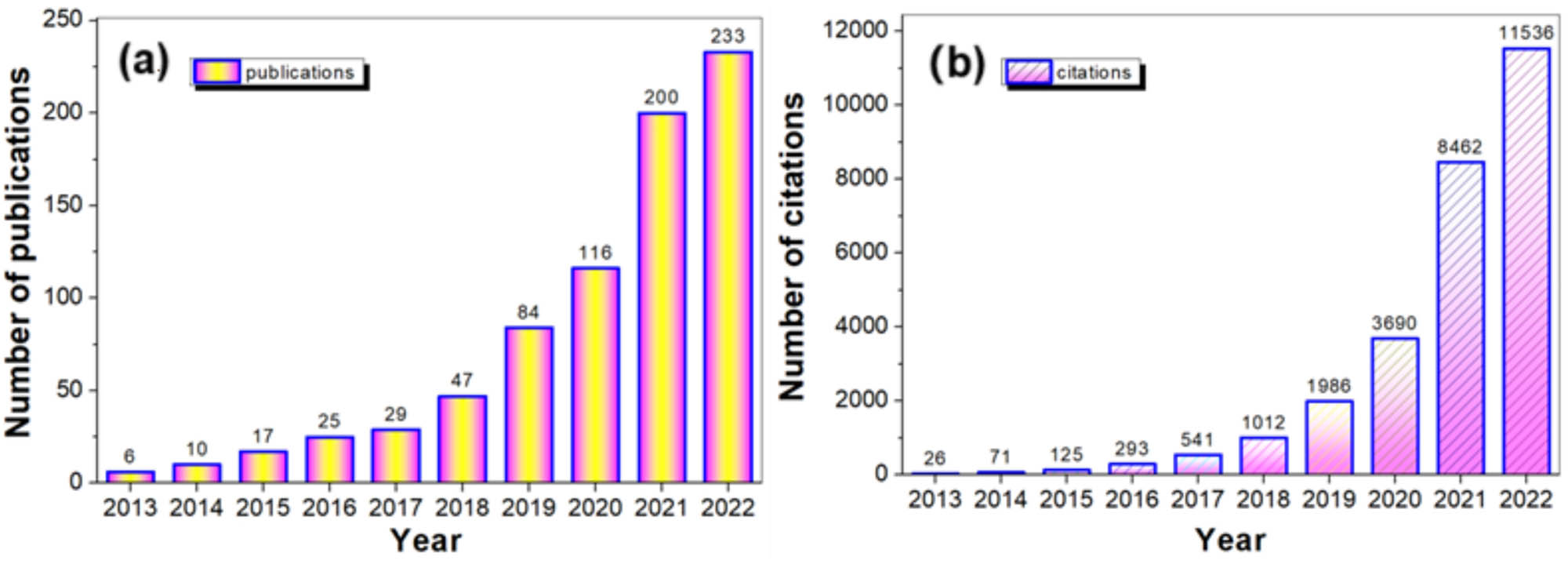
Number of publications (a) and number of citations (b) from 2013 to 2022 (data obtained on 15 January 2024, based on an investigation from WoS (Web of Science) using “metal-organic framework” and “Fenton” as searching terms).
However, the exploration of different MOF types across various applications, particularly in adapting them for Fenton-like systems, is still ongoing.
Nowadays, MOFs are widely used as catalysts in the elimination of environmental pollutants, such as antibiotics, dyes, and herbicides [9]. A restricted mass transfer rate results in a depletion of reactants within the active zone, which in turn leads to a reduction in the efficiency of the catalyst and a low utilization of the catalyst. To enhance the diffusion effect, it is essential to develop multistage porous structures comprising multistage pores, optimal interconnectivity, and a substantial number of active sites [10]. The interconnected pore structure of MOFs, however, facilitates the transport of reactants and access to active sites, which can effectively enhance the catalytic efficiency. The multi-component design of MOFs provides a greater number of active sites for catalytic reactions [11]. At the level of metal valence transformation, multi-component MOFs offer opportunities for effective contact between disparate metal sources and pollutants, the formation of more stable metal ion structures, and the modulation of intermetallic electron transfer. Moreover, the structural and chemical properties of MOFs can be specifically tailored and controlled according to the desired practical applications through the selection of organic ligands [12]. This property enables a more targeted removal of a specific pollutant in a Fenton-like reaction. Figure 2 illustrates the various strategies for synthesizing MOFs with catalytically active sites.
![Figure 2
Strategies for introduction of catalytically active sites into MOFs: (a) introduction of a linker by post-synthetic modification (PSM) or before assembly, (b) introduction of an active nanoparticle by PSM or by encapsulation (c), and utilization of carbonized MOF support to stabilize active sites (d). Reproduced with permission from [13]. Copyright 2020, American Chemical Society.](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_002.jpg)
Strategies for introduction of catalytically active sites into MOFs: (a) introduction of a linker by post-synthetic modification (PSM) or before assembly, (b) introduction of an active nanoparticle by PSM or by encapsulation (c), and utilization of carbonized MOF support to stabilize active sites (d). Reproduced with permission from [13]. Copyright 2020, American Chemical Society.
Notwithstanding the enhanced performance of MOFs, their catalytic efficiency remains suboptimal. The current status of MOF materials is characterized by the following challenges: (i) insufficient pore utilization results in low mass transfer efficiency, which is not conducive to the desorption and diffusion of pollutants from the catalyst surface into solution [14]; (ⅱ) Poor stability may result in the leaching of metal ions from the solution in large quantities, which could lead to the loss of active sites or poor reproducibility [15]; (ⅲ) the synthesis processes employed are often environmentally unfriendly or expensive, with some requiring significant quantities of solvents or precious metal raw materials [16]. This presents a challenge for industrial applications. This review presents a comprehensive introduction to the characteristics of common MOF catalysts, modification methods, and applications in catalysis. It also discusses the current challenges and difficulties faced by MOF materials and proposes a future development trend for MOF materials. It is the intention of this article to assist in the design of catalysts with optimized properties, thereby facilitating the large-scale application of MOF materials in Fenton-like processes.
2 Classification of common MOFs
2.1 Single-site MOFs
Since its discovery, MOFs have been recognized by the catalytic community as an excellent platform for isomer catalysis. In essence, MOFs can be viewed as molecules within a lattice, allowing homogeneous catalysts to extend throughout the structure, forming a periodic, catalytically active framework [13,17]. As a result, many synthesized MOFs function as single-site catalysts [18].
Tu et al. reported a novel MOF structure synthesized from CuI₃(HPyC)3] and CuII3(μ-OH)-(HPyC)3]2+ [19]. This innovative synthesis integrates metal ligands with zinc-based secondary building units in a single framework. MOFs retain the reversible Cu(i)/Cu(ii) redox properties, enhancing their catalytic performance. The catalytic efficiency of MOFs is largely influenced by the characteristics of their ligands and building units. The use of pyrazole ligands significantly stabilizes the resulting MOFs. Nikseresht et al. synthesized analogous [Cu3(BTC)2] catalysts via the ultrasonic method, confirming that copper atoms serve as the main active sites, with minimal contribution from other ligands [20]. Dhakshinamoorthy et al. utilized [Cu3(BTC)2] to catalyze the synthesis of Boro siloxanes from silane and pinene-boranes [21].
Fe(BTC) has proven to be an effective catalyst for the aerobic oxidation of cyclic and linear hydrocarbons, as shown by Nagarjun and Dhakshinamoorthy, without the need for peroxides or free radical initiators [22]. Han et al. demonstrated that materials of institut lavoisier (MIL)-100(Fe), synthesized without the use of hydrogen fluoride, exhibited excellent catalytic performance and stability in the acetalization of benzaldehyde with methanol [23]. The same approach was applied to MIL-101(Cr), which achieved a 35% yield of benzaldehyde in a benzyl alcohol oxidation test. Huang et al. investigated the catalytic activity of MIL-100(Fe ii) in the N,N-diethyl-p-phenylenediamine sulfate salt (DPD) reaction, finding that MIL-101(Fe ii) had a DPD affinity of 0.16 mM at 30°C [24]. The introduction of Fe²⁺ enhanced the formation of Fe(ii)-oxygen nodes, improving electron transfer between DPD and H₂O₂ and boosting the catalytic activity of MIL-101(Feii) in mimicking peroxidase activity. Alamgholiloo and Rostamnia used mixed-valence catalysts for the aza-Michael addition of amines to α and β-unsaturated compounds, employing open metal site (OMS)-MIL-100(Fe) Fe²⁺/Fe³⁺ [25].
MIL-100 treated under vacuum at 250°C produced up to 93% yield without additives and retained its activity after recovery. Han et al. also used vacuum-treated MIL-100(Fe) as a catalyst for selective ethylene tetramerization, showing the best catalytic performance at 250°C, with over 80% of the product being branched and cyclic C8 alkanes [26]. The co-catalyst influenced oligomerization by creating additional active sites. MIL-100(Cr) showed moderate catalytic activity for ethylene oligomerization, with a high selectivity of around 99% for C6, C8, and C10 olefins. X-ray Photoelectron Spectroscopy (XPS) analysis indicated that the reduction of Cr³⁺ to Cr²⁺ contributed to polymerization activity, with the highest performance at 250°C.
2.2 Bimetal MOFs
Recently, pollutants like chlorinated aromatic compounds and phenolic compounds have exacerbated water pollution, prompting a shift from Fe-based MOFs, which are insufficient for rapid degradation, to bimetallic MOF materials. Bimetallic ions offer multiple reaction sites and create electron-rich regions due to varying electron densities around the metal ions. This characteristic facilitates faster electron transfer in Fenton-like systems and enhances the activation of hydrogen peroxide for the degradation of organic pollutants [27].
For example, Ma et al. investigated Fe-Mn mixed oxide templates for the efficient and stable catalytic oxidation of 1,2-dichlorobenzene (DCB) [28]. In this work, Fe-Mn mixed oxide hollow microspheres were synthesized using a hard template method and evaluated for the catalytic oxidation of DCB, a model compound for polychlorinated dibenzodioxins and dibenzofurans (PCDDs/Fs). The FeMn20 hollow microspheres, with a Mn/(Fe + Mn) ratio of approximately 20%, exhibited the highest catalytic activity. The catalyst demonstrated excellent chlorine resistance, robust water resistance, and remarkable stability, showing no significant activity loss over 750 min of continuous operation. X-ray diffractometer, N2 sorption, transmission electron microscope (TEM), and XPS revealed that the high performance of FeMn20 was attributed to its large Brunauer-Emmett-Teller specific surface area, small crystallite size, and high surface-active oxygen concentration. This novel approach for preparing Fe-Mn mixed oxide hollow microspheres and the detailed structure-activity relationship provides valuable insights for designing and synthesizing mixed oxide catalysts for various reactions, including the catalytic oxidation of PCDD/Fs.
Liang et al. developed a new Fenton-like catalyst, CUMSs/MIL-101(Fe, Cu), incorporating MIL-101 with mixed valences of Fe(ii)/Fe(iii) and Cu(i)/Cu(ii) as coordinatively unsaturated metal sites (CUMSs) [29]. This catalyst was evaluated for its ability to degrade ciprofloxacin (CIP) through the catalytic activation of H₂O₂. The study found that CIP degradation occurs rapidly within a neutral pH range, with the catalytic activity of H₂O₂ being enhanced by a factor of 20 compared to MIL-101(Fe)/H₂O₂. The apparent rate constant for CUMSs/MIL-101(Fe, Cu) was 22 times higher than that of MIL-101(Fe)/H₂O₂ under various conditions.
To optimize CIP degradation, the effects of initial pH, catalyst dosage, H₂O₂ concentration, and coexisting anions were systematically investigated. Intermediates involved in the CIP degradation process were identified, and a proposed degradation pathway was suggested. The study revealed that the favorable thermodynamic reactions and π-cation interactions between Cu(i) and Fe(iii) enhance the redox cycles of Fe(ii)/Fe(iii) and Cu(i)/Cu(ii), thereby improving Fenton-like properties over a wide pH range. Density functional theory (DFT) calculations provided insights into the hydrogen atom dissociation process at the atomic level and the associated energy barriers. This research offers a novel approach for designing efficient, stable, and environmentally benign Fenton-like catalysts with enhanced remediation efficiency across a broad pH range (Figure 3).
![Figure 3
Possible mechanism of CIP degradation in CUMSs/MIL-101(Fe,Cu) based Fenton-like reaction. Reproduced with permission from [29]. Copyright 2021, Elsevier.](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_003.jpg)
Possible mechanism of CIP degradation in CUMSs/MIL-101(Fe,Cu) based Fenton-like reaction. Reproduced with permission from [29]. Copyright 2021, Elsevier.
Organic linkers are also crucial for optimizing catalyst performance. For instance, Chen et al. synthesized ZIF-67 by embedding cobalt in N-doped porous carbon [20]. The resulting composites, characterized by their layered porous carbon structure, nitrogen doping, and uniform Co dispersion, demonstrated superior electrocatalytic activity for both oxygen reduction reaction and oxygen evolution reaction. Additionally, the incorporation of multiple metals can enhance regional selectivity. Ramirez et al. investigated the effect of adding 14 different secondary metals (Fe, Cu, Mo, Li, Na, K, Mg, Ca, Zn, Ni, Co, Mn, Pt, and Rh) to Fe@C MOFMS for CO₂ hydrogenation to produce light olefins [30]. Their findings indicated that the catalytic performance could be tailored by varying the metal content in Fe MOFMS.
3 Modification methods of MOFs
3.1 Activation and pyrolysis
MOFs serving as sacrificial templates for creating heterogenous catalysts have recently attracted significant interest. Although initially met with criticism from both MOF researchers and the traditional catalysis community, numerous studies have since shown that the controlled decomposition of MOFs enables the synthesis of materials otherwise unattainable by conventional methods [31]. Through simple post-treatments, often involving pyrolysis, MOFs can be transformed into more stable materials. These MOF-derived structures (MOFMSs) retain key characteristics of the parent MOF, such as high porosity, tunable composition, and elevated metal content, making them highly suitable for heterogenous catalytic applications and often superior to traditional catalysts [32]. Additionally, this new class of MOFMSs offers nearly limitless possibilities for fine-tuning catalyst performance [33]. As a result, MOFMSs are gaining increasing popularity among researchers due to their remarkable properties.
The activation and pyrolysis of MOF materials in Fenton reactions have been extensively explored. For instance, Tang and Wang developed MIL-100(Fe) with Fe(ii)/Fe(iii) mixed-valence coordinatively unsaturated iron centers (CUS-MIL-100(Fe)) to improve the degradation efficiency of sulfamethazine [34]. Thermogravimetric analysis indicated that MIL-101(Fe) could be activated at 230°C, enhancing its performance (Figure 4). The incorporation of Fe(ii) and Fe(iii) CUSs significantly increased the specific surface area and created mesopores, which contributed to the superior sulfamethazine removal efficiency of CUS-MIL-100(Fe) (nearly 100%) compared to MIL-100(Fe) (17.6%).
![Figure 4
Schematic illustration of the structure evolution of MIL-100(Fe) before and after activation by pyrolysis (a), and comparison of the Fenton-like activity in sulfamethazine removal (b). Reproduced with permission from [34]. Copyright 2018, American Chemical Society.](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_004.jpg)
Schematic illustration of the structure evolution of MIL-100(Fe) before and after activation by pyrolysis (a), and comparison of the Fenton-like activity in sulfamethazine removal (b). Reproduced with permission from [34]. Copyright 2018, American Chemical Society.
The direct carbonization of parent MOFs in an inert atmosphere is a common method for producing metal nanoparticles embedded within a carbon matrix. This process yields highly dispersed nanoparticles encased in a porous carbon structure. Various MOFs, including those based on Fe, Zn, Co, and Cu, have been used for this purpose, with similar carbonization techniques applied across these materials. To control the final size of the nanoparticles, the carbonization temperature must be carefully managed. For example, Wezendonk et al. examined the structural changes in iron during the pyrolysis of the iron MOF Basolite F-300 using in situ X-ray Absorption Fine Structure, diffuse reflectance infrared Fourier transform spectroscopy, and Mössbauer spectroscopy [35]. Among all catalysts, Fe@C – 500 has the shortest activation time and reaches a 76% steady-state CO conversion level after ∼30 h of time-on-stream (TOS). The materials synthesized at 400 and 600°C reach similar conversion levels after around 60 h of TOS. They demonstrated that the carbonization temperature influenced the final dimensions, phase, and porosity of the Fe/C nanostructure. Specifically, pyrolysis at 400°C favored the formation of highly dispersed 2.5 nm epsilon carbides, while pyrolysis at 600°C produced 6.0 nm Hagg carbide particles. Particles larger than 28 nm underwent significant carburization and sintering at temperatures above this threshold (Figure 5).
![Figure 5
(a) Simulation of the effect of pyrolysis temperature on the Fe phase evolution, and (b) the calculated Fe phase distribution at certain pyrolysis temperatures. Reproduced with permission from [35]. Copyright 2016, American Chemical Society.](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_005.jpg)
(a) Simulation of the effect of pyrolysis temperature on the Fe phase evolution, and (b) the calculated Fe phase distribution at certain pyrolysis temperatures. Reproduced with permission from [35]. Copyright 2016, American Chemical Society.
Although activation and pyrolysis could efficiently enhance activity and stability [36], boost conductivity [37], and modify pore structure [38], the calcination temperature and atmosphere remained the key factors. A series of experiments were thus required to determine the optimal conditions. Consequently, pyrolytic activation was also confronted with the challenge of precisely governing the pyrolysis conditions. During this process, issues such as alterations in the environment of organic ligands or metal coordination, along with metal agglomeration, tended to occur. It reported that the presence of inorganic chlorine on the catalytic sites can lead to deactivation [39]. Increasing the reaction temperature can promote the desorption of inorganic chlorine, allowing the activity of the poisoned catalyst to be restored. Moreover, in the course of high-temperature pyrolysis, the pristine structure of MOFs is prone to being impaired. This gives rise to the collapse of their initially well-ordered pore architectures and alterations in the coordination surroundings of metal nodes and organic ligands. Subsequently, certain inherent characteristics of MOFs are forfeited, thereby impeding the full manifestation of their performance capabilities.
3.2 Amino-functionalization MOF structure
MOFs with intrinsic catalytic activity have been previously noted, and further enhancements often involve functional groups. For Fenton reactions, amino-functionalized materials are particularly prevalent. Liu et al. utilized NH₂-MIL-101(Fe) in a persulfate-activated system for degrading bisphenol F (BPF) [40]. This material demonstrated greater degradation efficiency compared to the parent MIL-101(Fe), showing a broader pH range adaptation and improved resistance to anion interference. Additionally, NH₂-MIL-101(Fe) was synthesized at room temperature for degrading bisphenol A (BPA). The amino groups enhanced the in situ formation of Fe(II) by modulating the Fe-oxo nodes within the MIL framework. The results indicated that NH₂-MIL-101(Fe) achieved a significantly higher BPA degradation rate (91% within 40 min) than MIL-101(Fe) (4.4%). DFT calculations demonstrated that the introduction of the –NH2 modification to NH2-MIL-101(Fe) could markedly enhance its electronic conductivity. This was evidenced by an increase in the Fermi level to −4.28 eV and a strengthening of the binding energy to PS to −1.19 eV. (Figure 6).
![Figure 6
Comparison of the degradation mechanism of BPF in NH2-MIL-101(Fe)/PS and MIL-101(Fe)/PS systems. Reproduced with permission from [40]. Copyright 2020, Elsevier.](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_006.jpg)
Comparison of the degradation mechanism of BPF in NH2-MIL-101(Fe)/PS and MIL-101(Fe)/PS systems. Reproduced with permission from [40]. Copyright 2020, Elsevier.
Wang et al. designed C2H6-selective MOF (Tb-MOF-76(NH2)) materials modified with amino groups with the objective of enhancing the C2H6/C2H4 separation performance of MOFs [41]. The introduction of amino groups (-NH2) resulted in a reduction in the pore size of Tb-MOF-76(NH2) (from 7.9 × 7.9 Å2 to 7.2 × 7.2 Å2), which led to an increase in host–guest interactions within the restricted pores. This, in turn, led to an enhancement in the C2H6 and C2H4 adsorption capacity, as well as the C2H6/C2H4 selectivity, of Tb-MOF-76(NH2) in comparison to the original material. In a recent publication, Huang et al. presented the employment of an amino-functionalized MOF MIL-125-NH2 for the photo modulated N-alkylation of various amines with aromatic halides. This approach enables the selective synthesis of secondary amines and imines under ambient conditions, with no need for harsh oxidants and additives [42]. Under dark conditions, the Lewis acid site of MIL-125-NH2 promotes the direct dehalogenation condensation of benzyl halides with a range of amines to produce secondary amines; upon illumination, the photogenerated oxygen radical of MIL-125-NH2 oxidizes benzyl halides to produce aldehydes, which are coupled with a range of amines via acceptorless dehydrogenative coupling (ADC) to produce imines.
Amination can regulate the surface charge and electronic structure of MOFs. The amino group can be used as a functional modification site to regulate the properties and expand the applications [43]. However, in the Fenton-like reaction, the amino group was susceptible to structural changes or decomposition due to external factors [44]. Furthermore, the synthesis process was relatively complicated, and the contact between the reactants and the active site might be hindered by the spatial site resistance effect [45].
3.3 Metal nanoparticle doping
A number of nano-metals have been observed to exhibit high activity levels, and the combination of these with organic frameworks is anticipated to result in a more effective catalytic degradation effect. For example, nano zero-valent iron (nZVI) is a type of particle that exhibits high activity and is prone to aggregation. The use of porous materials is necessary to enhance the stability of the system. Hou et al. modified MIL-101(Cr) with nZVI through impregnation of nZVI onto MIL-101(Cr) for the purposes of adsorption and degradation of tetracycline (TC) [46]. The use of MIL-101(Cr) provided a substantial surface area for nZVI coating, thereby enhancing the adsorption capacity of TC to a certain extent. Fortunately, the nZVI/MIL-101(Cr) composite exhibited comparable Fenton-like reactivity and reduced iron leaching. Moreover, the combination of the framework and nano-metal has the potential to effectively enhance the Lewis acid site of the material. The results demonstrated that nZVI/MIL-101(Cr) was capable of degrading 90% of the dye within 120 min.
Zhang et al. prepared Fe/Ni@ZIF-8 capable of effectively degrading ofloxacin (OFX) [47]. Under the optimal reaction conditions (temperature 318 K, pH 6.0, H2O2 concentration of 10 mM, and initial OFX concentration of 10 mg L−1), the removal rate of OFX was greater than 98%. The overall removal of OFX by Fe/Ni@ZIF-8 was achieved through a combination of oxidation and adsorption. Fe/Ni@ZIF-8 adsorbed OFX mainly via electrostatic and π–π bonding interactions, and Fenton oxidation oxidized and degraded OFX by generating −OH through the reaction between Fe2+ and H2O2 (Figure 7).
![Figure 7
Degradation mechanism of OFX in Fe/Ni@ZIF-8 during the catalytic reaction [47].](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_007.jpg)
Degradation mechanism of OFX in Fe/Ni@ZIF-8 during the catalytic reaction [47].
The loading of metal nanoparticles on MOFs offered a number of advantages. It had the potential to expand the number of active sites [48]. The porous structure of MOFs facilitates the dispersion of nanoparticles and enables electronic interactions, thereby optimizing the reaction and exhibiting a synergistic effect [49]. However, there are also some disadvantages associated with this approach. The loading stability is low due to the weak interaction between the two components, which may result in agglomeration and detachment [50]. Therefore, enhanced binding is required to address this issue. The restricted access of macromolecules to the microporous structure hinders mass transfer. Furthermore, residual solvents from the nanoparticle synthesis process may potentially act as impurities [51].
3.4 Co-catalysts loading
Ahmad et al. successfully synthesized MIL-88B-Fe catalysts (CUCs-MIL-88B-Fe) with mixed-valence coordinated unsaturated iron (Fe2+/Fe3+) centers on ultrathin Ti3C2 nanosheets through thermal activation under vacuum conditions [52]. Using two-dimensional ultrathin Ti3C2 nanosheets as co-catalysts can significantly enhance charge separation capabilities, especially for hole separation, because of the nanosheets’ low Fermi level (Figure 8). The catalyst showed an efficiency of more than 90% in removing both phenol and sulfamethoxazole.
![Figure 8
Schematic diagram of charge separation mechanism in CUCs-MIL-88B-Fe/Ti3C2 and H2O2 activation by CUCs-MIL-88B-Fe during catalytic reactions [52].](/document/doi/10.1515/rams-2025-0099/asset/graphic/j_rams-2025-0099_fig_008.jpg)
Schematic diagram of charge separation mechanism in CUCs-MIL-88B-Fe/Ti3C2 and H2O2 activation by CUCs-MIL-88B-Fe during catalytic reactions [52].
Li et al. synthesized MoS2 peony-like heterojunction composites with Fe-based MOF matrix (NH2-MIL-101 (Fe)) by a facile hydrothermal method [53]. The composites were able to remove 97.4% rhodamine B (50 ppm) and 99.9% BPA (20 ppm) within 10 min. Based on the experimental results and characterization analysis, the heterogeneous combination of catalyst and co-catalyst provided by the system was the main reason for the improved efficiency of the Fenton reaction.
Co-catalyst significantly improved charge separation of MOF; however, the unsatisfied stability of MOF might lead to the decomposition of composites [54]. In addition, the loading of co-catalysts required extra cots and the mass of loading should be carefully designed.
4 Performance of MOF-mediated catalysts in Fenton-like reaction
In recent times, MOFs have been the subject of extensive and rapid study in the field of environmental remediation. Of particular interest has been the use of MOFs as Fenton-like catalysts for the treatment of organic wastewater and antibacterial.
4.1 Organic pollutants
The Zhao group employed a cathode composed of MOF (2Fe/Co) on carbon aerogel, where iron and cobalt served as the metal centers and trimesic acid as the linker, to degrade rhodamine B (RhB) and dimethyl phthalate (DMP) [15]. They discovered that the application of visible light irradiation enhanced the removal efficiency of RhB by 52% within 45 min and DMP by 41% within 120 min, outperforming the electro-Fenton process. Lv and colleagues synthesized Fe(ii)@MIL-100(Fe) by incorporating Fe(ii) into MIL-100(Fe), resulting in a material with a high degradation capacity for methylene blue, achieving 70% decomposition within 3 h across a broad pH range of 3–8 [16]. It is proposed that the coexistence of Fe(ii) and Fe(iii) in the material could boost the generation of hydroxyl radicals (˙OH). The performance of select MOF-based catalysts is presented in Table 1.
Performance of MOF catalysts in Fenton-like reaction for organic pollutants degradation
| Sample | S BET area m2·g−1 | Fenton-like conditions | Target compound | Efficiency | Ref. |
|---|---|---|---|---|---|
| MIL-101(FeII 3, Mn) | — | Catalyst, 100 mg·L−1; pH = 6; H2O2, 20 mM; Temp., 35°C | Phenol | >99% (10 min) | [55] |
| MIL-100(Fe)/Fe3O4 | 1,433 | Catalyst, 0.1 g·L−1; H2O2, 98 mM; pH = 4; Temp., room temp. | Sodium sulfadiazine | >90% (240 min) | [56] |
| N-BiFeO3/NH2-MIL-53(Fe) | — | Catalyst, 200 mg·L−1; pH = 7; H2O2, 0.2 mL·L−1; Temp., room temp. | Tetracycline hydrochloride | >99% (60 min) | [57] |
| Cu2O/MIL(Fe/Cu) | 1,553 | Catalyst, 25 mg; H2O2, 49 mM; pH = 7.47; temp., 25°C | Thiacloprid | 100% (25 min) | [58] |
| MIL-101(Fe,Co) | 1,206 | Catalyst, 0.2 g·L−1; pH, 5; H2O2, 5 mM, temp., 25°C | CIP | 98% (30 min) | [59] |
| BMFe-4 | 26 | Catalyst, 0.5 g·L−1; pH = 5.5; H2O2, 5 mM | BPA | 99% (10 min) | [60] |
| Phenol | 59% (60 min) | ||||
| Methylene blue | 90.4% (120 min) | ||||
| Carbamazepine | 67% (80 min) | ||||
| MIPMIL-100(Fe)CUS | 60 | Catalyst, 1 g·L−1; PS, 0.3 g; pH = 2.8; temp., 25°C | Diethyl Phthalate | 13.64 mg·g−1 (120 min) | [61] |
| MIL-88B | 42 | Catalyst, 0.3 g·L−1; pH = 5; H2O2, 44 mM; | Thiacloprid | 83.3% (7 min) | [62] |
| MIL-53(Fe) | 88 | Catalyst, 0.5 g·L−1; H2O2, 192 mM; temp., 30°C | Methyl orange | pH = 2, 100% (70 min); pH = 12, 47% (120 min) | [63] |
| TiO2@NH2-MIL-88B(Fe) | 23.8 | Catalyst, 0.2 g·L−1; H2O2, 20 mM; pH = 7; temp., 25°C | Methylene blue | 100% (150 min) | [64] |
| r-MIL-88A | 24 | Catalyst, 0.015 g; H2O2, 0.1 mL; pH = 4.5; temp., 20°C | Phenol | 100% (15 min) | [65] |
| CoFe-alloy@N-doped graphitic carbon (CoFe@NC) | — | Catalyst, 0.1 g·L−1; PMS, 0.4 mM; pH = 5.9; temp., 25°C | Atrazine | 100% (50 min) | [66] |
| Prussian blue | — | Catalyst, 300 mg·L−1; H2O2, 50 mg·L−1; Temp., 25 °C | Methylene blue | 100% (30 min) | [67] |
| Fe1ZIF-8 | — | Catalyst, 20 mg; H2O2, 12 mM; pH = 3.0; temp., 40°C | Tar | 66.5% (240 min) | [68] |
| MSS@Co-CN | 13.9 | Catalyst, 30 mg; PMS, 1 mM; pH = 3.0; temp., 40°C | Tetracycline | 90% (20 min) | [69] |
4.2 Antibacterial
Liu et al. designed a new bimetallic hybrid nanoenzyme based on IRMOF-3 for repairing bacterial infection wounds [70]. It inhibits the growth of both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria by generating hydroxyl radicals (˙OH) through a Fenton-like reaction. When treated with 400 μg·mL−1 Fe3O4@MOF@Au nano enzymes and a low dose of H2O2, the antibacterial activities of E. coli and S. aureus are significantly suppressed, reaching 97 and 98%, respectively. Recently, it has been reported in the literature that the Fenton chemistry of MOFs has been successfully activated by a trace cobalt-doping strategy, leading to excellent antimicrobial efficacy [71]. It has been shown to achieve a kill rate of more than 99% against Escherichia coli and Staphylococcus aureus and can effectively promote wound healing in bacterial infections.
5 Summary and outlook
MOFs are emerging as a substitute for conventional porous materials in addressing new contaminants, leveraging their distinctive attributes such as exceptional porosity and surface area. By harnessing the redox-active characteristics of these hybrid materials and integrating them onto suitable substrates, the development of future technological devices with enhanced efficiency is anticipated.
The modification of MOFs is a critical area of research aimed at enhancing their properties and expanding their utility in various fields. Here are some key strategies for modifying MOFs:
Controlling the size and distribution of metal centers within the framework;
Exploring new organic ligands;
Defect engineering;
Morphology control.
While innovative and effective modification techniques can markedly enhance the functionality of MOFs, there remain significant obstacles to the large-scale deployment of MOFs:
Further investigation is required to ascertain the stability and reproducibility of MOFs in Fenton-like reactions. While MOF-based materials have been extensively studied, their stability remains a critical issue. Researchers need to assess whether MOFs can fully replace traditional homogeneous catalysts.
Future development of MOFs in industrial employment. Most research on MOFs synthesis is limited to labs, making it hard to get enough precursors for further processes. To produce MOFs on a large scale, researchers need to consider cost, material availability, solvent safety, yield, and equipment. Simple, reliable, affordable, scalable, and eco-friendly methods are urgently needed.
Additional measures are needed to reduce environmental risks from practical uses. The leaching rate of metals, such as Fe, from MOF materials in Fenton-like reactions is typically maintained at a dose of 2 mg·L−1. However, it remains unclear whether the metal leaching rate can be maintained at an environmentally benign level after repeated recycling, particularly given that acidic conditions accelerate metal leaching. Additionally, the stability of organic ligands in MOF is a significant concern.
Further study is required to gain a deeper understanding of the intrinsic mechanism of the Fenton-like reaction. Many studies lack detailed information on the intermediate products of pollutant degradation reactions. Structural stability, which impacts reuse rates, is a key concern, along with the potential for structural defects in MOFs. Addressing these issues could enhance MOF applications in fields like photocatalysis and electrocatalysis.
-
Funding information: This work was financially supported by the 2024 Science and Technology Planning Project of Henan Province [242102311257], Henan Province Major Science and Technology Special Project [231100310200] and Major Science and Technology Project of China National Tobacco Corporation [110202201005 (JY-05)].
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Mitsika, E. E., C. Christophoridis, and K. Fytianos. Fenton and Fenton-like oxidation of pesticide acetamiprid in water samples: kinetic study of the degradation and optimization using response surface methodology. Chemosphere, Vol. 93, No. 9, 2013, pp. 1818–1825.10.1016/j.chemosphere.2013.06.033Search in Google Scholar PubMed
[2] Neyens, E. and J. Baeyens. A review of classic Fenton’s peroxidation as an advanced oxidation technique. Journal of Hazardous materials, Vol. 98, No. 1, 2003, pp. 33–50.10.1016/S0304-3894(02)00282-0Search in Google Scholar PubMed
[3] Li, L., J. Han, X. Huang, S. Qiu, X. Liu, L. Liu, et al. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. Journal of Environmental Chemical Engineering, Vol. 11, 2023, id. 111217.10.1016/j.jece.2023.111217Search in Google Scholar
[4] Brillas, E., I. Sirés, and M. A. Oturan. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chemical Reviews, Vol. 10912, 2009, pp. 6570–6631.10.1021/cr900136gSearch in Google Scholar PubMed
[5] Antwi-Baah, R. and H. Liu. Recent hydrophobic metal-organic frameworks and their applications. Materials, Vol. 1111, 2018, id. 2250.10.3390/ma11112250Search in Google Scholar PubMed PubMed Central
[6] Ezugwu, C. I., J. M. Sonawane, and R. Rosal. Redox-active metal-organic frameworks for the removal of contaminants of emerging concern. Separation and Purification Technology, Vol. 284, 2022, id. 120246.10.1016/j.seppur.2021.120246Search in Google Scholar
[7] Murtaza, S. Z., H. T. Alqassem, R. Sabouni, and M. Ghommem. Degradation of micropollutants by metal organic framework composite-based catalysts: a review. Environmental Technology & Innovation, Vol. 29, 2023, id. 102998.10.1016/j.eti.2022.102998Search in Google Scholar
[8] Wang, J., C. Xue, W. Yao, J. Liu, X. Gao, R. Zong, et al. MOF-derived hollow TiO2@C/FeTiO3 nanoparticles as photoanodes with enhanced full spectrum light PEC activities. Applied Catalysis B: Environmental, Vol. 250, 2019, pp. 369–381.10.1016/j.apcatb.2019.03.002Search in Google Scholar
[9] Hu, T., L. Tang, H. Feng, J. Zhang, X. Li, Y. Zuo, et al. Metal-organic frameworks (MOFs) and their derivatives as emerging catalysts for electro-Fenton process in water purification. Coordination Chemistry Reviews, Vol. 451, 2022, id. 214277.10.1016/j.ccr.2021.214277Search in Google Scholar
[10] Li, X., D. Wu, T. Hua, X. Lan, S. Han, J. Cheng, et al. Micro/macrostructure and multicomponent design of catalysts by MOF-derived strategy: Opportunities for the application of nanomaterials-based advanced oxidation processes in wastewater treatment. Science of The Total Environment, Vol. 804, 2022, id. 150096.10.1016/j.scitotenv.2021.150096Search in Google Scholar PubMed
[11] Zheng, F., S. Cao, Z. Yang, Y. Sun, Z. Shen, Y. Wang, et al. Synthesis and catalytic application of MOF complexes containing noble metals. Energy & Fuels, Vol. 3813, 2024, pp. 11494–11520.10.1021/acs.energyfuels.4c01963Search in Google Scholar
[12] Qiu, J., X. Zhang, Y. Feng, X. Zhang, H. Wang, and J. Yao. Modified metal-organic frameworks as photocatalysts. Applied Catalysis B: Environmental, Vol. 231, 2018, pp. 317–342.10.1016/j.apcatb.2018.03.039Search in Google Scholar
[13] Bavykina, A., N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez, and J. Gascon. Metal–organic frameworks in heterogeneous catalysis: recent progress, new trends, and future perspectives. Chemical reviews, Vol. 12016, 2020, pp. 8468–8535.10.1021/acs.chemrev.9b00685Search in Google Scholar PubMed
[14] Xu, J., Y. Qi, C. Wang, and L. Wang. NH2-MIL-101(Fe)/Ni(OH)2-derived C,N-codoped Fe2P/Ni2P cocatalyst modified g-C3N4 for enhanced photocatalytic hydrogen evolution from water splitting. Applied Catalysis B: Environmental, Vol. 241, 2019, pp. 178–186.10.1016/j.apcatb.2018.09.035Search in Google Scholar
[15] Zhao, H., Y. Chen, Q. Peng, Q. Wang, and G. Zhao. Catalytic activity of MOF (2Fe/Co)/carbon aerogel for improving H2O2 and ·OH generation in solar photo–electro–Fenton process. Applied Catalysis B: Environmental, Vol. 203, 2017, pp. 127–137.10.1016/j.apcatb.2016.09.074Search in Google Scholar
[16] Lv, H., H. Zhao, T. Cao, L. Qian, Y. Wang, and G. Zhao. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. Journal of Molecular Catalysis A: Chemical, Vol. 400, 2015, pp. 81–89.10.1016/j.molcata.2015.02.007Search in Google Scholar
[17] Bajwa, R. A., U. Farooq, S. Ullah, M. Salman, S. Haider, and R. Hussain. Metal-organic framework (MOF) attached and their derived metal oxides (Co, Cu, Zn and Fe) as anode for lithium ion battery: A review. Journal of Energy Storage, Vol. 72, 2023, id. 108708.10.1016/j.est.2023.108708Search in Google Scholar
[18] Gao, D., H. Ji, R. Li, M. T. Munir, X. Wu, Y. Huang, et al. Advancing sustainable phosphorus removal and recovery with Metal-Organic frameworks (MOFs). Chemical Engineering Journal, Vol. 475, 2023, id. 145949.10.1016/j.cej.2023.145949Search in Google Scholar
[19] Tu, B., Q. Pang, H. Xu, X. Li, Y. Wang, and Z. Ma. Reversible redox activity in multicomponent metal–organic frameworks constructed from trinuclear copper pyrazolate building blocks. Journal of the American Chemical Society, Vol. 13923, 2017, pp. 7998–8007.10.1021/jacs.7b03578Search in Google Scholar PubMed
[20] Chen, B., G. Ma, Y. Zhu, and Y. Xia. Metal-organic-frameworks derived cobalt embedded in various carbon structures as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Scientific Reports, Vol. 7, 2017, id. 5266.10.1038/s41598-017-05636-ySearch in Google Scholar PubMed PubMed Central
[21] Dhakshinamoorthy, A., A. M. Asiri, P. Concepcion, and H. Garcia. Synthesis of borasiloxanes by oxidative hydrolysis of silanes and pinacolborane using Cu3(BTC)2 as a solid catalyst. Chemical communications, Vol. 53, No. 72, 2017, pp. 9998–10001.10.1039/C7CC05221ASearch in Google Scholar PubMed
[22] Nagarjun, N. and A. Dhakshinamoorthy. Liquid phase aerobic oxidation of cyclic and linear hydrocarbons using iron metal organic frameworks as solid heterogeneous catalyst. Molecular Catalysis, Vol. 463, 2019, pp. 54–60.10.1016/j.mcat.2018.11.012Search in Google Scholar
[23] Han, L., H. Qi, D. Zhang, G. Ye, W. Zhou, C. Hou, et al. A facile and green synthesis of MIL-100 (Fe) with high-yield and its catalytic performance. New Journal of Chemistry, Vol. 4122, 2017, pp. 13504–13509.10.1039/C7NJ02975FSearch in Google Scholar
[24] Huang, P., Q. Chang, G. Jiang, X. Wang, H. Zhu, and Q. Liu. Rapidly and ultra-sensitive colorimetric detection of H2O2 and glucose based on ferrous-metal organic framework with enhanced peroxidase-mimicking activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 285, 2023, id. 121943.10.1016/j.saa.2022.121943Search in Google Scholar PubMed
[25] Rostamnia, S. and H. Alamgholiloo. Synthesis and catalytic application of mixed valence iron (FeII/FeIII)-based OMS-MIL-100(Fe) as an efficient green catalyst for the aza-Michael reaction. Catalysis Letters, Vol. 148, 2018, pp. 2918–2928.10.1007/s10562-018-2490-5Search in Google Scholar
[26] Han, Y., Y. Zhang, Y. Zhang, A. Cheng, Y. Hu, and Z. Wang. Selective ethylene tetramerization with iron-based metal−organic framework MIL-100 to obtain C8 alkanes. Applied Catalysis A: General, Vol. 564, 2018, pp. 183–189.10.1016/j.apcata.2018.07.030Search in Google Scholar
[27] Zhang, S., M. Li, J. Wang, R. Zhang, X. Ma, and H. Tao. Bimetal-organic framework MIL-53 (Fe, Ni) stimulates peroxydisulfate to degrade rhodamine B: Properties and degradation mechanism. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 664, 2023, id. 131208.10.1016/j.colsurfa.2023.131208Search in Google Scholar
[28] Ma, X., J. Wen, H. Guo, and G. Ren. Facile template fabrication of Fe-Mn mixed oxides with hollow microsphere structure for efficient and stable catalytic oxidation of 1,2-dichlorobenzene. Chemical Engineering Journal, Vol. 382, No. 1, 2020, id. 122940.10.1016/j.cej.2019.122940Search in Google Scholar
[29] Liang, H., R. Liu, X. An, C. Hu, X. Zhang, and H. Liu. Bimetal-organic frameworks with coordinatively unsaturated metal sites for highly efficient Fenton-like catalysis. Chemical Engineering Journal, Vol. 414, 2021, id. 128669.10.1016/j.cej.2021.128669Search in Google Scholar
[30] Ramirez, A., L. Gevers, A. Bavykina, S. Ould-Chikh, and J. Gascon. Metal organic framework-derived iron catalysts for the direct hydrogenation of CO2 to short chain olefins. ACS Catalysis, Vol. 810, 2018, pp. 9174–9182.10.1021/acscatal.8b02892Search in Google Scholar
[31] Zhou, P., S. Zhang, Z. Ren, Y. Wang, Y. Zhang, and C. Huang. Study on the thermal decomposition behavior of ammonium perchlorate catalyzed by Zn-Co cooperation in MOF. Inorganic Chemistry Frontiers, Vol. 920, 2022, pp. 5195–5209.10.1039/D2QI00968DSearch in Google Scholar
[32] Liu, J., L. Zhao, H. Geng, B. Wang, X. Tong, Y. Li, et al. Fe-MOF-derived carbon compounds as catalysts for trichloroethylene degradation via persulfate oxidation: Role of precursor template and pyrolysis temperature. Journal of Environmental Chemical Engineering, Vol. 11, No. 5, 2023, id. 110649.10.1016/j.jece.2023.110649Search in Google Scholar
[33] Zhang, M., Z. Chen, J. Ruan, W. Shao, W. Wei, W. Zhang, et al. Functional polymers-assisted confined pyrolysis strategy to transform MOF into hierarchical Co/N-doped carbon for peroxymonosulfate advanced oxidation processes. Separation and Purification Technology, Vol. 305, 2023, id. 122407.10.1016/j.seppur.2022.122407Search in Google Scholar
[34] Tang, J. and J. Wang. Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine. Environmental Science & Technology, Vol. 52, No. 9, 2018, pp. 5367–5377.10.1021/acs.est.8b00092Search in Google Scholar PubMed
[35] Wezendonk, T. A., V. P. Santos, M. A. Nasalevich, Q. S. Warringa, A. I. Dugulan, A. Chojecki, et al. Elucidating the nature of Fe species during pyrolysis of the Fe-BTC MOF into highly active and stable Fischer–Tropsch catalysts. ACS catalysis, Vol. 6, No. 5, 2016, pp. 3236–3247.10.1021/acscatal.6b00426Search in Google Scholar
[36] Wang, F., Y. Gao, H. Fu, S. Liu, Y. Wei, P. Wang, et al. Almost 100% electron transfer regime over Fe-Co dual-atom catalyst toward pollutants removal: Regulation of peroxymonosulfate adsorption mode. Applied Catalysis B: Environmental, Vol. 339, 2023, id. 123178.10.1016/j.apcatb.2023.123178Search in Google Scholar
[37] Zhang, L., Y. Hu, J. Chen, W. Huang, J. Cheng, and Y. Chen. A novel metal organic framework-derived carbon-based catalyst for oxygen reduction reaction in a microbial fuel cell. Journal of Power Sources, Vol. 384, 2018, pp. 98–106.10.1016/j.jpowsour.2018.02.078Search in Google Scholar
[38] Rasaily, S., K. Baruah, D. Sharma, P. Lepcha, S. Biswas, A. N. Biswas, et al. Rationally designed manganese-based metal–organic frameworks as altruistic metal oxide precursors for noble metal-free oxygen reduction reaction. Inorganic Chemistry, Vol. 62, No. 7, 2023, pp. 3026–3035.10.1021/acs.inorgchem.2c03707Search in Google Scholar PubMed
[39] Jiang, T., X. Wang, J. Zhang, Y. Mai, and J. Chen. Efficient MnCeOx catalyst derived from MnCe-MOF for chlorobenzene oxidation: effects of metal ratio, pyrolysis atmosphere and pyrolysis temperature. Microporous and Mesoporous Materials, Vol. 368, 2024, id. 113035.10.1016/j.micromeso.2024.113035Search in Google Scholar
[40] Liu, Z., R. Su, X. Sun, W. Zhou, B. Gao, Q. Yue, et al. The obvious advantage of amino-functionalized metal-organic frameworks: As a persulfate activator for bisphenol F degradation. Science of the Total Environment, Vol. 741, 2020, id. 140464.10.1016/j.scitotenv.2020.140464Search in Google Scholar PubMed
[41] Wang, G. D., R. Krishna, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang, et al. Boosting ethane/ethylene separation by MOFs through the amino‐functionalization of pores. Angewandte Chemie, Vol. 134, No. 48, 2022, pp. 1–7.10.1002/ange.202213015Search in Google Scholar
[42] Huang, Y., Y. Li, D. Zhang, Y. Mai, F. Besenbacher, C. Dong, et al. Light-switchable N-alkylation using amine-functionalized MOF. Applied Catalysis B: Environment and Energy, Vol. 350, 2024, id. 123924.10.1016/j.apcatb.2024.123924Search in Google Scholar
[43] Liang, F., Z. Nie, Y. Xia, Y. Yan, X. Chen, B. Liu, et al. Decontamination of neutral aqueous systems containing organophosphate esters by zirconium-based metal organic frameworks with or without amino groups. Journal of Environmental Chemical Engineering, Vol. 10, No. 6, 2022, id. 108945.10.1016/j.jece.2022.108945Search in Google Scholar
[44] Feng, L., K. Wang, G. S. Day, M. R. Ryder, and H. Zhou. Destruction of metal–organic frameworks: positive and negative aspects of stability and lability. Chemical Reviews, Vol. 12023, 2020, pp. 13087–13133.10.1021/acs.chemrev.0c00722Search in Google Scholar PubMed
[45] Ahmad, M. Z., M. Navarro, M. Lhotka, B. Zornoza, C. Téllez, W. M. de Vos, et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. Journal of membrane science, Vol. 558, 2018, pp. 64–77.10.1016/j.memsci.2018.04.040Search in Google Scholar
[46] Hou, X., J. Shi, N. Wang, Z. Wen, M. Sun, J. Qu, et al. Removal of antibiotic tetracycline by metal-organic framework MIL-101(Cr) loaded nano zero-valent iron. Journal of Molecular Liquids, Vol. 313, 2020, id. 113512.10.1016/j.molliq.2020.113512Search in Google Scholar
[47] Zhang, T., P. Wu, G. Owens, and Z. Chen. Adsorption and fenton-like oxidation of ofloxacin in wastewater using hybrid MOF bimetallic Fe/Ni nanoparticles. Chemosphere, Vol. 307, 2022, id. 135936.10.1016/j.chemosphere.2022.135936Search in Google Scholar PubMed
[48] Zhang, Y. and S. Park. Stabilization of dispersed CuPd bimetallic alloy nanoparticles on ZIF-8 for photoreduction of Cr(VI) in aqueous solution. Chemical Engineering Journal, Vol. 369, 2019, pp. 353–362.10.1016/j.cej.2019.03.083Search in Google Scholar
[49] Mukoyoshi, M. and H. Kitagawa. Nanoparticle/metal–organic framework hybrid catalysts: elucidating the role of the MOF. Chemical Communications, Vol. 5877, 2022, pp. 10757–10767.10.1039/D2CC03233CSearch in Google Scholar PubMed
[50] Habib, N. R., E. Asedegbega-Nieto, A. M. Taddesse, and I. Diaz. Non-noble MNP@MOF materials: synthesis and applications in heterogeneous catalysis. Dalton Transactions, Vol. 5030, 2021, pp. 10340–10353.10.1039/D1DT01531ASearch in Google Scholar
[51] Yang, Q., Q. Xu, and H. Jiang. Metal-organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis. Chemical Society Reviews, Vol. 4615, 2017, pp. 4774–4808.10.1039/C6CS00724DSearch in Google Scholar
[52] Ahmad, M., X. Quan, S. Chen, and H. Yu. Tuning Lewis acidity of MIL-88B-Fe with mix-valence coordinatively unsaturated iron centers on ultrathin Ti3C2 nanosheets for efficient photo-Fenton reaction. Applied Catalysis B: Environmental, Vol. 264, 2020, id. 118534.10.1016/j.apcatb.2019.118534Search in Google Scholar
[53] Li, Z., Y. Gu, and F. Li. Heterogeneous fenton system with dual working mechanisms for aqueous pollutants degradation. Journal of Environmental Chemical Engineering, Vol. 10, No. 3, 2022, id. 107686.10.1016/j.jece.2022.107686Search in Google Scholar
[54] Yang, C., Z. Xue, and J. Wen. Recent advances in MOF-based materials for remediation of heavy metals and organic pollutants: insights into performance, mechanisms, and future opportunities. Sustainability, Vol. 15, No. 8, 2023, id. 6686.10.3390/su15086686Search in Google Scholar
[55] Huang, P., Q. Chang, G. Jiang, K. Xiao, and X. Wang. MIL-101 (FeII3,Mn) with dual-reaction center as Fenton-like catalyst for highly efficient peroxide activation and phenol degradation. Separation and Purification Technology, Vol. 306, 2023, id. 122582.10.1016/j.seppur.2022.122582Search in Google Scholar
[56] Tian, H., J. Peng, Q. Du, X. Hui, and H. He. One-pot sustainable synthesis of magnetic MIL-100(Fe) with novel Fe3O4 morphology and its application in heterogeneous degradation. Dalton Transactions, Vol. 47, No. 10, 2018, pp. 3417–3424.10.1039/C7DT04819JSearch in Google Scholar PubMed
[57] Xiang, Q., Z. Yu, P. Wang, N. He, Q. Tan, Q. Wang, et al. Construction of Z-scheme N-doped BiFeO3/NH2-MIL-53(Fe) with the synergy of hydrogen peroxide and visible-light-driven photo-Fenton degradation of organic contaminants. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 654, 2022, id. 130112.10.1016/j.colsurfa.2022.130112Search in Google Scholar
[58] Zhong, Z., M. Li, J. Fu, Y. Wang, Y. Muhammad, S. Li, et al. Construction of Cu-bridged Cu2O/MIL(Fe/Cu) catalyst with enhanced interfacial contact for the synergistic photo-Fenton degradation of thiacloprid. Chemical Engineering Journal, Vol. 395, 2020, id. 125184.10.1016/j.cej.2020.125184Search in Google Scholar
[59] Liang, H., R. Liu, C. Hu, X. An, X. Zhang, H. Liu, et al. Synergistic effect of dual sites on bimetal-organic frameworks for highly efficient peroxide activation. Journal of Hazardous Materials, Vol. 406, 2021, id. 124692.10.1016/j.jhazmat.2020.124692Search in Google Scholar PubMed
[60] Zhang, B., L. Zhang, K. Akiyama, P. A. Bingham, Y. Zhou and S. Kubuki. Self-assembly of nanosheet-supported Fe-MOF heterocrystals as a reusable catalyst for boosting advanced oxidation performance via radical and nonradical pathways. ACS Applied Materials & Interfaces, Vol. 1319, 2021, pp. 22694–22707.10.1021/acsami.1c06149Search in Google Scholar PubMed
[61] Li, X., J. Wan, Y. Wang, H. Chi, Z. Yan, and S. Ding. Selective removal and persulfate catalytic decomposition of diethyl phthalate from contaminated water on modified MIL100 through surface molecular imprinting. Chemosphere, Vol. 240, 2020, id. 124875.10.1016/j.chemosphere.2019.124875Search in Google Scholar PubMed
[62] Geng, N., W. Chen, H. Xu, M. Ding, T. Lin, Q. Wu, et al. Insights into the novel application of Fe-MOFs in ultrasound-assisted heterogeneous Fenton system: Efficiency, kinetics and mechanism. Ultrasonics Sonochemistry, Vol. 72, 2021, id. 105411.10.1016/j.ultsonch.2020.105411Search in Google Scholar PubMed PubMed Central
[63] Dinh Du, P., H. Thanh Danh, P. Ngoc Hoai, N. M. Thanh, V. Thang Nguyen, and D. Quang Khieu. Heterogeneous UV/fenton‐like degradation of methyl orange using iron terephthalate MIL‐53 catalyst. Journal of Chemistry, Vol. 2020, No. 1, 2020, id. 1474357.10.1155/2020/1474357Search in Google Scholar
[64] Li, Y., J. Jiang, Y. Fang, Z. Cao, D. Chen, N. Li, et al. TiO2 nanoparticles anchored onto the metal-organic framework NH2-MIL-88B(Fe) as an adsorptive photocatalyst with enhanced fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustainable Chemistry & Engineering, Vol. 612, 2018, pp. 16186–16197.10.1021/acssuschemeng.8b02968Search in Google Scholar
[65] Liao, X., F. Wang, F. Wang, Y. Cai, Y. Yao, B. Teng, et al. Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal. Applied Catalysis B: Environmental, Vol. 259, 2019, id. 118064.10.1016/j.apcatb.2019.118064Search in Google Scholar
[66] Sun, X., H. Qi, S. Mao, and Z. Sun. Atrazine removal by peroxymonosulfate activated with magnetic CoFe alloy@N-doped graphitic carbon encapsulated in chitosan carbonized microspheres. Chemical Engineering Journal, Vol. 423, 2021, id. 130169.10.1016/j.cej.2021.130169Search in Google Scholar
[67] Liu, X., W. Wang, H. Lin, Y. Shen, Q. Fang, and F. Liu. Construction of hierarchical Prussian blue microcrystal with high sunlight absorption for efficient photo-thermal degradation of organic pollutants. Separation and Purification Technology, Vol. 269, 2021, id. 118724.10.1016/j.seppur.2021.118724Search in Google Scholar
[68] Assila, O., N. Vilaça, A. R. Bertão, A. M. Fonseca, P. Parpot, O. S. Soares, et al. Optimization of iron-ZIF-8 catalysts for degradation of tartrazine in water by Fenton-like reaction. Chemosphere, Vol. 339, 2023, id. 139634.10.1016/j.chemosphere.2023.139634Search in Google Scholar PubMed
[69] Fang, C., L. Nie, H. Chen, and Y. Yang. Magnetic recyclable Co-MOF derivate-modified steel slag for tetracycline removal by integrated adsorption and Fenton-like catalysis. Journal of Water Process Engineering, Vol. 63, 2024, id. 105434.10.1016/j.jwpe.2024.105434Search in Google Scholar
[70] Liu, C., X. Zhao, Z. Wang, Y. Zhao, R. Li, X. Chen, et al. Metal-organic framework-modulated Fe3O4 composite Au nanoparticles for antibacterial wound healing via synergistic peroxidase-like nanozymatic catalysis. Journal of Nanobiotechnology, Vol. 21, No. 1, 2023, id. 427.10.1186/s12951-023-02186-6Search in Google Scholar PubMed PubMed Central
[71] Qin, J., J. Su, H. Li, L. Zhang, and K. Jiang. Fenton chemistry activation in metal-organic frameworks for synergistic bacteria eradication. Chemical Engineering Journal, Vol. 497, 2024, id. 154413.10.1016/j.cej.2024.154413Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review Articles
- Utilization of steel slag in concrete: A review on durability and microstructure analysis
- Technical development of modified emulsion asphalt: A review on the preparation, performance, and applications
- Recent developments in ultrasonic welding of similar and dissimilar joints of carbon fiber reinforcement thermoplastics with and without interlayer: A state-of-the-art review
- Unveiling the crucial factors and coating mitigation of solid particle erosion in steam turbine blade failures: A review
- From magnesium oxide, magnesium oxide concrete to magnesium oxide concrete dams
- Properties and potential applications of polymer composites containing secondary fillers
- A scientometric review on the utilization of copper slag as a substitute constituent of ordinary Portland cement concrete
- Advancement of additive manufacturing technology in the development of personalized in vivo and in vitro prosthetic implants
- Recent advance of MOFs in Fenton-like reaction
- A review of defect formation, detection, and effect on mechanical properties of three-dimensional braided composites
- Non-conventional approaches to producing biochars for environmental and energy applications
- Review of the development and application of aluminum alloys in the nuclear industry
- Advances in the development and characterization of combustible cartridge cases and propellants: Preparation, performance, and future prospects
- Recent trends in rubberized and non-rubberized ultra-high performance geopolymer concrete for sustainable construction: A review
- Cement-based materials for radiative cooling: Potential, material and structural design, and future prospects
- A comprehensive review: The impact of recycling polypropylene fiber on lightweight concrete performance
- A comprehensive review of preheating temperature effects on reclaimed asphalt pavement in the hot center plant recycling
- Exploring the potential applications of semi-flexible pavement: A comprehensive review
- A critical review of alkali-activated metakaolin/blast furnace slag-based cementitious materials: Reaction evolution and mechanism
- Dispersibility of graphene-family materials and their impact on the properties of cement-based materials: Application challenges and prospects
- Research progress on rubidium and cesium separation and extraction
- A step towards sustainable concrete with the utilization of M-sand in concrete production: A review
- Studying the effect of nanofillers in civil applications: A review
- Studies on the anticorrosive effect of phytochemicals on mild steel, carbon steel, and stainless-steel surfaces in acid and alkali medium: A review
- Nanotechnology for calcium aluminate cement: thematic analysis
- Towards sustainable concrete pavements: a critical review on fly ash as a supplementary cementitious material
- Optimizing rice husk ash for ultra-high-performance concrete: a comprehensive review of mechanical properties, durability, and environmental benefits
- Research Articles
- Investigation of the corrosion performance of HVOF-sprayed WC-CoCr coatings applied on offshore hydraulic equipment
- A systematic review of metakaolin-based alkali-activated and geopolymer concrete: A step toward green concrete
- Evaluation of color matching of three single-shade composites employing simulated 3D printed cavities with different thicknesses using CIELAB and CIEDE2000 color difference formulae
- Novel approaches in prediction of tensile strain capacity of engineered cementitious composites using interpretable approaches
- Effect of TiB2 particles on the compressive, hardness, and water absorption responses of Kulkual fiber-reinforced epoxy composites
- Analyzing the compressive strength, eco-strength, and cost–strength ratio of agro-waste-derived concrete using advanced machine learning methods
- Tensile behavior evaluation of two-stage concrete using an innovative model optimization approach
- Tailoring the mechanical and degradation properties of 3DP PLA/PCL scaffolds for biomedical applications
- Optimizing compressive strength prediction in glass powder-modified concrete: A comprehensive study on silicon dioxide and calcium oxide influence across varied sample dimensions and strength ranges
- Experimental study on solid particle erosion of protective aircraft coatings at different impact angles
- Compatibility between polyurea resin modifier and asphalt binder based on segregation and rheological parameters
- Fe-containing nominal wollastonite (CaSiO3)–Na2O glass-ceramic: Characterization and biocompatibility
- Relevance of pore network connectivity in tannin-derived carbons for rapid detection of BTEX traces in indoor air
- A life cycle and environmental impact analysis of sustainable concrete incorporating date palm ash and eggshell powder as supplementary cementitious materials
- Eco-friendly utilisation of agricultural waste: Assessing mixture performance and physical properties of asphalt modified with peanut husk ash using response surface methodology
- Benefits and limitations of N2 addition with Ar as shielding gas on microstructure change and their effect on hardness and corrosion resistance of duplex stainless steel weldments
- Effect of selective laser sintering processing parameters on the mechanical properties of peanut shell powder/polyether sulfone composite
- Impact and mechanism of improving the UV aging resistance performance of modified asphalt binder
- AI-based prediction for the strength, cost, and sustainability of eggshell and date palm ash-blended concrete
- Investigating the sulfonated ZnO–PVA membrane for improved MFC performance
- Strontium coupling with sulphur in mouse bone apatites
- Transforming waste into value: Advancing sustainable construction materials with treated plastic waste and foundry sand in lightweight foamed concrete for a greener future
- Evaluating the use of recycled sawdust in porous foam mortar for improved performance
- Improvement and predictive modeling of the mechanical performance of waste fire clay blended concrete
- Polyvinyl alcohol/alginate/gelatin hydrogel-based CaSiO3 designed for accelerating wound healing
- Research on assembly stress and deformation of thin-walled composite material power cabin fairings
- Effect of volcanic pumice powder on the properties of fiber-reinforced cement mortars in aggressive environments
- Analyzing the compressive performance of lightweight foamcrete and parameter interdependencies using machine intelligence strategies
- Selected materials techniques for evaluation of attributes of sourdough bread with Kombucha SCOBY
- Establishing strength prediction models for low-carbon rubberized cementitious mortar using advanced AI tools
- Investigating the strength performance of 3D printed fiber-reinforced concrete using applicable predictive models
- An eco-friendly synthesis of ZnO nanoparticles with jamun seed extract and their multi-applications
- The application of convolutional neural networks, LF-NMR, and texture for microparticle analysis in assessing the quality of fruit powders: Case study – blackcurrant powders
- Study of feasibility of using copper mining tailings in mortar production
- Shear and flexural performance of reinforced concrete beams with recycled concrete aggregates
- Advancing GGBS geopolymer concrete with nano-alumina: A study on strength and durability in aggressive environments
- Leveraging waste-based additives and machine learning for sustainable mortar development in construction
- Study on the modification effects and mechanisms of organic–inorganic composite anti-aging agents on asphalt across multiple scales
- Morphological and microstructural analysis of sustainable concrete with crumb rubber and SCMs
- Structural, physical, and luminescence properties of sodium–aluminum–zinc borophosphate glass embedded with Nd3+ ions for optical applications
- Eco-friendly waste plastic-based mortar incorporating industrial waste powders: Interpretable models for flexural strength
- Bioactive potential of marine Aspergillus niger AMG31: Metabolite profiling and green synthesis of copper/zinc oxide nanocomposites – An insight into biomedical applications
- Preparation of geopolymer cementitious materials by combining industrial waste and municipal dewatering sludge: Stabilization, microscopic analysis and water seepage
- Seismic behavior and shear capacity calculation of a new type of self-centering steel-concrete composite joint
- Sustainable utilization of aluminum waste in geopolymer concrete: Influence of alkaline activation on microstructure and mechanical properties
- Optimization of oil palm boiler ash waste and zinc oxide as antibacterial fabric coating
- Tailoring ZX30 alloy’s microstructural evolution, electrochemical and mechanical behavior via ECAP processing parameters
- Comparative study on the effect of natural and synthetic fibers on the production of sustainable concrete
- Microemulsion synthesis of zinc-containing mesoporous bioactive silicate glass nanoparticles: In vitro bioactivity and drug release studies
- On the interaction of shear bands with nanoparticles in ZrCu-based metallic glass: In situ TEM investigation
- Developing low carbon molybdenum tailing self-consolidating concrete: Workability, shrinkage, strength, and pore structure
- Experimental and computational analyses of eco-friendly concrete using recycled crushed brick
- High-performance WC–Co coatings via HVOF: Mechanical properties of steel surfaces
- Mechanical properties and fatigue analysis of rubber concrete under uniaxial compression modified by a combination of mineral admixture
- Experimental study of flexural performance of solid wood beams strengthened with CFRP fibers
- Eco-friendly green synthesis of silver nanoparticles with Syzygium aromaticum extract: characterization and evaluation against Schistosoma haematobium
- Predictive modeling assessment of advanced concrete materials incorporating plastic waste as sand replacement
- Self-compacting mortar overlays using expanded polystyrene beads for thermal performance and energy efficiency in buildings
- Enhancing frost resistance of alkali-activated slag concrete using surfactants: sodium dodecyl sulfate, sodium abietate, and triterpenoid saponins
- Equation-driven strength prediction of GGBS concrete: a symbolic machine learning approach for sustainable development
- Empowering 3D printed concrete: discovering the impact of steel fiber reinforcement on mechanical performance
- Advanced hybrid machine learning models for estimating chloride penetration resistance of concrete structures for durability assessment: optimization and hyperparameter tuning
- Influence of diamine structure on the properties of colorless and transparent polyimides
- Post-heating strength prediction in concrete with Wadi Gyada Alkharj fine aggregate using thermal conductivity and ultrasonic pulse velocity
- Experimental and RSM-based optimization of sustainable concrete properties using glass powder and rubber fine aggregates as partial replacements
- Special Issue on Recent Advancement in Low-carbon Cement-based Materials - Part II
- Investigating the effect of locally available volcanic ash on mechanical and microstructure properties of concrete
- Flexural performance evaluation using computational tools for plastic-derived mortar modified with blends of industrial waste powders
- Foamed geopolymers as low carbon materials for fire-resistant and lightweight applications in construction: A review
- Autogenous shrinkage of cementitious composites incorporating red mud
- Mechanical, durability, and microstructure analysis of concrete made with metakaolin and copper slag for sustainable construction
- Special Issue on AI-Driven Advances for Nano-Enhanced Sustainable Construction Materials
- Advanced explainable models for strength evaluation of self-compacting concrete modified with supplementary glass and marble powders
- Analyzing the viability of agro-waste for sustainable concrete: Expression-based formulation and validation of predictive models for strength performance
- Special Issue on Advanced Materials for Energy Storage and Conversion
- Innovative optimization of seashell ash-based lightweight foamed concrete: Enhancing physicomechanical properties through ANN-GA hybrid approach
- Production of novel reinforcing rods of waste polyester, polypropylene, and cotton as alternatives to reinforcement steel rods
Articles in the same Issue
- Review Articles
- Utilization of steel slag in concrete: A review on durability and microstructure analysis
- Technical development of modified emulsion asphalt: A review on the preparation, performance, and applications
- Recent developments in ultrasonic welding of similar and dissimilar joints of carbon fiber reinforcement thermoplastics with and without interlayer: A state-of-the-art review
- Unveiling the crucial factors and coating mitigation of solid particle erosion in steam turbine blade failures: A review
- From magnesium oxide, magnesium oxide concrete to magnesium oxide concrete dams
- Properties and potential applications of polymer composites containing secondary fillers
- A scientometric review on the utilization of copper slag as a substitute constituent of ordinary Portland cement concrete
- Advancement of additive manufacturing technology in the development of personalized in vivo and in vitro prosthetic implants
- Recent advance of MOFs in Fenton-like reaction
- A review of defect formation, detection, and effect on mechanical properties of three-dimensional braided composites
- Non-conventional approaches to producing biochars for environmental and energy applications
- Review of the development and application of aluminum alloys in the nuclear industry
- Advances in the development and characterization of combustible cartridge cases and propellants: Preparation, performance, and future prospects
- Recent trends in rubberized and non-rubberized ultra-high performance geopolymer concrete for sustainable construction: A review
- Cement-based materials for radiative cooling: Potential, material and structural design, and future prospects
- A comprehensive review: The impact of recycling polypropylene fiber on lightweight concrete performance
- A comprehensive review of preheating temperature effects on reclaimed asphalt pavement in the hot center plant recycling
- Exploring the potential applications of semi-flexible pavement: A comprehensive review
- A critical review of alkali-activated metakaolin/blast furnace slag-based cementitious materials: Reaction evolution and mechanism
- Dispersibility of graphene-family materials and their impact on the properties of cement-based materials: Application challenges and prospects
- Research progress on rubidium and cesium separation and extraction
- A step towards sustainable concrete with the utilization of M-sand in concrete production: A review
- Studying the effect of nanofillers in civil applications: A review
- Studies on the anticorrosive effect of phytochemicals on mild steel, carbon steel, and stainless-steel surfaces in acid and alkali medium: A review
- Nanotechnology for calcium aluminate cement: thematic analysis
- Towards sustainable concrete pavements: a critical review on fly ash as a supplementary cementitious material
- Optimizing rice husk ash for ultra-high-performance concrete: a comprehensive review of mechanical properties, durability, and environmental benefits
- Research Articles
- Investigation of the corrosion performance of HVOF-sprayed WC-CoCr coatings applied on offshore hydraulic equipment
- A systematic review of metakaolin-based alkali-activated and geopolymer concrete: A step toward green concrete
- Evaluation of color matching of three single-shade composites employing simulated 3D printed cavities with different thicknesses using CIELAB and CIEDE2000 color difference formulae
- Novel approaches in prediction of tensile strain capacity of engineered cementitious composites using interpretable approaches
- Effect of TiB2 particles on the compressive, hardness, and water absorption responses of Kulkual fiber-reinforced epoxy composites
- Analyzing the compressive strength, eco-strength, and cost–strength ratio of agro-waste-derived concrete using advanced machine learning methods
- Tensile behavior evaluation of two-stage concrete using an innovative model optimization approach
- Tailoring the mechanical and degradation properties of 3DP PLA/PCL scaffolds for biomedical applications
- Optimizing compressive strength prediction in glass powder-modified concrete: A comprehensive study on silicon dioxide and calcium oxide influence across varied sample dimensions and strength ranges
- Experimental study on solid particle erosion of protective aircraft coatings at different impact angles
- Compatibility between polyurea resin modifier and asphalt binder based on segregation and rheological parameters
- Fe-containing nominal wollastonite (CaSiO3)–Na2O glass-ceramic: Characterization and biocompatibility
- Relevance of pore network connectivity in tannin-derived carbons for rapid detection of BTEX traces in indoor air
- A life cycle and environmental impact analysis of sustainable concrete incorporating date palm ash and eggshell powder as supplementary cementitious materials
- Eco-friendly utilisation of agricultural waste: Assessing mixture performance and physical properties of asphalt modified with peanut husk ash using response surface methodology
- Benefits and limitations of N2 addition with Ar as shielding gas on microstructure change and their effect on hardness and corrosion resistance of duplex stainless steel weldments
- Effect of selective laser sintering processing parameters on the mechanical properties of peanut shell powder/polyether sulfone composite
- Impact and mechanism of improving the UV aging resistance performance of modified asphalt binder
- AI-based prediction for the strength, cost, and sustainability of eggshell and date palm ash-blended concrete
- Investigating the sulfonated ZnO–PVA membrane for improved MFC performance
- Strontium coupling with sulphur in mouse bone apatites
- Transforming waste into value: Advancing sustainable construction materials with treated plastic waste and foundry sand in lightweight foamed concrete for a greener future
- Evaluating the use of recycled sawdust in porous foam mortar for improved performance
- Improvement and predictive modeling of the mechanical performance of waste fire clay blended concrete
- Polyvinyl alcohol/alginate/gelatin hydrogel-based CaSiO3 designed for accelerating wound healing
- Research on assembly stress and deformation of thin-walled composite material power cabin fairings
- Effect of volcanic pumice powder on the properties of fiber-reinforced cement mortars in aggressive environments
- Analyzing the compressive performance of lightweight foamcrete and parameter interdependencies using machine intelligence strategies
- Selected materials techniques for evaluation of attributes of sourdough bread with Kombucha SCOBY
- Establishing strength prediction models for low-carbon rubberized cementitious mortar using advanced AI tools
- Investigating the strength performance of 3D printed fiber-reinforced concrete using applicable predictive models
- An eco-friendly synthesis of ZnO nanoparticles with jamun seed extract and their multi-applications
- The application of convolutional neural networks, LF-NMR, and texture for microparticle analysis in assessing the quality of fruit powders: Case study – blackcurrant powders
- Study of feasibility of using copper mining tailings in mortar production
- Shear and flexural performance of reinforced concrete beams with recycled concrete aggregates
- Advancing GGBS geopolymer concrete with nano-alumina: A study on strength and durability in aggressive environments
- Leveraging waste-based additives and machine learning for sustainable mortar development in construction
- Study on the modification effects and mechanisms of organic–inorganic composite anti-aging agents on asphalt across multiple scales
- Morphological and microstructural analysis of sustainable concrete with crumb rubber and SCMs
- Structural, physical, and luminescence properties of sodium–aluminum–zinc borophosphate glass embedded with Nd3+ ions for optical applications
- Eco-friendly waste plastic-based mortar incorporating industrial waste powders: Interpretable models for flexural strength
- Bioactive potential of marine Aspergillus niger AMG31: Metabolite profiling and green synthesis of copper/zinc oxide nanocomposites – An insight into biomedical applications
- Preparation of geopolymer cementitious materials by combining industrial waste and municipal dewatering sludge: Stabilization, microscopic analysis and water seepage
- Seismic behavior and shear capacity calculation of a new type of self-centering steel-concrete composite joint
- Sustainable utilization of aluminum waste in geopolymer concrete: Influence of alkaline activation on microstructure and mechanical properties
- Optimization of oil palm boiler ash waste and zinc oxide as antibacterial fabric coating
- Tailoring ZX30 alloy’s microstructural evolution, electrochemical and mechanical behavior via ECAP processing parameters
- Comparative study on the effect of natural and synthetic fibers on the production of sustainable concrete
- Microemulsion synthesis of zinc-containing mesoporous bioactive silicate glass nanoparticles: In vitro bioactivity and drug release studies
- On the interaction of shear bands with nanoparticles in ZrCu-based metallic glass: In situ TEM investigation
- Developing low carbon molybdenum tailing self-consolidating concrete: Workability, shrinkage, strength, and pore structure
- Experimental and computational analyses of eco-friendly concrete using recycled crushed brick
- High-performance WC–Co coatings via HVOF: Mechanical properties of steel surfaces
- Mechanical properties and fatigue analysis of rubber concrete under uniaxial compression modified by a combination of mineral admixture
- Experimental study of flexural performance of solid wood beams strengthened with CFRP fibers
- Eco-friendly green synthesis of silver nanoparticles with Syzygium aromaticum extract: characterization and evaluation against Schistosoma haematobium
- Predictive modeling assessment of advanced concrete materials incorporating plastic waste as sand replacement
- Self-compacting mortar overlays using expanded polystyrene beads for thermal performance and energy efficiency in buildings
- Enhancing frost resistance of alkali-activated slag concrete using surfactants: sodium dodecyl sulfate, sodium abietate, and triterpenoid saponins
- Equation-driven strength prediction of GGBS concrete: a symbolic machine learning approach for sustainable development
- Empowering 3D printed concrete: discovering the impact of steel fiber reinforcement on mechanical performance
- Advanced hybrid machine learning models for estimating chloride penetration resistance of concrete structures for durability assessment: optimization and hyperparameter tuning
- Influence of diamine structure on the properties of colorless and transparent polyimides
- Post-heating strength prediction in concrete with Wadi Gyada Alkharj fine aggregate using thermal conductivity and ultrasonic pulse velocity
- Experimental and RSM-based optimization of sustainable concrete properties using glass powder and rubber fine aggregates as partial replacements
- Special Issue on Recent Advancement in Low-carbon Cement-based Materials - Part II
- Investigating the effect of locally available volcanic ash on mechanical and microstructure properties of concrete
- Flexural performance evaluation using computational tools for plastic-derived mortar modified with blends of industrial waste powders
- Foamed geopolymers as low carbon materials for fire-resistant and lightweight applications in construction: A review
- Autogenous shrinkage of cementitious composites incorporating red mud
- Mechanical, durability, and microstructure analysis of concrete made with metakaolin and copper slag for sustainable construction
- Special Issue on AI-Driven Advances for Nano-Enhanced Sustainable Construction Materials
- Advanced explainable models for strength evaluation of self-compacting concrete modified with supplementary glass and marble powders
- Analyzing the viability of agro-waste for sustainable concrete: Expression-based formulation and validation of predictive models for strength performance
- Special Issue on Advanced Materials for Energy Storage and Conversion
- Innovative optimization of seashell ash-based lightweight foamed concrete: Enhancing physicomechanical properties through ANN-GA hybrid approach
- Production of novel reinforcing rods of waste polyester, polypropylene, and cotton as alternatives to reinforcement steel rods

