Abstract
C14H18O7, orthorhombic, P212121 (no. 19), a = 4.3538(6) Å, b = 12.7630(19) Å, c = 26.493(4) Å, V = 1,472.2(4) Å3, Z = 4, R gt (F) = 0.0414, wR ref (F 2) = 0.1043, T = 296(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.19 × 0.15 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.11 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 11,557, 2,748, 0.039 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1960 |
| N(param)refined: | 194 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Diamond 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.2531 (12) | 0.3586 (3) | −0.03413 (10) | 0.0820 (13) |
| H1A | 0.0477 | 0.3582 | −0.0473 | 0.123* |
| H1B | 0.3596 | 0.2970 | −0.0454 | 0.123* |
| H1C | 0.3591 | 0.4198 | −0.0459 | 0.123* |
| C2 | 0.0917 (9) | 0.4383 (2) | 0.04178 (12) | 0.0579 (9) |
| C3 | 0.1012 (8) | 0.4357 (2) | 0.09784 (10) | 0.0474 (8) |
| C4 | 0.2571 (8) | 0.3577 (2) | 0.12362 (10) | 0.0468 (8) |
| H4 | 0.3544 | 0.3041 | 0.1060 | 0.056* |
| C5 | 0.2670 (7) | 0.3602 (2) | 0.17595 (10) | 0.0441 (7) |
| C6 | 0.1262 (7) | 0.4418 (2) | 0.20184 (10) | 0.0417 (7) |
| C7 | −0.0367 (8) | 0.5184 (2) | 0.17540 (11) | 0.0458 (8) |
| C8 | −0.0497 (8) | 0.5149 (2) | 0.12334 (10) | 0.0489 (8) |
| H8 | −0.1591 | 0.5655 | 0.1056 | 0.059* |
| C9 | 0.5440 (10) | 0.1986 (3) | 0.18127 (12) | 0.0674 (10) |
| H9A | 0.3864 | 0.1596 | 0.1643 | 0.101* |
| H9B | 0.6408 | 0.1550 | 0.2061 | 0.101* |
| H9C | 0.6943 | 0.2215 | 0.1572 | 0.101* |
| C10 | −0.3476 (9) | 0.6719 (2) | 0.17966 (12) | 0.0619 (9) |
| H10A | −0.2196 | 0.7096 | 0.1564 | 0.093* |
| H10B | −0.4303 | 0.7197 | 0.2041 | 0.093* |
| H10C | −0.5126 | 0.6391 | 0.1616 | 0.093* |
| C11 | −0.0880 (7) | 0.4045 (2) | 0.28092 (10) | 0.0500 (8) |
| H11A | −0.0901 | 0.3292 | 0.2758 | 0.060* |
| H11B | −0.2820 | 0.4326 | 0.2691 | 0.060* |
| C12 | −0.0486 (8) | 0.4284 (2) | 0.33539 (11) | 0.0471 (8) |
| C13 | −0.2301 (10) | 0.3800 (3) | 0.41709 (10) | 0.0693 (11) |
| H13A | −0.3647 | 0.4389 | 0.4239 | 0.083* |
| H13B | −0.0278 | 0.3963 | 0.4302 | 0.083* |
| C14 | −0.3509 (11) | 0.2845 (3) | 0.44159 (12) | 0.0866 (13) |
| H14A | −0.5495 | 0.2683 | 0.4279 | 0.130* |
| H14B | −0.3682 | 0.2962 | 0.4773 | 0.130* |
| H14C | −0.2134 | 0.2271 | 0.4355 | 0.130* |
| O1 | 0.2427 (6) | 0.35965 (16) | 0.02034 (7) | 0.0672 (7) |
| O2 | −0.0373 (9) | 0.5048 (2) | 0.01778 (8) | 0.1019 (12) |
| O3 | −0.1698 (6) | 0.59389 (17) | 0.20468 (7) | 0.0590 (6) |
| O4 | 0.1574 (5) | 0.44972 (15) | 0.25340 (7) | 0.0471 (5) |
| O5 | 0.4110 (6) | 0.28758 (16) | 0.20538 (7) | 0.0570 (6) |
| O6 | 0.0991 (8) | 0.4973 (2) | 0.35315 (8) | 0.0834 (9) |
| O7 | −0.2130 (6) | 0.36104 (16) | 0.36309 (7) | 0.0593 (6) |
1 Source of materials
The mixture of methyl 4-hydroxy-3,5-dimethoxybenzoate (2.12 g, 0.01 mol), ethyl bromoacetate (2.03 g, 0.012 mol), K2CO3 (2.76 g, 0.02 mol) and acetone (15 mL) was reacted at 70 °C for 24 h. After the reaction was completed (monitored by TLC), colorless crystals were obtained by slow cooling. The product was filtered, and washed with water 3 times respectively. Yield 90 % (based on methyl 4-hydroxybenzoate). Elemental Anal. Calcd. (%) for C14H18O7 (298.29): C, 56.37; H, 6.08. Found (%): C, 55.53; H, 6.16.
2 Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms.
3 Comment
Syringic acid, 4-hydroxy-3,5-dimethoxybenzoic acid, is a phenolic acid of benzoic acid categorie, which has antibacterial activities, sedative activities and local anesthetic effects. 5 , 6 , 7 , 8 Methyl syringate is considered as a symbolic component of Manuka honey, and it is also a functional component of its antioxidant and antibacterial activity. 9 , 10 , 11 It also is an effective bacterial and fungal laccase phenol medium and an agonist of TRPA1. 12 Because of its strong activities and its wide applications, the synthesis and application of syringic acid derivatives have attracted much attention. 13 , 14 Saeed et al. have reported the synthesis and crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate. 15 Nie et al. have reported the synthesis and crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate. 16 We still focused on the synthesis and antibacterial activities of preservatives.
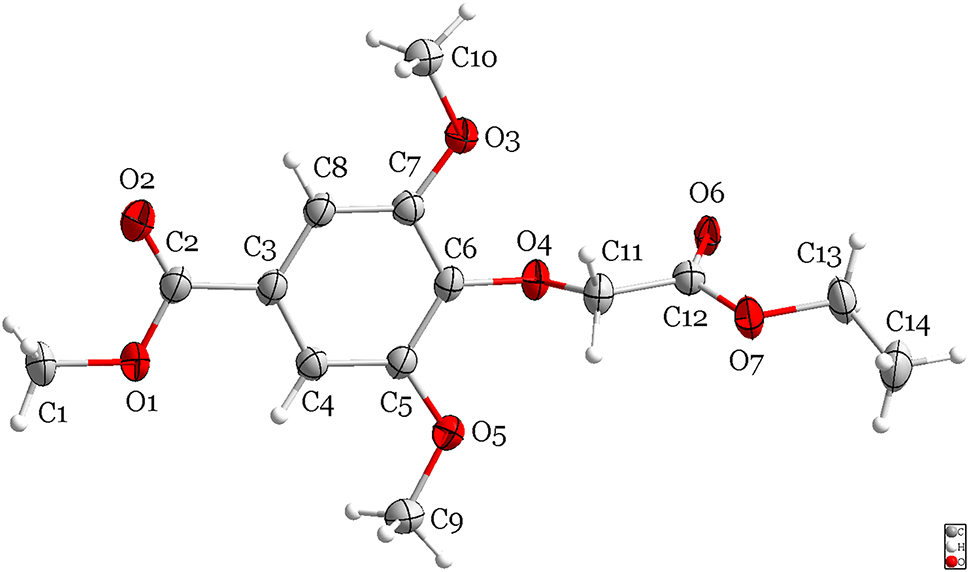
In the molecules of the title structure bond lengths and angles are very similar to those given in the literature. 15 , 16 , 17 In the title structure, the part of methyl 4-hydroxy-3,5-dimethoxybenzoic acid is approximately planar. The dihedral angle formed by the C3–C8 plane with the carboxlate group O1–C2–O2 plane is 2.5°. The torsion angles of C4–C5–O5–C9 and C8–C7–O3–C10 are −4.5(4)° and 3.2(4)°. The part of 2-ethoxy-2-oxoethoxy group is perpendicular to methyl 4-hydroxy-3,5-dimethoxybenzoic acid and the torsion angles of C5–C6–O4–C11, C6–O4–C11–C12 and C12–O7–C13–C14 are −95.6(3)°, −173.5(2)° and −165.8(3)°. The dihedral angle formed by the C3–C8 plane with the carboxylate group O6–C12–O7 plane is 71.218(107)°.
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by the National Natural Science Foundation of China (Grant Number 32360365), the Key Laboratory of State Forestry and Grassland Administration on Highly-Efficient Utilization of Forestry Biomass Resources in Southwest China (Grant Number 2022–KF12).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHEXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Liu, F.; Jiang, F. B.; Li, Y. T.; Liu, R. M.; Wu, Z. Y.; Yan, C. W. Cocrystallization with Syringic Acid Presents a New Opportunity for Effectively Reducing the Hepatotoxicity of Isoniazid. Drug Dev. Ind. Pharm. 2020, 46, 988–995. https://doi.org/10.1080/03639045.2020.1764024.Search in Google Scholar PubMed
6. Cordelia, M. J.; Sumathy, A. Syringic Acid (4-hydroxy-3,5-Dimethoxybenzoic Acid) Inhibits Adipogenesis and Promotes Lipolysis in 3T3–L1 Adipocytes. Nat. Prod. Res. 2020, 34, 3432–3436. https://doi.org/10.1080/14786419.2019.1573820.Search in Google Scholar PubMed
7. Wang, J. R.; Ma, L.; Li, W. F.; Tang, X. H.; Zhao, G.; Peng, L. X.; Zhao, J. L. Effect of Trace Elements on the Flavonoids and Phenolic Acids in Tartary Buckwheat Sprouts. Acta Agric. Univ. Jiangxiensis 2017, 39, 55–63.Search in Google Scholar
8. Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C. M.; Suresh, K. C. Syringic Acid (SA) – A Review of its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557; https://doi.org/10.1016/j.biopha.2018.09.069.Search in Google Scholar PubMed
9. Main, E. N.; Huang, J. C.; Bowlin, G. L. Methyl Syringate: A Primary Driving Factor in Manuka Honeys Ability to Ameliorate Neutrophil Intracellular ROS Activity and NETosis. Front. Biosci. 2024, 29, 255. https://doi.org/10.31083/j.fbl2907255.Search in Google Scholar PubMed
10. Ahn, D.; Kwon, J. H.; Song, S. Y.; Lee, J. Y.; Yoon, S. Y.; Chung, S. J. Methyl Syringate Stimulates Glucose Uptake by Inhibiting Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Life 2023, 13, 1372. https://doi.org/10.3390/life13061372.Search in Google Scholar PubMed PubMed Central
11. Elamine, Y.; Lyoussi, B.; Miguel, M. G.; Anjos, O.; Estevinho, L.; Alaiz, M.; Girón–Calle, J.; Martín, J.; Vioque, J. Physicochemical Characteristics and Antiproliferative and Antioxidant Activities of Moroccan Zantaz Honey Rich in Methyl Syringate. Food Chem. 2020, 339, 128098. https://doi.org/10.1016/j.foodchem.2020.128098.Search in Google Scholar PubMed
12. Park, J.; Shim, M. K.; Jin, M.; Rhyu, M. R.; Lee, Y. Methyl Syringate, a TRPA1 Agonist Represses Hypoxia-Induced Cyclooxygenase-2 in Lung Cancer Cells. Phytomedicine 2016, 23, 324–329. ISSN 0944–7113 https://doi.org/10.1016/j.phymed.2016.01.009.Search in Google Scholar PubMed
13. Zhou, X. L.; Huang, S. X.; Wang, P. C.; Luo, Q.; Huang, X.; Xu, Q.; Qin, J. K.; Liang, C. Q.; Chen, X. A Syringic Acid Derivative and Two Iridoid Glycosides from the Roots of Stachys Geobombycis and Their Antioxidant Properties. Nat. Prod. Res. 2017, 31, 681–686. https://doi.org/10.1080/14786419.2017.1405413.Search in Google Scholar PubMed
14. Chen, P. S.; Chen, H. M.; Zhang, G. F.; Chen, W. J.; Chen, W. X. Synthesis, Characterization and Interaction of Syringic Acid–Copper Complex with DNA. Chin. J. Chem. 2018, 81, 170–174.Search in Google Scholar
15. Saeed, A.; Khera, R. A.; Bolte, M. Methyl 3,4,5-Trimethoxybenzoate. Acta Crystallogr. 2007, E63, o4582. https://doi.org/10.1107/S1600536807054384.Search in Google Scholar
16. Xiong, C. L.; Lan, Y. D.; Song, X. Y.; Xiong, W. M.; Nie, X. L. Crystal Structure of Methyl 4-Acetoxy-3,5-Dimethoxybenzoate, C12H14O6. Z. Kristallogr. N. Cryst. Struct 2021, 236, 573–575. https://doi.org/10.1515/ncrs-2020–0632.10.1515/ncrs-2020-0632Search in Google Scholar
17. Liu, S. J.; Guo, C. M.; Wang, G. S.; Li, W. J.; Nie, X. L. Crystal Structure of Methyl 4-(2-Ethoxy-2-Oxoethoxy)-3-Methoxybenzoate, C13H16O6. Z. Kristallogr. N. Cryst. Struct 2024, 239, 129–130. https://doi.org/10.1515/ncrs-2023–0471.10.1515/ncrs-2023-0471Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10


