Abstract
C60H78Sn2, cubic, Pa3̄ (no. 206), a = 17.621(2) Å, V = 5,472(2) Å3, Z = 4, R gt (F) = 0.0214, wR ref (F 2) = 0.0600, T = 296 (2) K.
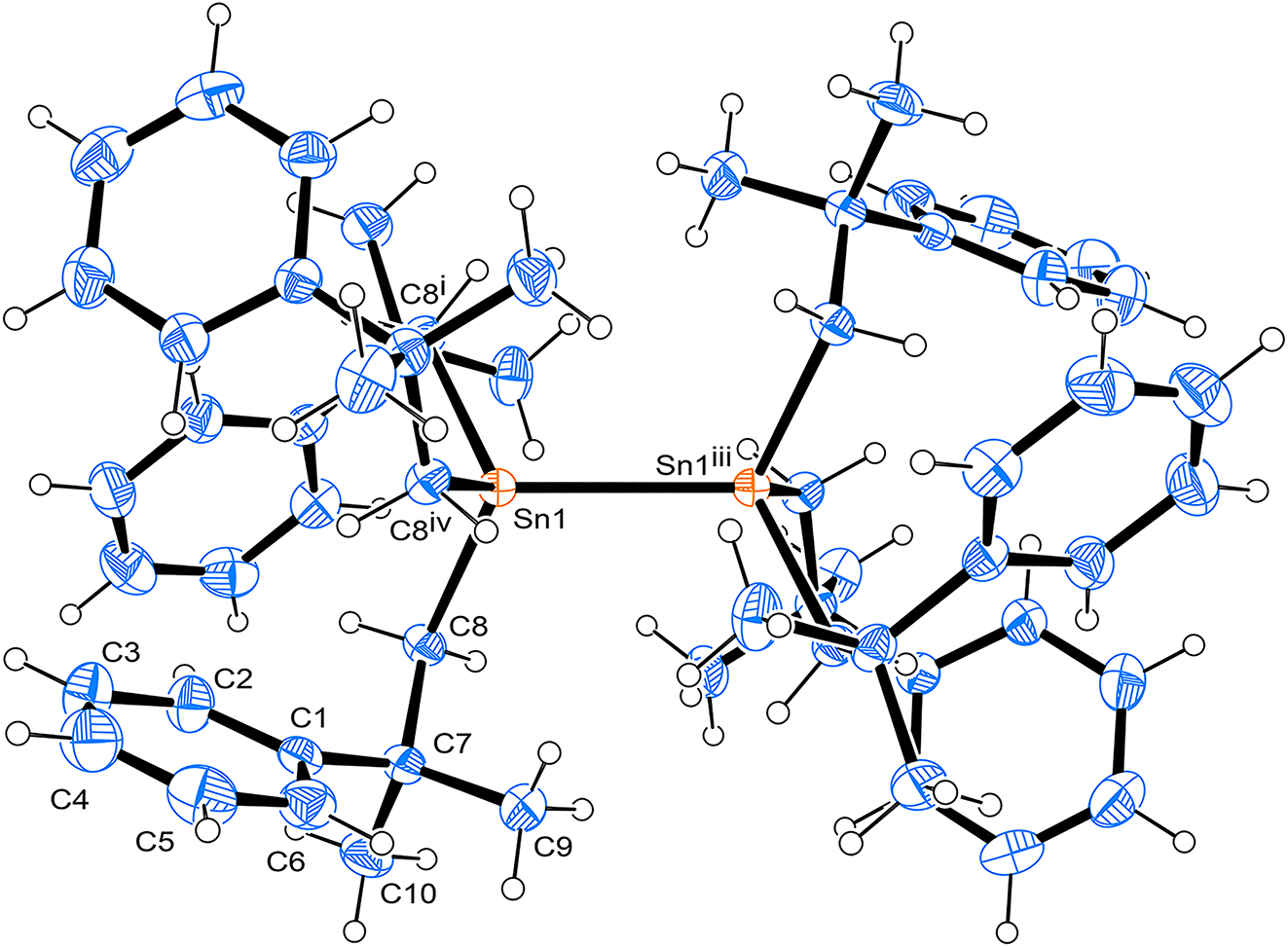
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size | 0.22 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.95 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 27.0°, >99 % |
| N(hkl)measured , N(hkl)unique, R int: | 31,064, 2002, 0.031 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 1,673 |
| N(param)refined: | 96 |
| Programs: | Bruker, 1 SHELX, 2 , 3 WinGX/ORTEP 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.50776 (10) | 0.73737 (10) | 0.12487 (9) | 0.0399 (4) |
| C2 | 0.44649 (11) | 0.74656 (13) | 0.17385 (13) | 0.0547 (5) |
| H2 | 0.438570 | 0.710909 | 0.211959 | 0.066* |
| C3 | 0.39698 (14) | 0.80724 (14) | 0.16750 (15) | 0.0703 (6) |
| H3 | 0.356436 | 0.811733 | 0.200983 | 0.084* |
| C4 | 0.40755 (15) | 0.86076 (14) | 0.11194 (17) | 0.0753 (7) |
| H4 | 0.374099 | 0.901312 | 0.107234 | 0.090* |
| C5 | 0.46768 (16) | 0.85382 (14) | 0.06371 (16) | 0.0716 (7) |
| H5 | 0.475276 | 0.890096 | 0.026139 | 0.086* |
| C6 | 0.51792 (13) | 0.79296 (12) | 0.07005 (13) | 0.0564 (5) |
| H6 | 0.558913 | 0.789596 | 0.036971 | 0.068* |
| C7 | 0.56184 (10) | 0.66992 (11) | 0.13443 (10) | 0.0409 (4) |
| C8 | 0.51755 (10) | 0.59442 (10) | 0.14220 (10) | 0.0389 (4) |
| H8A | 0.482151 | 0.600772 | 0.183878 | 0.047* |
| H8B | 0.553786 | 0.556072 | 0.157887 | 0.047* |
| C9 | 0.61864 (12) | 0.66363 (13) | 0.06891 (13) | 0.0600 (6) |
| H9A | 0.591628 | 0.657920 | 0.021947 | 0.090* |
| H9B | 0.650728 | 0.620317 | 0.076791 | 0.090* |
| H9C | 0.649154 | 0.708721 | 0.066943 | 0.090* |
| C10 | 0.60724 (12) | 0.68265 (13) | 0.20853 (12) | 0.0600 (6) |
| H10A | 0.635994 | 0.728749 | 0.204518 | 0.090* |
| H10B | 0.641134 | 0.640743 | 0.216485 | 0.090* |
| H10C | 0.572763 | 0.686348 | 0.250547 | 0.090* |
| Sn1 | 0.45321 (2) | 0.54679 (2) | 0.04679 (2) | 0.03107 (8) |
1 Source of material
All chemicals were purchased from commercial sources and used as received without further purification. The fenbutatin oxide (1 mmol, 1.053 g) and cinnamic acid (2 mmol, 0.296 g) were dissolved in MeOH (50 mL). The mixture was refluxed for 10 h. Finally, the title crystal was precipitated by controlling slowly cooling.
2 Experimental details
All H-atoms bound to C atoms were placed geometrically and refined using a riding model with common isotropic displacement factors U iso (H) = 1.2 or 1.5 U eq (parent C-atom).
3 Comment
Organotin compounds have been extensively studied in various fields, including industry, agriculture, medicine and so on. 5 , 6 In recent years, they have garnered significant attention for their exceptional performance in organic synthesis and nonlinear optics. 7 , 8 In general, bulky sterically hindered substituents attached to the tin atom typically have a significant impact on the properties of organotin compounds. Additionally, the unique characteristics of Sn–Sn bonds have prompted their investigation in organic synthesis. 9
Single-crystal structure analysis revealed that the title compound crystallized in the cubic space group Pa3̄. The title molecule in the crystal structure is located on a special position. The ORTEP diagram is presented in Figure. Bond lengths and angles are all in the expected ranges. The bond length of Sn1–Sn1‴ is 2.8562(5) Å (‴ = -x+1,-y+1,-z), which is longer than those reported in the literature. 10 , 11 , 12 The observed phenomenon may be attributed to the significant steric hindrance posed by the 2-methyl-2-phenylpropyl group, which is similar to the situation reported in literature 13 regarding bulky o-methylphenyl groups.
Funding source: The Innovation and Entrepreneurship Training Program for College students
Award Identifier / Grant number: S202410699644
Funding source: China International College Students’ Innovation Competition Program
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the Innovation and Entrepreneurship Training Program for College students (No. S202410699644) and China International College Students’ Innovation Competition Program.
References
1. Bruker SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WinGX and ORTEP for Windows: an Update. J. Appl. Cryst. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Hu, L.; Wang, H.; Xia, T.; Fang, B.; Shen, Y.; Zhang, Q.; Tian, X.; Zhou, H.; Wu, J.; Tian, Y. Two-photon-active Organotin(IV) Complexes for Antibacterial Function and Superresolution Bacteria Imaging. Inorg. Chem. 2018, 57, 6340–6348; https://doi.org/10.1021/acs.inorgchem.8b00413.Search in Google Scholar PubMed
6. Patra, S. A.; Sahu, G.; Pattanayak, P. D.; Sasamori, T.; Dinda, R. Mitochondria-targeted Luminescent Organotin(IV) Complexes: Synthesis, Photophysical Characterization, and Live Cell Imaging. Inorg. Chem. 2022, 61, 16914–16928; https://doi.org/10.1021/acs.inorgchem.2c02959.Search in Google Scholar PubMed
7. Elangovan, S.; Randt, T.; Irran, E.; Klare, H. F. T.; Oestreich, M. Synthesis of a Cationic Cobalt-Selenolate Complex for Cooperative Sn—H Bond Activation: Solvent-dependent Stereoselectivity in Alkyne Hydrostannylation. Organometallics 2024, 43, 1619–1624; https://doi.org/10.1021/acs.organomet.4c00236.Search in Google Scholar
8. Luo, M. B.; Lai, H. D.; Huang, S. L.; Zhang, J.; Lin, Q. Pseudotetrahedral Organotin-Capped Chalcogenidometalate Supermolecules with Optical Limiting Performance. J. Am. Chem. Soc. 2024, 146, 7690–7697; https://doi.org/10.1021/jacs.3c14333.Search in Google Scholar PubMed
9. Liu, Z.; Tan, H.; Fu, T.; Xia, Y.; Qiu, D.; Zhang, Y.; Wang, J. Pd(0)-catalyzed Carbene Insertion into Si—Si and Sn – Sn Bonds. J. Am. Chem. Soc. 2015, 137, 12800–12803; https://doi.org/10.1021/jacs.5b09135.Search in Google Scholar PubMed
10. Preut, H.; Haupt, H. J.; Huber, F. Die Kristall- und Molekularstruktur des Hexaphenyl-distannans. Z. Anorg. Allg. Chem. 1973, 396, 81–89; https://doi.org/10.1002/zaac.19733960109.Search in Google Scholar
11. Bauer, J. O. The Crystal Structure of the First Ether Solvate of Hexaphenyldistannane [(Ph3 Sn)2. 2 THF]. Main Group Met. Chem. 2020, 43, 1–6; https://doi.org/10.1515/mgmc-2020-0001.Search in Google Scholar
12. Zarl, E.; Baumgartner, J.; Decker, K.; Fischer, R.; Seibt, B.; Uhlig, F. Solvent Influence in Reactions of Fluoroalkyl Sulfonic Acids with Phenyldistannanes. Phosphorus Sulfur. 2008, 183, 1923–1934; https://doi.org/10.1080/10426500701804910.Search in Google Scholar
13. Schneider–Koglin, C.; Behrends, K.; Dräger, M. Über gemischte gruppe 14-gruppe 14-bindungen: VI. Hexa-o-tolylethan-analoga o–Tol6 Sn2, o–Tol6 PbSn und o–Tol6 Pb2: ein vergleich von bindungsstärke und polarität in der reihung Sn—Sn, Pb—Sn, Pb—Pb. J. Organomet. Chem. 1993, 448, 29–38; https://doi.org/10.1016/0022-328x(93)80063-h.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10

