Abstract
C31H37NO7, monoclinic, P21 (no. 4), a = 8.1269(1) Å, b = 11.0527(1) Å, c = 16.3586(2) Å, β = 97.973(1)°, V = 1455.19(3) Å3, Z = 2, Rgt (F) = 0.0467, wRref (F 2) = 0.1308, T = 293(2) K.
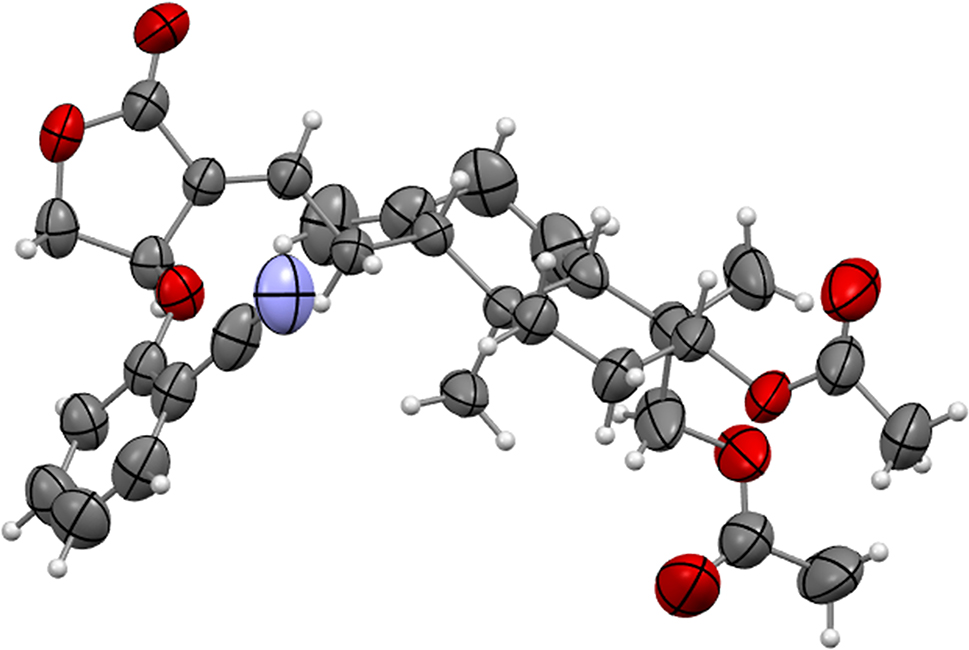
A part of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.12 × 0.10 mm |
| Wavelength: μ: |
Cu Kα radiation (1.54184 Å) 0.70 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
XtaLAB Synergy, ω
66.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 42561, 5127, 0.068 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4702 |
| N(param)refined: | 356 |
| Programs: | CrysAlisPRO, 1 Olex2 2 , 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.6012 (3) | 0.6137 (3) | 0.89752 (17) | 0.0566 (6) |
| C2 | 0.4303 (3) | 0.6300 (3) | 0.89772 (17) | 0.0621 (7) |
| C3 | 0.3182 (4) | 0.5324 (4) | 0.8733 (2) | 0.0755 (10) |
| C4 | 0.3714 (5) | 0.7408 (4) | 0.9217 (2) | 0.0832 (11) |
| H4 | 0.257709 | 0.752352 | 0.920608 | 0.100* |
| C5 | 0.4775 (6) | 0.8316 (4) | 0.9467 (3) | 0.0928 (12) |
| H5 | 0.437260 | 0.904698 | 0.963945 | 0.111* |
| C6 | 0.6459 (5) | 0.8160 (4) | 0.9465 (2) | 0.0853 (10) |
| H6 | 0.718186 | 0.879327 | 0.963013 | 0.102* |
| C7 | 0.7083 (4) | 0.7088 (3) | 0.9224 (2) | 0.0683 (8) |
| H7 | 0.822131 | 0.699648 | 0.922591 | 0.082* |
| C8 | 0.8221 (3) | 0.4772 (3) | 0.87548 (16) | 0.0522 (6) |
| H8 | 0.875186 | 0.536187 | 0.842791 | 0.063* |
| C9 | 0.9123 (3) | 0.4671 (3) | 0.96346 (18) | 0.0625 (7) |
| H9A | 1.018735 | 0.508065 | 0.968148 | 0.075* |
| H9B | 0.846627 | 0.503853 | 1.001997 | 0.075* |
| C10 | 0.8967 (4) | 0.2732 (3) | 0.91209 (19) | 0.0675 (8) |
| C11 | 0.8328 (3) | 0.3522 (3) | 0.84333 (16) | 0.0533 (6) |
| C12 | 0.7903 (3) | 0.3135 (3) | 0.76617 (17) | 0.0564 (6) |
| H12 | 0.806448 | 0.231882 | 0.755841 | 0.068* |
| C13 | 0.7192 (3) | 0.3899 (3) | 0.69534 (16) | 0.0545 (6) |
| H13A | 0.766487 | 0.470460 | 0.702390 | 0.065* |
| H13B | 0.600252 | 0.396931 | 0.695378 | 0.065* |
| C14 | 0.7518 (3) | 0.3395 (2) | 0.61170 (15) | 0.0489 (5) |
| H14 | 0.706400 | 0.257255 | 0.608419 | 0.059* |
| C15 | 0.9337 (4) | 0.3270 (3) | 0.6039 (2) | 0.0700 (8) |
| C16 | 1.0561 (4) | 0.3723 (6) | 0.6556 (3) | 0.1031 (15) |
| H16A | 1.165233 | 0.362582 | 0.645623 | 0.124* |
| H16B | 1.033673 | 0.414072 | 0.702103 | 0.124* |
| C17 | 0.9629 (5) | 0.2591 (5) | 0.5282 (3) | 0.0938 (12) |
| H17A | 0.922709 | 0.176775 | 0.531332 | 0.113* |
| H17B | 1.081111 | 0.255776 | 0.525136 | 0.113* |
| C18 | 0.8740 (5) | 0.3200 (5) | 0.4513 (2) | 0.0912 (12) |
| H18A | 0.887251 | 0.271298 | 0.403346 | 0.109* |
| H18B | 0.924052 | 0.398382 | 0.444631 | 0.109* |
| C19 | 0.6885 (4) | 0.3365 (3) | 0.45646 (17) | 0.0604 (7) |
| H19 | 0.648566 | 0.254713 | 0.465943 | 0.073* |
| C20 | 0.6591 (3) | 0.4089 (2) | 0.53565 (15) | 0.0496 (6) |
| C21 | 0.7232 (5) | 0.5393 (3) | 0.5383 (2) | 0.0721 (9) |
| H21A | 0.715062 | 0.573790 | 0.591434 | 0.108* |
| H21B | 0.657610 | 0.586031 | 0.496281 | 0.108* |
| H21C | 0.837171 | 0.539849 | 0.528795 | 0.108* |
| C22 | 0.4726 (3) | 0.4088 (3) | 0.54030 (17) | 0.0601 (7) |
| H22A | 0.438915 | 0.327352 | 0.552659 | 0.072* |
| H22B | 0.451118 | 0.460638 | 0.585455 | 0.072* |
| C23 | 0.3670 (5) | 0.4516 (4) | 0.4613 (2) | 0.0801 (9) |
| H23A | 0.250278 | 0.444616 | 0.467297 | 0.096* |
| H23B | 0.390591 | 0.536030 | 0.451829 | 0.096* |
| C24 | 0.4025 (5) | 0.3771 (3) | 0.38809 (18) | 0.0728 (9) |
| H24 | 0.368931 | 0.293360 | 0.396649 | 0.087* |
| C25 | 0.5846 (5) | 0.3765 (3) | 0.37335 (18) | 0.0742 (9) |
| C26 | 0.1788 (5) | 0.3555 (4) | 0.2761 (2) | 0.0871 (11) |
| C27 | 0.0722 (7) | 0.4237 (5) | 0.2103 (3) | 0.1130 (16) |
| H27A | 0.017421 | 0.488508 | 0.234838 | 0.170* |
| H27B | −0.009424 | 0.370210 | 0.181794 | 0.170* |
| H27C | 0.139911 | 0.456294 | 0.172017 | 0.170* |
| C28 | 0.6036 (7) | 0.2826 (4) | 0.3056 (2) | 0.1012 (14) |
| H28A | 0.709845 | 0.292585 | 0.287136 | 0.152* |
| H28B | 0.517116 | 0.294233 | 0.260023 | 0.152* |
| H28C | 0.595653 | 0.202588 | 0.327536 | 0.152* |
| C29 | 0.6409 (8) | 0.5033 (4) | 0.3477 (2) | 0.1088 (17) |
| H29A | 0.581730 | 0.565017 | 0.374074 | 0.131* |
| H29B | 0.758712 | 0.513203 | 0.366311 | 0.131* |
| C30 | 0.5844 (6) | 0.6319 (4) | 0.2364 (2) | 0.0859 (10) |
| C31 | 0.5579 (9) | 0.6481 (5) | 0.1454 (3) | 0.126 (2) |
| H31A | 0.656627 | 0.624804 | 0.123287 | 0.189* |
| H31B | 0.533224 | 0.731385 | 0.132445 | 0.189* |
| H31C | 0.466675 | 0.598399 | 0.121665 | 0.189* |
| N1 | 0.2240 (4) | 0.4560 (5) | 0.8564 (3) | 0.1049 (12) |
| O1 | 0.6463 (2) | 0.50224 (18) | 0.87444 (13) | 0.0593 (5) |
| O2 | 0.9359 (3) | 0.3401 (2) | 0.98130 (12) | 0.0692 (6) |
| O3 | 0.9139 (5) | 0.1656 (3) | 0.91437 (17) | 0.1045 (10) |
| O4 | 0.2956 (4) | 0.4253 (2) | 0.31613 (16) | 0.0983 (9) |
| O5 | 0.1614 (5) | 0.2510 (3) | 0.2906 (2) | 0.1191 (12) |
| O6 | 0.6096 (5) | 0.5189 (3) | 0.25982 (14) | 0.1034 (10) |
| O7 | 0.5770 (6) | 0.7125 (4) | 0.2845 (2) | 0.1312 (14) |
1 Source of materials
To the solution of andrographolide (9.95 g, 28.4 mmol) and 2,2-dimethoxypropane (24 mL, 195.3 mmol) in 20 mL of anhydrous dichloromethane, PPTS (0.71 g, 2.8 mmol) was added and the reaction mixture was heated at 40 °C. The reaction was monitored by TLC and then treated with ethyl acetate and sat. NaHCO3 after the reaction was completed. The organic phase was washed with brine, dried over anhydrous Na2SO4, and then filtered organic solution was evaporated to dryness. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 1:1) to produce 3,19-acetonylidene andrographolide (10.01 g, 24.7 mmol) as a white solid 4 . Under N2 atmosphere, compound 3,19-acetonylidene andrographolide (3.94 g, 10.1 mmol), PPh3 (3.97 g, 15.1 mmol) and 2-hydroxybenzonitrile (1.80 g, 15.1 mmol) were dissolved in 30 mL of anhydrous THF. The solution was cooled to 0 °C and then treated with DIAD (3 mL, 15.1 mmol) in 5 mL of anhydrous THF. The reaction was stirred overnight at room temperature after being stirred at 0 °C for 1 h. After distilling of the volatile solvents, the residue was dissolved in ethyl acetate and washed with brine about 5 times and dried over anhydrous Na2SO4. The filtered organic solution was evaporated to dryness and the residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 5:1) to give 14-(R)-(2′-cyano-phenoxy)-3,19-isopropylidene andrographolide (3.05 g, 6.2 mmol) as a white solid 4 . Compound 14-(R)-(2′-cyano-phenoxy)-3,19-isopropylidene andrographolide (2.45 g, 5.0 mmol) was added in 15 mL of methanol and then treated with p-toluenesulphonic acid monohydrate (0.10 g, 0.5 mmol) at 20 °C for 30 min. Diluted by ethyl acetate and washed with sat. NaHCO3, brine, the organic phase was dried over anhydrous Na2SO4, filtered, evaporated by a rotavap to dryness. Compound 14-(R)-(2′-cyano-phenoxy)-andrographolide (2.03 g, 4.5 mmol) was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 10:7) 4 . Compound 14-(R)-(2′-cyano-phenoxy)-andrographolide (1.81 g, 4.0 mmol) was added in 10 mL of acetic anhydride and then treated with zinc chloride (0.05 g, 0.4 mmol) at 60 °C for 1 h. The solution was cooled to room temperature and then diluted by ethyl acetate and washed with sat. NaHCO3, brine, the organic phase was dried over anhydrous Na2SO4, The filtered organic solution was evaporated to dryness and the residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 4:1) to give 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl-isopropylidene andrographolide (1.50 g, 2.8 mmol) as a white solid. Crystals of the title compound were obtained by recrystallization from a 1:1 system of ethyl acetate: petroleum ether.
2 Experimental details
X-ray reflections on compound cocrystal were collected on a XtaLAB Synergy HyPix diffractometer. All H atoms attached to carbon were included using a riding-model using Olex2, 2 with C–H = 0.93–0.98 Å, and their Uiso values were set to 1.2 U eq. The structure was solved with the OLEX2 3 structure solution program.
3 Comment
The title compound, 14-R-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide is an important derivative of natural active substances. To the best of our knowledge, there is no example of an title compound that has been reported so far. The asymmetric unit of the title structure contains one 14-R-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide molecule. The bond lengths and angles within the structure of 14-R-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide are unexceptional and lie within the expected ranges. In details, the C–O distances within 4-isobutoxybenzaldehyde oxime have values between 1.191(6)–1.463(4) Å. The C26–O5 distance is 1.191(6) Å and the C30–O7 distance is 1.195(5) Å, which suggest an O=C double bond. So esterification is as expected. Andrographolide is a diterpenoid lactone bioactive agent used in traditional medicine in China. 5 It is derived from the plant leaves of Andrographis paniculata. Andrographolide has many pharmacological actions, such as antiviral, antiinflammatory, anticancer, 6 , 7 and antimalarial. 8 Despite being safe at high doses of 17 g/kg per day in humans, the efficacy of andrographolide is limited by poor bioavailability of 2.67 % (reported in rats). 9 This is partly owing to its poor aqueous solubility (46 mg/L). 10 A significant drop in the bioavailability of andrographolide is due to transformation to four metabolites. The main metabolite of andrographolide was identified as 14-deoxy-12-R-sulfoandrographolide. 11 The title compound is obtained by linking 2′-cyano-phenoxy to andrographolide so that andrographolide will not take this reaction. Then andrographolide can have better bioavailability.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: The work was supported by General Project for Natural Science Research in Jiangsu Province Universities (23KJD350004), 2023 Jiangsu Vocational College Student Innovation and Entrepreneurship Cultivation Plan Project (G-2023–1731), and 2023 Jiangsu Province University “Blue Project” Excellent Teaching Team.
References
1. Agilent Technologies: CrysAlisPRO; Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Puschmann, H.; Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K. Olex2–A Complete Package for Molecular Crystallography. Acta Crystallogr. A 2011, 67, C593; https://doi.org/10.1107/s0108767311084996.Search in Google Scholar
4. Liu, Z.; Law, W.-K.; Wang, D.; Nie, X.; Sheng, D.; Song, G.; Guo, K.; Wei, P.; Ouyang, P.; Wong, C.-W.; Zhou, G.-C. Synthesis and Discovery of Andrographolide Derivatives as Non-steroidal Farnesoid X Receptor (FXR) Antagonists. RSC Adv. 2014, 4, 13533–13545; https://doi.org/10.1039/c3ra46715e.Search in Google Scholar
5. Samy, P. R.; Thwin, M. M.; Gopalakrishnakone, P. Phytochemistry, Pharmacology and Clinical Use of Andrographis Paniculata. Nat. Prod. Commun. 2007, 2, 607–618; https://doi.org/10.1177/1934578x0700200519.Search in Google Scholar
6. Woo, A. Y. H.; Waye, M. M. Y.; Tsui, S. K. W.; Yeung, S. T. W.; Cheng, C. H. K. Andrographolide Up-Regulates Cellular-Reduced Glutathione Level and Protects Cardiomyocytes against Hypoxia/reoxygenation Injury. J. Pharmacol. Exp. Therapeut. 2008, 325, 226; https://doi.org/10.1124/jpet.107.133918.Search in Google Scholar PubMed
7. Zhao, F.; He, E. Q.; Wang, L.; Liu, K. J. Anti-tumor Activities of Andrographolide, a Diterpene from Andrographis Paniculata, by Inducing Apoptosis and Inhibiting VEGF Level. J. Asian. Nat. Prod. Res. 2008, 10, 467–473; https://doi.org/10.1080/10286020801948334.Search in Google Scholar PubMed
8. Kirti, M.; Dash, A. P.; Nrisingha, D. Andrographolide: A Novel Antimalarial Diterpene Lactone Compound from Andrographis Paniculata and its Interaction with Curcumin and Artesunate. J. Trop. Med. 2011, 2011, 579518; https://doi.org/10.1155/2011/579518.Search in Google Scholar PubMed PubMed Central
9. Ye, L.; Wang, T.; Tang, L.; Liu, W.; Yang, Z.; Zhou, J.; Zheng, Z.; Cai, Z.; Hu, M.; Liu, Z. Poor Oral Bioavailability of a Promising Anticancer Agent Andrographolide Is Due to Extensive Metabolism and Efflux by P-Glycoprotein. J. Pharm. Sci. 2011, 100, 5007–5017; https://doi.org/10.1002/jps.22693.Search in Google Scholar PubMed
10. Chen, M.; Xie, C.; Liu, L. Solubility of Andrographolide in Various Solvents from (288.2 to 323.2) K. J. Chem. Eng. Data 2010, 55, 5297–5298; https://doi.org/10.1021/je100344z.Search in Google Scholar
11. Cui, L.; Qiu, F.; Wang, N.; Yao, X. Four New Andrographolide Metabolites in Human Urine. Chem. Pharm. Bull. 2004, 35, 772–775; https://doi.org/10.1248/cpb.52.772.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10

