Abstract
Tb0.82Sm0.18Ni0.83Co0.17Mg, orthorhombic, Cmcm (no. 63), a = 3.6707(4) Å, b = 17.723(1) Å, c = 3.9863(4) Å, V = 259.33 (4) Å3, Z = 4, R gt (F) = 0.0334, wR ref (F 2) = 0.0861, T = 293 (2) K.
A part of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Grey plate |
| Size: | 0.07 × 0.06 × 0.01 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 33.2 mm−1 |
| Diffractometer, scan mode: | Xcalibur Oxford Diffraction, ω |
| θ max, completeness: | 26.4°, 97 % |
| N(hkl) measured , N(hkl) unique, R int: | 749, 171, 0.056 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 163 |
| N(param) refined: | 14 |
| Programs: | SHELX, 1 , 2 publCIF, 3 DIAMOND 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Tba | 0.0000 | 0.08753 (5) | 0.7500 | 0.0151 (5) |
| Smb | 0.0000 | 0.08753 (5) | 0.7500 | 0.0151 (5) |
| Nic | 0.5000 | 0.95664 (14) | 0.7500 | 0.0173 (6) |
| Cod | 0.5000 | 0.95664 (14) | 0.7500 | 0.0173 (6) |
| Mg | 0.5000 | 0.2023 (4) | 0.2500 | 0.0209 (13) |
-
aOccupancy: 0.82, bOccupancy: 0.18, cOccupancy: 0.83, dOccupancy: 0.17.
1 Source of material
The Tb0.82Sm0.18Ni0.83Co0.17Mg alloy was prepared in Ta-crucibles by induction melting. The purity of constituent elements was more than 99.98 wt%. The reaction between the metals was thermally activated in an induction furnace at 1,100 °C. After the melting, the sample was rapidly cooled down to room temperature by removing the crucible from the furnace. For homogenization, the alloy was then annealed at 400 °C for 240 h. After this treatment, the sample was separated from the tantalum crucible and the small prismatic single crystals were isolated from the alloy. The density of the alloy was determined using the volumetric method. For the Tb0.82Sm0.18Ni0.83Co0.17Mg phase, the measured density is 6.17(2) g/cm3, which is less than 2 % of the difference from the X-ray data-based theoretical density.
2 Experimental details
The crystal structure of Tb0.82Sm0.18Ni0.83Co0.17Mg was studied by a single crystal X-ray diffraction (diffractometer Xcalibur Oxford Diffraction, CCD-detector, Mo-Kα1-radiation, ω-scan mode). Absorption correction was performed by an analytical method. The solution of the crystal structure was carried out by Direct Methods of full matrix least squares refinement on F 2. The statistical mixture of Tb/Sm and Ni/Co atoms occupies two 4c sites. Crystal data and details of the structure refinement for Tb0.82Sm0.18Ni0.83Co0.17Mg are given in Table 1. The atomic positions, isotropic and anisotropic thermal displacement parameters are given in Table 2.
3 Comment
The interaction of magnesium with rare earth elements and 3d-metals is characterized by the formation of intermetallics, some of which are prone to hydrogen absorption. 5 , 6 , 7 , 8 , 9 The high entropy Tb0.82Sm0.18Ni0.83Co0.17Mg phase was received from ternary compound TiNiMg 10 by partial substitution of terbium by samarium and nickel by cobalt atoms. This modification was carried out in order to increase the hydrogen sorption activity of this material. The crystal structure of the Tb0.82Sm0.18Ni0.83Co0.17Mg phase has been determined by single crystal X-ray diffraction data analysis. The unit cell and coordination polyhedra of atoms are given in Figure 1 (top). In this phase the smallest Ni/Co atoms are enclosed into three capped trigonal prisms. The largest Tb/Sm atoms are enclosed in 17-vertex polyhedra, namely as polarly and equatorially capped pentagonal prisms. The closest arrangement around Mg atoms has the form of a distorted rhombic dodecahedron with coordination number 14. The structure of the title compound can be described by applying the intergrowth concept on the base of the AlB2, CsCl and W simple fragments (Figure 1, middle).
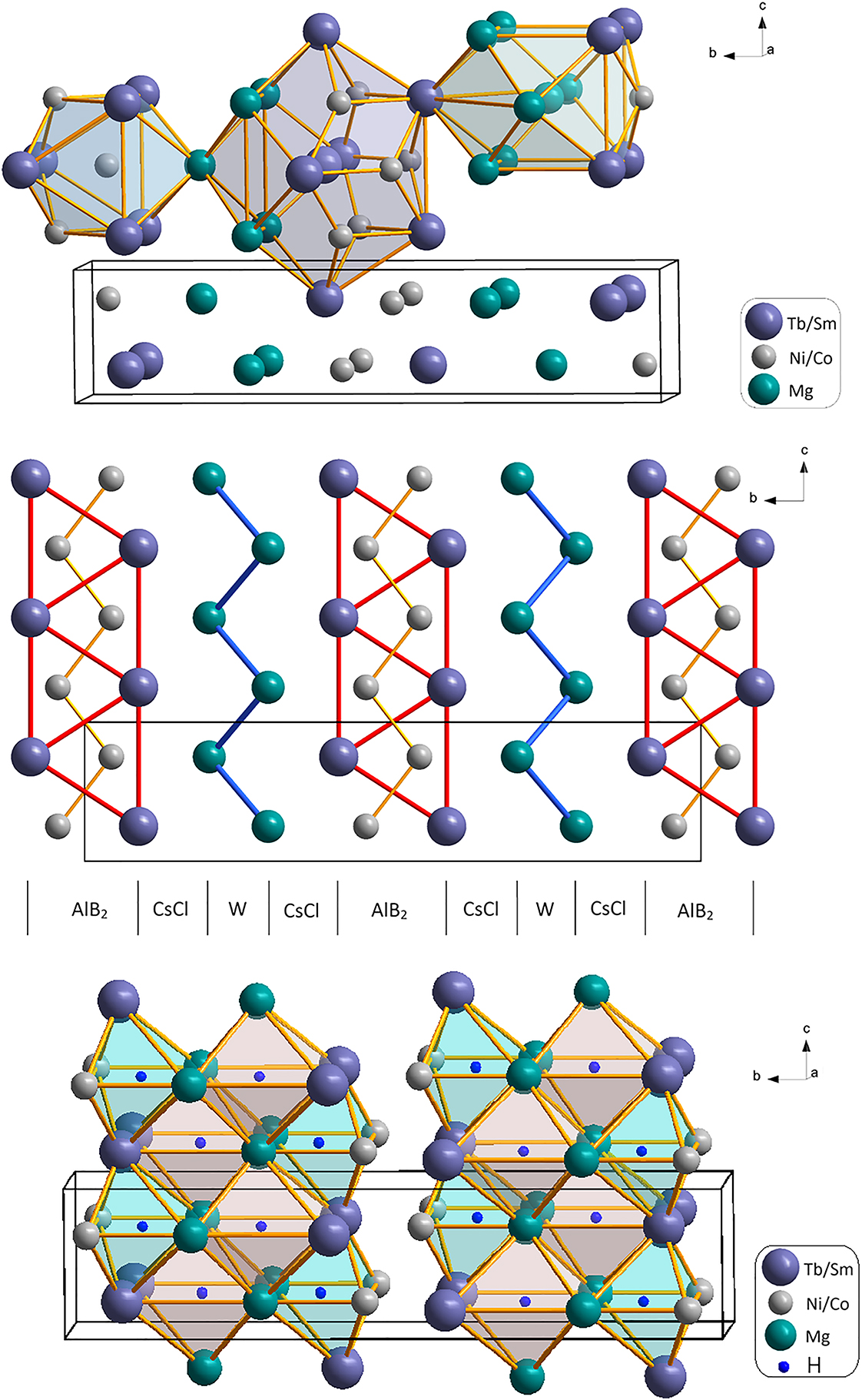
The studies of hydrogen sorption properties show a hydrogen sorption capacity of up to 0.83 wt% H2, which corresponds to the formula of the hydride Tb0.82Sm0.18Ni0.83Co0.17MgH2.0. The theoretical basics of the analysis of voids in crystal structures by means of a crystallochemical approach give positional parameters of two octahedral voids (sites 4c: x = 1/2, y = 0.380, z = 3/4 and x = 1/2, y = 0.380, z = 3/4) which can be occupied by hydrogen atoms. The mutual stacking of hydrogen-filled octahedra is shown in Figure 1 (bottom). For the hydrogenated phase the unit cell dimension increases up to a = 3.680(2) Å, b = 17.731(5) Å, c = 3.991(3) Å.
Acknowledgement
We gratefully acknowledge support by the National Research Foundation of Ukraine (2022.01/0064).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/S0108767307043930.Search in Google Scholar PubMed
2. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Westrip, S. P. publCIF: Software for Editing, Validating and Formatting Crystallographic Information Files. J. Appl. Crystallogr. 2010, 43, 920–925. https://doi.org/10.1107/S0021889810022120.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 3.1f.; Crystal Impact: Bonn, Germany, 2008.Search in Google Scholar
5. Shtender, V. V.; Denys, R. V.; Zavaliy, I. Y.; Zelinska, O. Y.; Paul-Boncour, V.; Pavlyuk, V. V. Phase Equilibria in the Tb–Mg–Co System at 500 °C, Crystal Structure and Hydrogenation Properties of Selected Compounds. J. Solid State Chem. 2015, 232, 228–235. https://doi.org/10.1016/j.jssc.2015.09.031.Search in Google Scholar
6. Pavlyuk, V.; Marciniak, B.; Różycka-Sokołowska, E. The Isothermal Section of the Phase Diagram of Ce–Mg–Zn Ternary System at 470 K. Intermetallics 2012, 20, 8–15. https://doi.org/10.1016/j.intermet.2011.08.013.Search in Google Scholar
7. Pavlyuk, V.; Solokha, P.; Dmytriv, G.; Marciniak, B.; Paul-Boncour, V. The Heusler-type Alloy MgZn2Ce. Acta Crystallogr. 2007, E63, i161. https://doi.org/10.1107/S1600536807028899.Search in Google Scholar
8. Solokha, P.; De Negri, S.; Pavlyuk, V.; Saccone, A. Anti-Mackay Polyicosahedral Clusters in La–Ni–Mg Ternary Compounds: Synthesis and Crystal Structure of the La43Ni17Mg5 New Intermetallic Phase. Inorg. Chem. 2009, 48, 11586–11593. https://doi.org/10.1021/ic901422v.Search in Google Scholar PubMed
9. Solokha, P.; De Negri, S.; Pavlyuk, V.; Eck, B.; Dronskowski, R.; Saccone, A. 3D [Ag–Mg] Polyanionic Frameworks in the La4Ag10Mg3 and La4Ag10.3Mg12 New Ternary Compounds. J. Solid State Chem. 2010, 183, 2995–3001. https://doi.org/10.1016/j.jssc.2010.10.018.Search in Google Scholar
10. Solokha, P.; De Negri, S.; Pavlyuk, V.; Saccone, A. The Novel Intermetallic Phases TbNiMg and Tb4+xNi2Mg3−x (x = 0.2): Synthesis, Crystal Structure and Peculiarities. Intermetallics 2010, 18, 719–724. https://doi.org/10.1016/j.intermet.2009.11.012.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10

