Abstract
C19H14N2O2, monoclinic, P21/n (no. 14), a = 11.3111(5) Å, b = 7.2906(3) Å, c = 18.0875(10) Å, β = 102.935(2)°V = 1,453.73(12) Å3, Z = 4, Rgt (F) = 0.0545, wRref (F 2) = 0.1389, T = 150 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
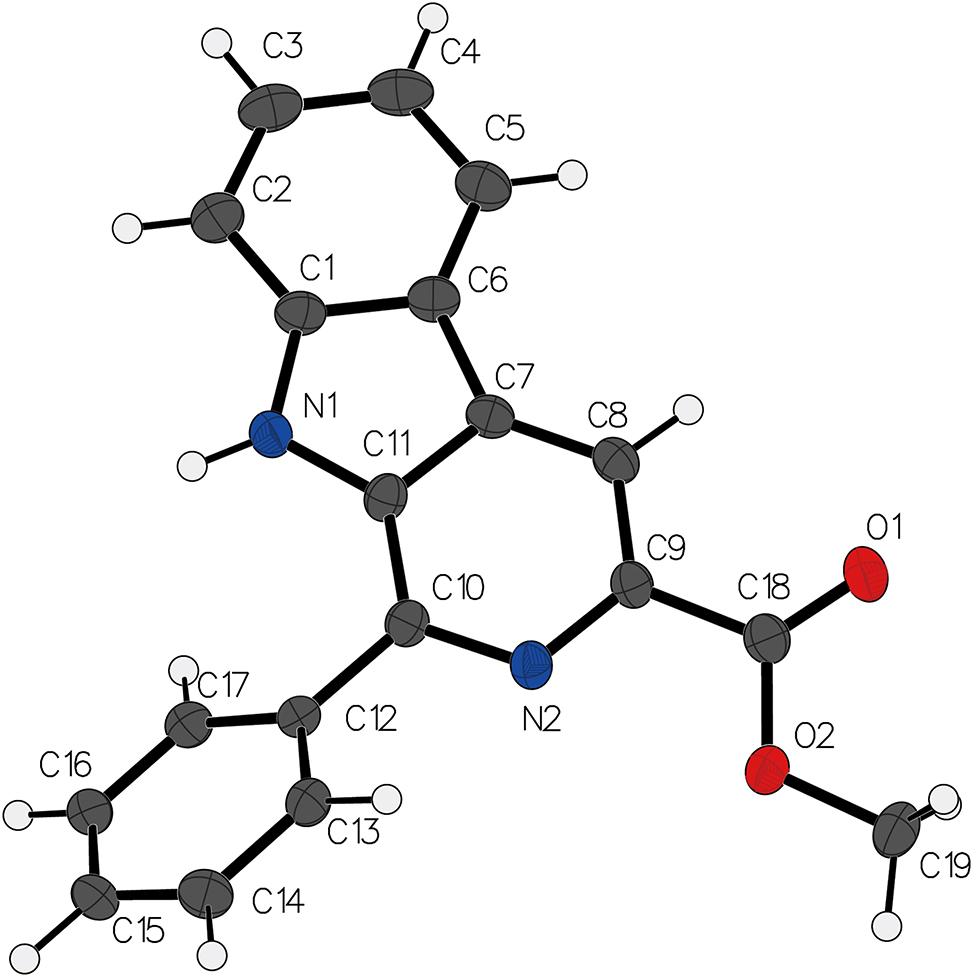
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.08 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE, φ and ω |
| θ max, completeness: | 26.0°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 10,087, 2,812, 0.085 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1,853 |
| N(param)refined: | 209 |
| Programs: | Bruker 1 , SHELX 2 , 3 , Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso */U eq |
|---|---|---|---|---|
| C1 | 0.1410 (2) | 0.6852 (3) | 0.54911 (13) | 0.0240 (6) |
| C2 | 0.0573 (2) | 0.7632 (3) | 0.48928 (14) | 0.0309 (6) |
| H2 | −0.022128 | 0.794584 | 0.494122 | 0.037* |
| C3 | 0.0942 (2) | 0.7933 (3) | 0.42256 (14) | 0.0333 (7) |
| H3 | 0.039024 | 0.847512 | 0.380913 | 0.040* |
| C4 | 0.2103 (3) | 0.7464 (3) | 0.41453 (14) | 0.0339 (7) |
| H4 | 0.232535 | 0.767712 | 0.367628 | 0.041* |
| C5 | 0.2933 (2) | 0.6691 (3) | 0.47434 (14) | 0.0293 (6) |
| H5 | 0.372599 | 0.638181 | 0.469100 | 0.035* |
| C6 | 0.2584 (2) | 0.6374 (3) | 0.54257 (13) | 0.0241 (6) |
| C7 | 0.3204 (2) | 0.5697 (3) | 0.61665 (13) | 0.0221 (5) |
| C8 | 0.4372 (2) | 0.5100 (3) | 0.64865 (13) | 0.0234 (6) |
| H8 | 0.497626 | 0.503812 | 0.619758 | 0.028* |
| C9 | 0.4620 (2) | 0.4600 (3) | 0.72446 (13) | 0.0215 (5) |
| C10 | 0.2671 (2) | 0.5224 (3) | 0.73927 (13) | 0.0214 (5) |
| C11 | 0.2353 (2) | 0.5765 (3) | 0.66288 (13) | 0.0214 (5) |
| C12 | 0.1810 (2) | 0.5238 (3) | 0.79032 (13) | 0.0214 (5) |
| C13 | 0.2208 (2) | 0.5743 (3) | 0.86635 (13) | 0.0258 (6) |
| H13 | 0.302957 | 0.609362 | 0.885050 | 0.031* |
| C14 | 0.1418 (2) | 0.5739 (4) | 0.91458 (14) | 0.0314 (6) |
| H14 | 0.169979 | 0.608292 | 0.966195 | 0.038* |
| C15 | 0.0217 (2) | 0.5235 (3) | 0.88795 (14) | 0.0309 (6) |
| H15 | −0.032702 | 0.524463 | 0.921050 | 0.037* |
| C16 | −0.0187 (2) | 0.4717 (3) | 0.81268 (14) | 0.0294 (6) |
| H16 | −0.100834 | 0.436256 | 0.794324 | 0.035* |
| C17 | 0.0601 (2) | 0.4715 (3) | 0.76440 (14) | 0.0260 (6) |
| H17 | 0.031772 | 0.435366 | 0.713019 | 0.031* |
| C18 | 0.5854 (2) | 0.3917 (3) | 0.76081 (14) | 0.0245 (6) |
| C19 | 0.7064 (2) | 0.2410 (4) | 0.86703 (15) | 0.0338 (7) |
| H19A | 0.736619 | 0.157553 | 0.833004 | 0.051* |
| H19B | 0.698172 | 0.174387 | 0.912670 | 0.051* |
| H19C | 0.763623 | 0.342675 | 0.881388 | 0.051* |
| N1 | 0.12679 (17) | 0.6445 (3) | 0.62153 (11) | 0.0250 (5) |
| H1 | 0.060068 | 0.659505 | 0.638212 | 0.030* |
| N2 | 0.38040 (17) | 0.4664 (3) | 0.76895 (11) | 0.0227 (5) |
| O1 | 0.67253 (15) | 0.4035 (3) | 0.73280 (10) | 0.0345 (5) |
| O2 | 0.58916 (14) | 0.3127 (2) | 0.82863 (9) | 0.0264 (4) |
1 Source of materials
To a solution of 3-chloro-1-phenyl-9H-pyrido[3,4-b]indole (2.78 g, 10 mmol) in methanol (25 mL) were added 1,1′-bis(diphenylphosphino)ferrocene-palladium(II) dichloride dichloromethane complex (408 mg, 0.5 mmol) and triethylamine (3.0 g, 30 mmol). Then, carbon monoxide gas (5 atm) was introduced into the reaction system and stirred at 60 °C for 3 h, until the TLC indicated the reaction was completed. The solvent was evaporated using a rotary evaporator, yielding the crude product that was purified. For crystal growth, the crude product was dissolved in a minimal amount of hot methanol and slowly cooled to room temperature.
2 Experimental details
Hydrogen atoms were assigned isotropic displacement factors: U iso(H) = 1.2 times U eq(C). All hydrogen atoms were refined as being bonded to their respective parent atoms.
3 Comment
Pyrido[3,4-b]indoles, which consist of a fused indole ring with a pyridine component, have been discovered to disrupt DNA replication and transcription, evoke strong neuropharmacological effects, and exhibit excellent cytotoxicity against cancer cells, fungi, and bacteria. 5 Numerous crystal structures of pyrido[3,4-b]indole derivatives have been reported in the literature. 6 , 7 , 8 , 9 , 10 , 11 , 12 In this paper, we report a crystal structure of a pyrido[3,4-b]indole derivative and provide a detailed discussion, thereby offering a foundation for drug development based on the reported crystal structure.
In the crystal structure of the titled compound, the skeleton atoms of the pyrido[3,4-b]indole moiety are almost coplanar, forming a conjugated structure consistent with many previously reported crystal structures of pyrido[3,4-b]indole derivatives. 13 , 14 , 15 , 16 , 17 , 18 , 19 Additionally, the atoms in the aliphatic group within this crystal structure are also nearly coplanar with the skeleton atoms of the pyrido[3,4-b]indole moiety, with the dihedral angle C8–C9–C18–O1 being only 12.5(4)°. The benzene ring in the crystal structure is significantly twisted relative to the pyrido[3,4-b]indole moiety, with a dihedral angle of 39.5°. Furthermore, no significant intermolecular hydrogen bonds were observed in the crystal structure.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Natural Science Foundation of Shannxi Province (2024JC–YBMS-733), Scientific research plan project of Shaanxi Provincial Department of Education (23JK0321), the Xianyang key laboratory of molecular imaging and drug synthesis (2021QXNL–PT-0008), the 2023 key research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles Among U. S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. https://doi.org/10.1021/jm501100b.Search in Google Scholar PubMed
6. Yu, G.; Li, Y.-Z.; Cao, L.-Y.; Zhang, X.-M.; Wang, K.; Hu, H.-W. 1-Ethyl-2-Oxo-5-Phenyl-1,6-Dihydro-2H-Oxazolo[4′,5′:5,6]Pyrido[3,4-b]Indole Methanol Hemisolvate. Acta Crystallogr. 2004, E60, o1271–o1272. https://doi.org/10.1107/s1600536804015193.Search in Google Scholar
7. Leboeuf, M.; Cavé, A.; Forgacs, P.; Provost, J.; Chiaroni, A.; Riche, C. Alkaloids of the Annonaceae. Part 33. Annomontine and Methoxyannomontine, Two New Pyrimidine-β-Carboline-Type Alkaloids from Annona montana. J. Chem. Soc., Perkin Trans. 1982, 1, 1205–1208. https://doi.org/10.1039/p19820001205.Search in Google Scholar
8. Yokomori, Y.; Sekido, K.; Wu, T.-S.; Tien, H.-J.; Hirokawa, S. The Crystal and Molecular Structure of 1-(2-Amino-4-Pyrimidinyl)-β-Carboline. Bull. Chem. Soc. Jpn. 2006, 55, 2236–2238. https://doi.org/10.1246/bcsj.55.2236.Search in Google Scholar
9. He, L.; Li, Y.; Tan, C.-P.; Ye, R.-R.; Chen, M.-H.; Cao, J.-J.; Ji, L.-N.; Mao, Z.-W. Cyclometalated Iridium(iii) Complexes as Lysosome-Targeted Photodynamic Anticancer and Real-Time Tracking Agents. Chem. Sci. 2015, 6, 5409–5418. https://doi.org/10.1039/c5sc01955a.Search in Google Scholar PubMed PubMed Central
10. Huang, Y.-Q.; Song, H.-J.; Liu, Y.-X.; Wang, Q.-M. Dehydrogenation of N-Heterocycles by Superoxide Ion Generated Through Single-Electron Transfer. Chem. Eur. J. 2018, 24, 2065–2069. https://doi.org/10.1002/chem.201705202.Search in Google Scholar PubMed
11. Dai, J.; Dan, W.; Ren, S.; Shang, C.; Wang, J. Design, Synthesis and Biological Evaluations of Quaternization Harman Analogues as Potential Antibacterial Agents. Eur. J. Med. Chem. 2018, 160, 23–36. https://doi.org/10.1016/j.ejmech.2018.10.012.Search in Google Scholar PubMed
12. Wang, Z.-X.; Xiang, J.-C.; Cheng, Y.; Ma, J.-T.; Wu, Y.-D.; Wu, A.-X. Direct Biomimetic Synthesis of β-Carboline Alkaloids from Two Amino Acids. J. Org. Chem. 2018, 83, 12247–12254. https://doi.org/10.1021/acs.joc.8b01668.Search in Google Scholar PubMed
13. Sheng, T.; Kong, M.; Wang, Y.; Wu, H.; Gu, Q.; Chuang, A. S.; Li, S.; Gao, X. Discovery and Preliminary Mechanism of 1-Carbamoyl β-Carbolines as New Antifungal Candidates. Eur. J. Med. Chem. 2021, 222, 113563. https://doi.org/10.1016/j.ejmech.2021.113563.Search in Google Scholar PubMed
14. Zhang, H.; Zhang, R.-H.; Liao, X.-M.; Yang, D.; Wang, Y.-C.; Zhao, Y.-L.; Xu, G.-B.; Liu, C.-H.; Li, Y.-J.; Liao, S.-G.; Zhou, M. Discovery of β-Carboline Derivatives as a Highly Potent Cardioprotectant Against Myocardial Ischemia-Reperfusion Injury. J. Med. Chem. 2021, 64, 9166–9181. https://doi.org/10.1021/acs.jmedchem.1c00384.Search in Google Scholar PubMed
15. Letessier, J.; Detert, H.; Götz, K.; Opatz, T. Microwave-Assisted Synthesis of 1,3-Disubstituted β-Carbolines from α-(Alkylideneamino)Nitriles and Gramine. Synthesis 2012, 44, 747–754. https://doi.org/10.1055/s-0031-1289675.Search in Google Scholar
16. Sang, J.; Feng, L.; Hu, R.; Chen, J.; Shang, D.; Bao, Q.; Rao, W. Sc(OTf) 3-Catalyzed C2-Selective Cyanation/Defluorination Cascade of Perfluoroalkylated 3-Indolylmethanols and Application to the Synthesis of 3-Fluoro(Perfluoroalkyl)-β-Carbolines. Org. Lett. 2021, 23, 7666–7671. https://doi.org/10.1021/acs.orglett.1c02932.Search in Google Scholar PubMed
17. Reniers, J.; Robert, S.; Frederick, R.; Masereel, B.; Vincent, S.; Wouters, J. Synthesis and Evaluation of β-Carboline Derivatives as Potential Monoamine Oxidase Inhibitors. Bioorg. Med. Chem. 2011, 19, 134–144. https://doi.org/10.1016/j.bmc.2010.11.041.Search in Google Scholar PubMed
18. Pal, B.; Jaisankar, P.; Sesha Giri, V.; Mondal, S.; Mukherjee, M. Unusual Formation of β-Carboline Dimers Under Bischler–Napieralski Reaction Conditions: An Old Reaction with a New Direction. Tetrahedron Lett. 2004, 45, 6489–6492. https://doi.org/10.1016/j.tetlet.2004.06.118.Search in Google Scholar
19. Kusurkar, R. S.; Alkobati, N. A. H.; Gokule, A. S.; Puranik, V. G. Use of the Pictet–Spengler Reaction for the Synthesis of 1,4-Disubstituted-1,2,3,4-Tetrahydro-β-Carbolines and 1,4-Disubstituted-β-Carbolines: Formation of γ-Carbolines. Tetrahedron 2008, 64, 1654–1662. https://doi.org/10.1016/j.tet.2007.12.008.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

