Abstract
C21H18ClFO3, monoclinic, P21/c (no. 14), a = 34.657(5) Å, b = 6.0214(8) Å, c = 8.2301(11) Å, β = 93.883(5)°, V = 1713.5(4) Å3, Z = 4, Rgt (F) = 0.0683, wR ref (F 2) = 0.1435, T = 205(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.11 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.25 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 27.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 30,930, 3,940, 0.095 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3,058 |
| N(param)refined: | 237 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.56867 (9) | 0.6643 (6) | 0.2273 (4) | 0.0235 (7) |
| H1 | 0.559856 | 0.800577 | 0.183236 | 0.028* |
| C2 | 0.54551 (9) | 0.5333 (6) | 0.3171 (4) | 0.0255 (7) |
| C3 | 0.55694 (9) | 0.3314 (6) | 0.3815 (4) | 0.0254 (7) |
| H3 | 0.540310 | 0.246131 | 0.442098 | 0.031* |
| C4 | 0.59387 (9) | 0.2575 (6) | 0.3540 (3) | 0.0197 (6) |
| H4 | 0.602112 | 0.118872 | 0.395847 | 0.024* |
| C5 | 0.61908 (8) | 0.3827 (5) | 0.2661 (3) | 0.0178 (6) |
| C6 | 0.60570 (9) | 0.5863 (6) | 0.2046 (3) | 0.0194 (6) |
| C7 | 0.65894 (8) | 0.2981 (5) | 0.2404 (4) | 0.0191 (6) |
| H7A | 0.661194 | 0.141883 | 0.273022 | 0.023* |
| H7B | 0.664127 | 0.309462 | 0.125058 | 0.023* |
| C8 | 0.72348 (9) | 0.4139 (5) | 0.2960 (4) | 0.0184 (6) |
| C9 | 0.75061 (9) | 0.5581 (5) | 0.4041 (4) | 0.0194 (6) |
| H9 | 0.748188 | 0.510047 | 0.518034 | 0.023* |
| C10 | 0.73774 (9) | 0.8004 (6) | 0.3920 (4) | 0.0262 (8) |
| H10A | 0.737979 | 0.849733 | 0.279860 | 0.039* |
| H10B | 0.755298 | 0.891514 | 0.460339 | 0.039* |
| H10C | 0.711775 | 0.814028 | 0.428080 | 0.039* |
| C11 | 0.79217 (9) | 0.5148 (5) | 0.3638 (3) | 0.0179 (6) |
| C12 | 0.81306 (8) | 0.6578 (6) | 0.2744 (3) | 0.0185 (6) |
| H12 | 0.801849 | 0.792884 | 0.238691 | 0.022* |
| C13 | 0.85128 (8) | 0.6062 (5) | 0.2347 (3) | 0.0166 (6) |
| C14 | 0.86826 (8) | 0.4021 (5) | 0.2886 (3) | 0.0164 (6) |
| C15 | 0.84630 (9) | 0.2582 (6) | 0.3820 (3) | 0.0184 (6) |
| H15 | 0.857114 | 0.122752 | 0.419161 | 0.022* |
| C16 | 0.80965 (9) | 0.3126 (5) | 0.4194 (3) | 0.0191 (6) |
| H16 | 0.795793 | 0.214793 | 0.482722 | 0.023* |
| C17 | 0.90624 (9) | 0.3481 (5) | 0.2466 (3) | 0.0180 (6) |
| H17 | 0.917794 | 0.214372 | 0.283389 | 0.022* |
| C18 | 0.92607 (9) | 0.4908 (6) | 0.1526 (3) | 0.0189 (6) |
| C19 | 0.90920 (9) | 0.6949 (5) | 0.0995 (3) | 0.0196 (7) |
| H19 | 0.923017 | 0.792041 | 0.035427 | 0.024* |
| C20 | 0.87305 (8) | 0.7509 (6) | 0.1408 (3) | 0.0191 (6) |
| H20 | 0.862383 | 0.887895 | 0.106347 | 0.023* |
| C21 | 0.98148 (9) | 0.2552 (6) | 0.1569 (4) | 0.0288 (8) |
| H21A | 0.966883 | 0.127575 | 0.115182 | 0.043* |
| H21B | 0.983216 | 0.251878 | 0.274962 | 0.043* |
| H21C | 1.007280 | 0.251069 | 0.118051 | 0.043* |
| Cl1 | 0.63552 (2) | 0.74993 (15) | 0.09212 (9) | 0.02457 (19) |
| F1 | 0.50929 (6) | 0.6088 (4) | 0.3409 (3) | 0.0400 (6) |
| O1 | 0.68645 (6) | 0.4323 (4) | 0.3386 (2) | 0.0201 (5) |
| O2 | 0.73216 (6) | 0.2976 (4) | 0.1861 (3) | 0.0278 (6) |
| O3 | 0.96238 (6) | 0.4547 (4) | 0.1014 (3) | 0.0225 (5) |
1 Source of materials
Naproxen acylchloride was synthesized according to the literature method. 4 2-Chloro-4-fluorobenzyl alcohol (0.01 mol, 1.60 g) and 4-(dimethylamino)-pyridin (DMAP, 0.0015 mol, 0.18 g) were dissolved in dry tetrahydrofuran (20 mL) and triethylamine (0.015 mol, 2 mL). The solution of naproxen acylchloride in dry tetrahydrofuran was dropwise added at 0 °C. The reaction mixture was stirred for 2 h at room temperature. The reaction mixture was filtrated to remove the solid and the filtrate was concentrated under vacuum to remove the solvent. The residue was dissolved in dichloromethane, successively washed with 5 % NaOH solution and water to pH = 7, and dried with anhydrous Na2SO4 The solution was filtrated, and concentrated under vacuum to obtain crude product. The crude product was purified by recrystallization in ethanol. The crystals were obtained from tetrahydrofuran solution.
2 Experimental details
The U iso values of hydrogen atoms were set to be 1.5U eq of the carrier atom for methyl H atoms and 1.2U eq for the remaining H atoms.
3 Comment
Naproxen, a powerful non-steroidal anti-inflammatory drug (NSAID), inhibits both COX-1 and COX-2 enzymes, essential elements of the cyclooxygenase pathway. Naproxen frequently induces gastrointestinal issues, including gastroduodenal ulcers. The carboxyl group in naproxen might be responsible for these adverse effects. Studies indicate that modifying this carboxyl group through esterification can lessen these gastrointestinal reactions. 5 , 6 Naproxen is presently employed in clinical settings to manage or mitigate pain and inflammation associated with conditions like rheumatoid arthritis 7 and gout. 8 Moreover, it has certain therapeutic effects on migraines 9 but may cause certain side effects. Additionally, research indicates that naproxen has a preventive effect on Lynch syndrome. 10 Consequently, creating derivatives of naproxen that have reduced side effects is crucial.
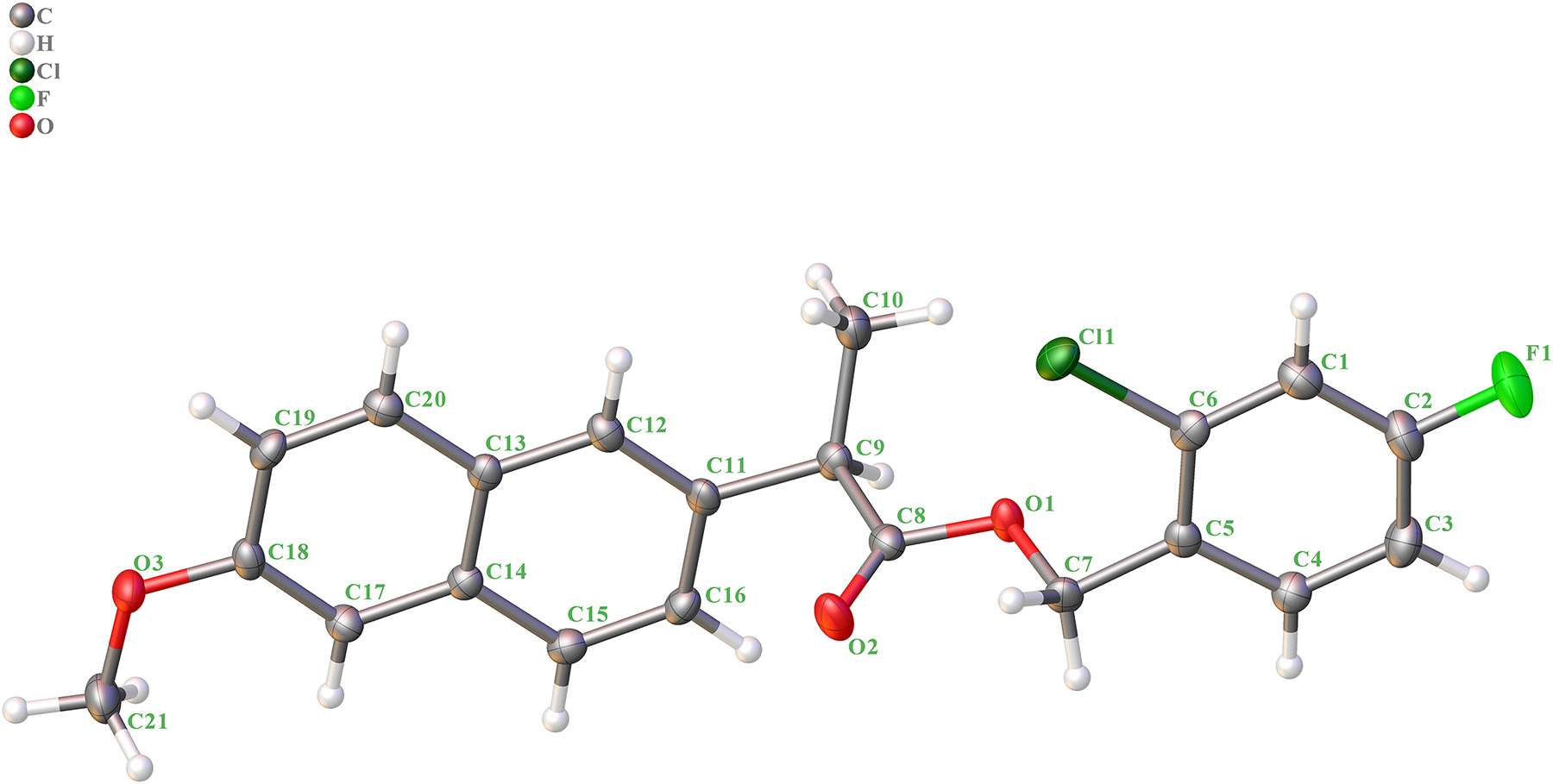
The title compound contains one naphthyl ring and one phenyl ring. The bond distances of C–O are 1.453(3) Å (C7–O1), 1.358(4) Å (C8–O1), 1.198(4) Å (C8–O2), 1.371(4) Å (C18–O3) and 1.431(4) Å (C21–O3). The bond distance of C8–O2 are shorter than others, indicating a double bond. The bond distance of C–Cl are 1.739(3) Å (C6–Cl1) and the bond distance of C–F are 1.362(4) Å (C2–F1). The dihedral angels of ring 1 (C1–C2–C3–C4–C5–C6) and ring 2 (C11–C12–C13–C14–C15–C16), ring 1 (C1–C2–C3–C4–C5–C6) and ring 3 (C13–C14–C17–C18–C19–C20), and ring 2 (C11–C12–C13–C14–C15–C16) and ring 3 (C13–C14–C17–C18–C19–C20) are 0.8(1)°, 2.1(1)° and 1.33(8)°. The other bond distances and angles are in their normal ranges according to the previously reported compounds. 11 , 12
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Research funding: None declared.
References
1. Bruker. SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Huang, Z.; Velázquez, C. A.; Abdellatif, K. R. A.; Chowdhury, M. A.; Reisz, J. A.; DuMond, J. F.; King, S. B.; Knaus, E. E. Ethanesulfohydroxamic Acid Ester Prodrugs of Nonsteroidal Anti-inflammatory Drugs (NSAIDs): Synthesis, Nitric Oxide and Nitroxyl Release, Cyclooxygenase Inhibition, Anti-inflammatory, and Ulcerogenicity Index Studies. J. Med. Chem. 2011, 54, 1356–1364; https://doi.org/10.1021/jm101403g.Search in Google Scholar PubMed
5. Ullah, N.; Huang, Z.; Sanaee, F.; Rodriguez-Dimitrescu, A.; Aldawsari, F.; Jamali, F.; Bhardwaj, A.; Islam, N. U.; Velázquez-Martínez, C. A. NSAIDs Do Not Require the Presence of a Carboxylic Acid to Exert Their Anti-inflammatory Effect – Why Do We Keep Using it? J. Enzyme Inhib. Med. Chem. 2015, 31, 1018–1028; https://doi.org/10.3109/14756366.2015.1088840.Search in Google Scholar PubMed
6. de Carvalho Bertozo, L.; Tadeu, H. C.; Sebastian, A.; Maszota-Zieleniak, M.; Samsonov, S. A.; Ximenes, V. F. Role for Carboxylic Acid Moiety in NSAIDs: Favoring the Binding at Site II of Bovine Serum Albumin. Mol. Pharmaceutics 2024, 21, 2501–2511; https://doi.org/10.1021/acs.molpharmaceut.4c00044.Search in Google Scholar PubMed
7. Brogden, R. N.; Heel, R. C.; Speight, T. M.; Avery, G. S. Naproxen up to Date: A Review of its Pharmacological Properties and Therapeutic Efficacy and Use in Rheumatic Diseases and Pain States. Drugs 1979, 18, 241–277; https://doi.org/10.2165/00003495-197918040-00001.Search in Google Scholar PubMed
8. Janssens, H. J.; Janssen, M.; van de Lisdonk, E. H.; van Riel, P. L.; van Weel, C. Use of Oral Prednisolone or Naproxen for the Treatment of Gout Arthritis: A Double-Blind, Randomised Equivalence Trial. Lancet 2008, 371, 1854–1860; https://doi.org/10.1016/s0140-6736(08)60799-0.Search in Google Scholar PubMed
9. Suthisisang, C. C.; Poolsup, N.; Suksomboon, N.; Lertpipopmetha, V.; Tepwitukgid, B. Meta-Analysis of the Efficacy and Safety of Naproxen Sodium in the Acute Treatment of Migraine. Headache 2010, 50, 808–818; https://doi.org/10.1111/j.1526-4610.2010.01635.x.Search in Google Scholar PubMed
10. Reyes-Uribe, L.; Wu, W.; Gelincik, O.; Bommi, P. V.; Francisco-Cruz, A.; Solis, L. M.; Lynch, P. M.; Lim, R.; Stoffel, E. M.; Kanth, P.; Samadder, N. J.; Mork, M. E.; Taggart, M. W.; Milne, G. L.; Marnett, L. J.; Vornik, L.; Liu, D. D.; Revuelta, M.; Chang, K.; Vilar, E.; Kopelovich, L.; Wistuba, I. I.; Lee, J. J.; Sei, S.; Shoemaker, R. H.; Szabo, E.; Richmond, E.; Umar, A.; Perloff, M.; Brown, P. H.; Lipkin, S. M. Naproxen Chemoprevention Promotes Immune Activation in Lynch Syndrome Colorectal Mucosa. Gut 2020, 70, 555–566; https://doi.org/10.1136/gutjnl-2020-320946.Search in Google Scholar PubMed PubMed Central
11. Liang, D.; Yang, X. H.; Sun, W.; Wang, W. N.; Yang, J. Z.; Liu, Y. Y.; Wang, G. S. Synthesis, Crystal Structure and Biological Activities of Naproxen-Eugenol Ester Progrug. Chem. Res. Chin. Univ . 2013, 29, 245–248; https://doi.org/10.1007/s40242-013-2266-9.Search in Google Scholar
12. Hashimoto, M.; Nakamura, Y.; Hamada, K. Structure of 4-Chlorobenzyl Alcohol. Acta Crystallogr. 1988, C44, 482–484; https://doi.org/10.1107/s0108270187010965.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

