Abstract
To investigate the efficacy of modified clay minerals to remediate heavy metals from industrial wastewater, two natural clay sediments dominated by kaolinite were selected. Since the kaolinite clay has low cation exchange capacity, some modifications were made using unusual treatments thermal transformation and acid activation techniques were used to increase exchangeability properties for producing modified kaolinite. The increased exchangeability was demonstrated through various methods. Results of X-Ray diffraction analysis verified the transformation of Kaolinite as indicated from disappearing all the diffractogram peaks due to kaolinite. In infra-red spectroscopy, the presence of a broad band with little change in the intensity in the region coupled with broad Si-O bending vibration band and Si-O-Al compound vibration bands explain the extent of structural disorder as a response of modification treatment. From a mineral structural viewpoint, destruction through heat treatment exposes directed –OH bonds located between the tetrahedral and octahedral layers (amorphization). It has been observed that after an acidification treatment, the –OH groups become less stable and lead to the newly formed vacant sites during the modification treatments accommodate extra structural water; thereby; broadening the –OH bands in the I.R spectrum.
Scanning Electron Microscope study has clearly demonstrated that, transformation in kaolinite structure from from hexagonal original shape to edgeless shape and values of Cation Exchange Capacity C.E.Cwere increased from 8.2 to 18.41 Meq/100g and from 12.66 to 28.53 Meq/100g in both Sinai and Aswan sediment, respectively. Result of the present study has indicated that crystalling structure of kaolinte tranformed to collapsed structure after modification treatment and the new structure can absorb a large amounts of pollutant metallic ions including Zn and cu from wastewater.
Observed increase of metal ions removed by modified clays are due to increase of exchange sites produced by the acid leaching on a collapsed kaolinite framework. A comparison of relative removing values between Zn and Cu revealed that the removing kinetics of Cu is more favored under identical molar concentrations than Zn.
1 Introduction
As the world’s population continues to grow, there are been a continous rise in the demand for clean potable water. At the same time the availability and uncontaminated surface and ground water resources are not only limited but also exhausting at an alarming rate.
According to [1] deterioration of water quality was consider to be one of the seven most critical challenges of water.
Several techniques were employed to remove the contaminants and eliminate or reduce the pollutants and prepare the water for release into the environment safely. Most of these techniques required extensive handling of material and excessive time to achieve removal of solids, oils and metal ions from the water.
Currently, the most common used techiques make use of several typed techiques make use of several types of material that are used to adsorb wastewater contaminants; among theses, clays were found to have remarkable affinity for heavy metals ions. Through ion exchange process the metal ions get bounded to the clays by electrostatic forces of attraction between the metal ions in solution and the anionic surfaces on the clay particles, [2, 3].
Although Kaolinite clay mineral has least exchange-ability properties, several researchers confirmed that modified kaolinite have potential ability to adsorp metal ions from solution [4, 5, 6].
A modified kaolinite amorphous derivative (metakaolinite Al2(OH)4Si2O5 ) which is produced from calcination of kaolinite at 600∘C, absorbs heavy metals and could be used in the treatment of toxic metal pollution in water [7, 8]. [4, 9, 10] reported that the porous structure of collapsed modified kaolinte structure can adsorb a large amount of metallic ions such as Pb, Cd and Cu from wastewater. [11] reported that sodium-capturing capacity has been significantly increased, by applying modified kaolinite.
In the view of above, present study was conducted with a view to (i) develop modified kaolinite from local clay to increase it’s ion exchange capacity, and (ii) to compare the results for estimating the efficacy of modified clay over non-modified clay for remediation of heavy metals from the industrial wastewater.
2 Sampling and Preparation
2.1 Clay sediments
Two clay sediments samples were collected from both Aswan , South of western desert, Egypt and El Tih plateau , Sinai, Eastern desert, Egypt.
The clay was washed with distilled water to remove soluble impurities and wet sieved through 350 mesh sieve (45 μm), homogenized and $#x003C;45 μm fraction was collected. The clay then was reacted with 0.5 N Ca(NO3)2 and rinsed with distilled water to eliminate excess salts, [12].
Each clay sample was divided into two portions, the former was retained as a raw sample and the later portion was modified as follows:
Heating the clay sample to 600∘C for 2 hrs. before subjecting to acid treatment by refluxing with 2M HCl at 80∘C for 20 min. The sample was washed until free of chloride ions using double distilled water. Thus a set of 4 samples (two raw and two modified) was generated as follows:
Aswan kaolinite raw (Ar)
Aswan modified kaolinite (Am)
Sinai kaolinite raw (Sr)
Sinai modified kaolinite (Sm)
2.2 Wastewater
Industrial non-treated wastewater was collected from Abha industrial city, Iron and Steel small factory, Abha, south-weste of Saudi Arabia. Wastewater samples were prepared according to [13] for both chemical analysis and heavy metals determination.
3 Analytical Methods
3.1 Physical and chemical analyses
According to [14], various analyses of clay sediments were carried out as follows: particle size analyses (pipette method), calcium carbonate (volumetric calcimeter method), organic carbon (walkely and Black method), and cation exchange capacity (sodium saturation method). Deternination of heavy metals in water were carried out by Inductive Coupled Plasma – Optical Emission (ICP-OES), whereas pH and EC were determined in water as well as clay sediments using glass electrode and electrical conductivity methods, respectively.
3.2 Mineralogical analyses
3.2.1 X-Ray Diffraction (XRD) analysis
A thin slurry of the sample solution was aliquot into a glass slide, air dried at room temperature and subjected to a Philips X- ray unit (PW 1830 fitted with a scintillation counter model PW3020. The XRD analysis was done using Ni filter and Cu-Kα radiation. At KV=40, MA=20, Gain=60, Range=1000, time constant during scans were 2, and chart drive 2 cm/min.
3.2.2 I.R spectroscopy
Infra red absorption spectra in region of 400 cm−1 to 4000 cm−1 were recorded using KBr sample pellets. The samples prepared as pellet method following the procedure of [15].
3.2.3 Scanning electron microscope (S.E.M)
The electron microscopy was performed in the Central Lab of Faculity of Agriculture Ain Shams University for Ar and Am clays after samples preparation according to [16].
3.3 Procedure of Heavy metals uptake
Fifty milliliters of non-treated Industrial wastewater was equilibrated with 0.5 gm clay sample for 5, 30, 60, 90, 120, 180 and 240 min. intervals. Initial pH was adjusted at 5.5 before starting the experiments. After centrifugation, the solution was analyzed for Cu and Zn using ICP-OES. Heavy metals released by clay minerals were calculated by the difference between initial concentration of the heavy metals and their concentration at equilibrium.
4 Results and Discussion
4.1 Chemical characterization of wastewater
Analytical results have indicated that the wastewater was alkaline as well as saline. In which, sodium and chlorine were dominant cation and anion respectively, Table 1.
Some chemical properties of wastewater used (mg/L).
| pH | EC ds/m | Ca2+ | Mg2+ | Na+ | K+ | Cl− | Fe | Ni | Cd | Zn | Cu | Pb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.15 | 2.64 | 134 | 44 | 324 | 27.4 | 511 | 209.7 | 319.4 | 10 | 0.3 | 10.2 | 110 | 102 | 1.46 |
The Copper, cadmium and zinc were the dominant contaminants in water.
4.2 Characterization of clay sediments
Table 2 summarised the observed physical and chemical characteristics of the sediments Both locations are non-saline, non-calcareous, moderate alkaline and weak in orgnic carbon. Values of Cation Exchange Capacity (CEC) are related to clay content in each sediment.
Some physical and chemical properties of sediments used.
| Clay sediments | EC ds/m | CaCO3 % | pH 1:2.5 | O.C % | C.E.C meq/100g | Coarse Sand% | Fine Sand% | Silt % | Clay % | Textural Class |
|---|---|---|---|---|---|---|---|---|---|---|
| Aswan | 1.01 | 8.21 | 8.1 | 0.02 | 12.66 | 3.35 | 6.44 | 18.67 | 71.54 | Clay |
| Sinai | 0.21 | 7.15 | 7.9 | 0.034 | 8.20 | 4.18 | 8.21 | 26.61 | 61.00 | Clay |
4.2.1 XRD analysis
X- ray diffraction was carried out to identify the mineralogical composition of the used sediments and to study the changes after modification treatments. Obtained diffraction patterns were illustrated in Figure 1. The results revealed that the kaolinite is predominant in both used sediments. This could be noted from the intensity peaks at 7.13, 4.64, 3.83, 3.56, 2.73, 1.66 and 1.48 Å. Besides, quartz is also presents in the two sediments as indicated by the peak at 3.34, 1.82 and 1.54 Å.
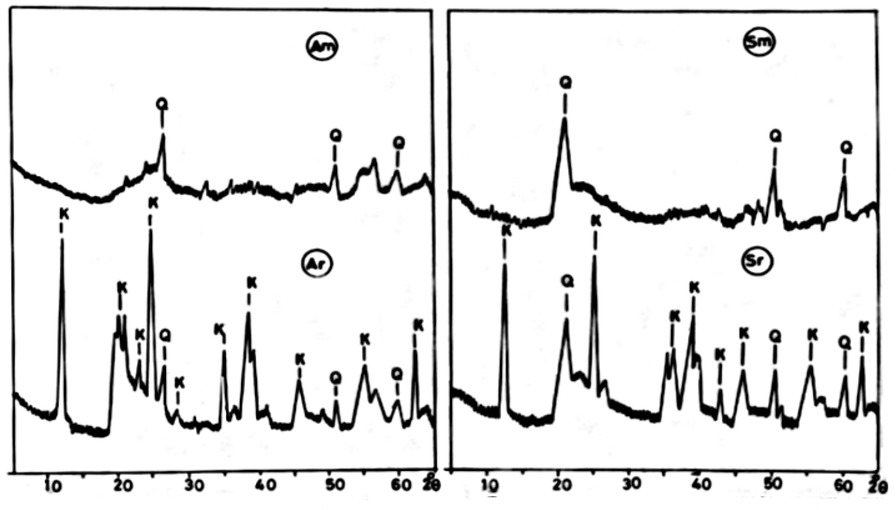
XRD analysis of raw and modified clay samples
After modification process, all the peaks in the diffractograms indicating kaolinite were disappeared, indicating its transformation to modified kaolinite. On the other hand the diffraction patterns caused did not change indicating relative stability of quartz during treatments.
4.2.2 Infra-red absorption spectrometry
To ensure the mineralogical composition of raw and modified clays, infra-red analysis spectroscopic study of the samples was carried out. The results of this study are shown in Figure 2, and the band assignments has been provided in Table 3. The raw sediments have exhibited all the characteristic bands of both kaolinite and quartz indicated by 3696, 3621, 913, 754 cm-1 and 793, 694 cm-1 , respectively, [15]. The band at 3654 cm−1 indicated that kaolinite shows disorder form. On the other hand, the modified clays show following differences;
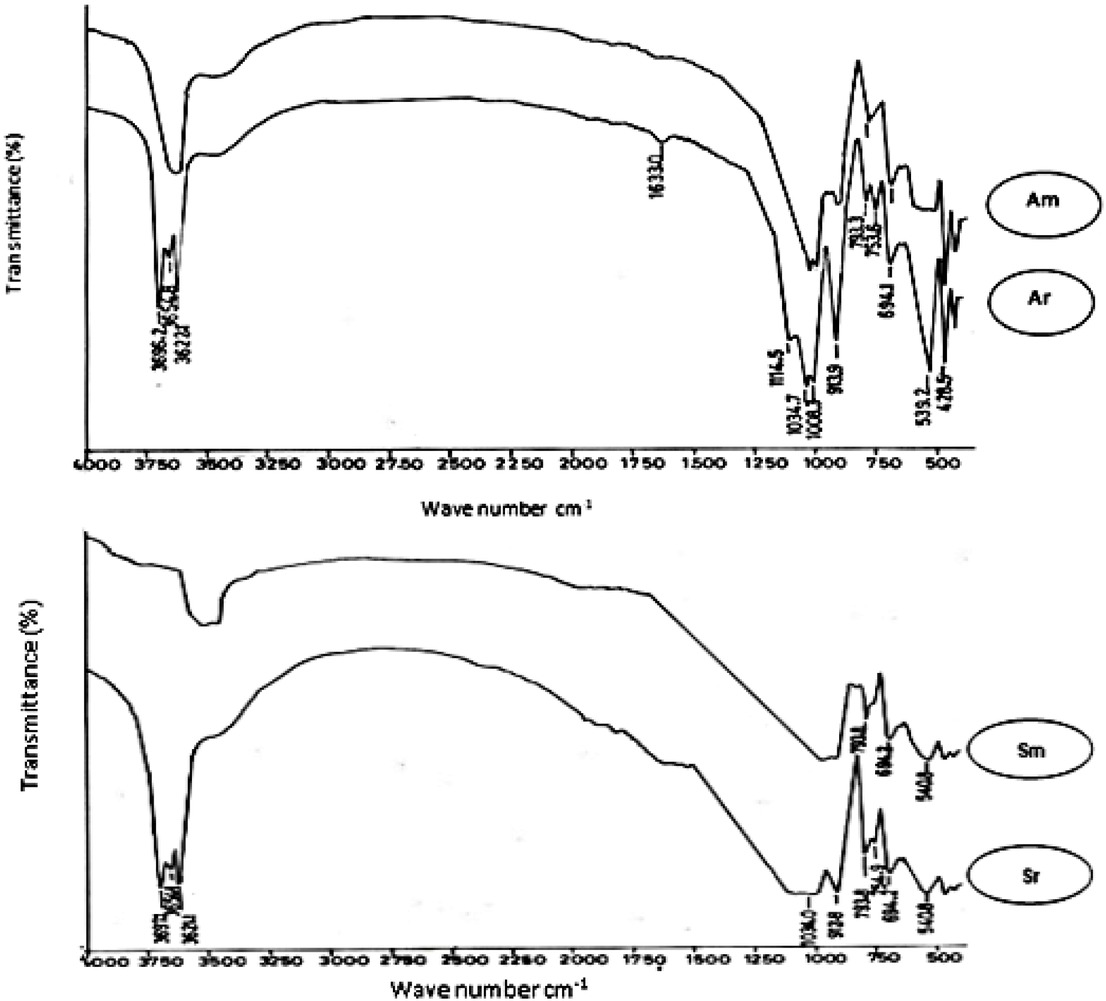
LR Spectra of raw and modified clay samples
Band assignments of kaolinite sediments.
| Wavelength (cm−1) | Assignments | References |
|---|---|---|
| ~3700 | OH stretching hydroxyl sheet | [9] |
| 3620 | Inner OH stretching vibration | [9] |
| 1114 –1034 - 1008 | Si-O bending vibration | [11] |
| 913 | Al-OH bending | [9] |
| 753 and 540 | Si-O-Al compound vibration | [11] |
| 797 - 697 | Silica, quartz | [9] |
The Absence of vibration band at 913 cm−1 indicating breaking of Al-OH bonds and increasing availability of –OH groups in the structure.
The persistence of 1008, 1034, 1114 cm−1 band indicating presence of Si-O bonds in the structure.
As the result of modification treatment the disordered kaolinite structure was shown in the region of Si-O and Si-O-Al bands with change in the intensity. From a mineral structural viewpoint, destruction through heat treatment exposes directed –OH bonds located between the tetrahedral and octahedral layers (amorphization) Figure 3. After acidification treatment, the –OH groups become less stable. The newly formed vacant sites during the modification treatments accommodate extra structural water; thereby; broadening the –OH bands in the I.R spectrum, [17] confirmed the same interpretation.
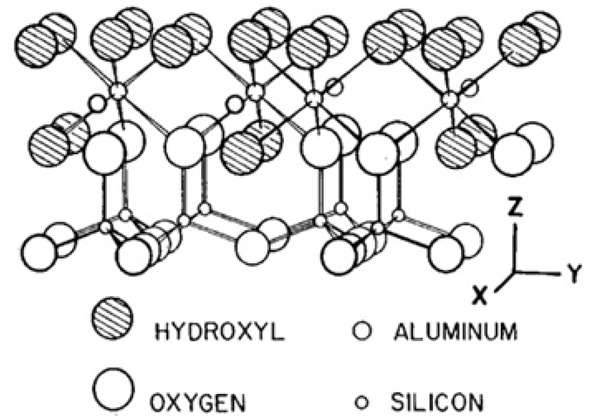
Ideal structure of kaolinite mineral
4.2.3 Scanning Electron Microscope (SEM)
The scanning electron microscopic study was carried out for both raw and modified Aswan clay samples, Figure 4(a).

Scanning electron microscope of Aswan raw and modified clay sediment
The presence of hexagonal shape particles with clear edges confirmed the presence of kaolinite in unmodified clay sample Figure 4(a). After modification treatments, transformation in kaolinite structure was observed from hexagonal shape to edgeless shape Figure 4(b). The shapes are more pronounced at the higher magnification see, Figure 4(c).
4.3 Cation Exchange Capacity
In order to assess the exchangeability of clay samples after modification treatments, C.E.C values were measured for raw and modified clay samples, which are shown in Table 4. The modification treatments nearly, duplicated the C.E.C values in the clay samples, which shows remarkable change from 8.2 to 18.41 Meq/100g and from 12.66 to 28.53 Meq/100g in Sinai and Aswan sediments, respectively.
Cation exchange capacity (Meq/100g) of raw and modified clays
| Clay sediments | Sr | Sm | Ar | Am |
|---|---|---|---|---|
| C.E.C | 8.20 | 18.41 | 12.66 | 28.53 |
The obtained results are similar to those published by [12] who reported four-fold increase in CEC values after the thermal treatment at 600∘C followed by acid activation treatment of the samples.
The observed increase in C.E.C after modification treatments is indicative of the increasing number of exchangeable sites caused during the modification treatments. Acid attack cleans up the mineral by leaching of metal ions from collapsed lattice structure due to the disorder caused by the transition of kaolinite to metakaolinite by using thermal treatment. In this respect, [18] has mentioned that unless a little surface change was observed on calcining kaolinite to less than 550∘C, a marked change in the exchange kinetics can not be expected.
4.4 Heavy metals uptake
The effect of equilibration time on the sorption of copper and zinc was analyzed kinetically over a range of 0-240 min. From theoritical point of view, adsorption process requires long equilibration time, while the practical approach needs a short contact time, this in accordance with [19]. The removing kinetics of Cu and Zn ions by raw and modified clay samples were monitored as a function of equilibration time and plotted in Figure 5. All metal ions attain a near equilibrium condition within the first 90 minutes and attains near-complete reomval in about 2 hrs time.
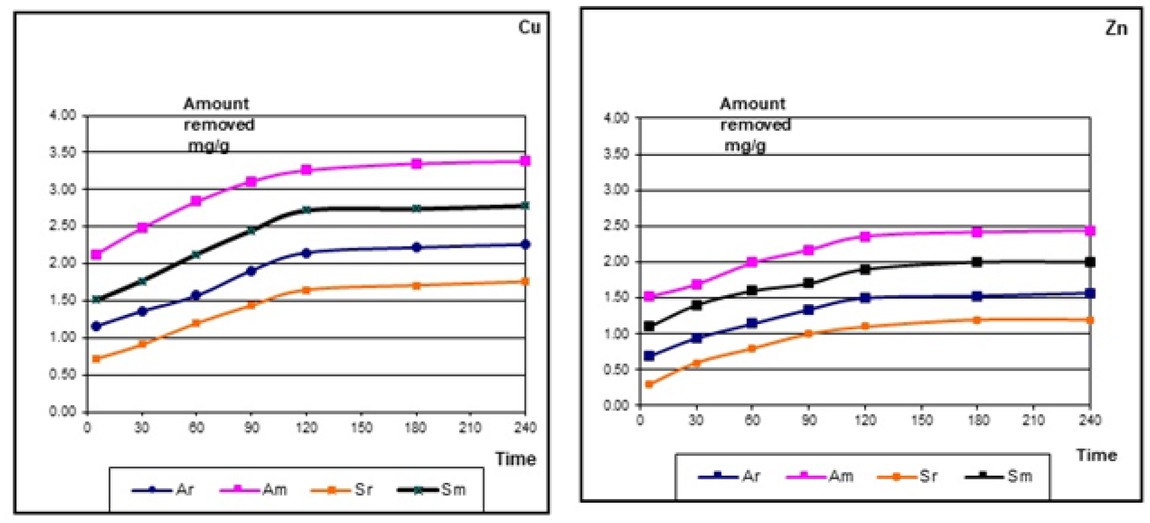
Amount of heavy metals removed (mg/g) by raw and modified clay
The btained results indicate that the modified clay sample have rendered maximum ion removal [2.78(Cu) and 2.00 (Zn) mg/g] for Sm clay; representing about [27.25%and 18.18%] from initial wastewater concentration, respectively.
Besides, 3.38(Cu) and 2.44(Zn)mg/g for Am clay represents about 33.14%and 22.18%from initial wastewater concentration, respectively. The observed increase of removed metal ions by modified clays are due to the increasing exchange sites produced after acid leaching on a collapsed kaolinite structure.
A comparison of relative removing values in Figures 5 has revealed that the removing kinetics of copper are more favored under identical molar concentrations than zinc.
This may be due to the formation of Cu(OH)+ and Cu(H2O4)2+ complex which can stabilize copper ions. It was also suggested that Cu2+ ions largely occupy the planar external surface rather than the edges of platelets. Moreover, zinc can form comparatively stable chloride complexes in solution which might not get re-adsorbed resulting into lower sorption kinetics for Zn as compared to Cu.
Several studies such as [20, 21]. Have confirmed previous finding. For e.g. [21] have shown that higher surfacecharge is the more selective for exchange process in favor of Cu with respect to Zn.
Also, [22] studied Laterite adsorbent and concluded the this sorpent can be preferred for removal of copper confirming the prsent finding.
However, on the contrary, [23] have observed that zinc is better removed than cobber by expanded vermiculite. The authors argued about the selectivity of zinc to get adsorped in the expanded vermiculite structure than copper. Nevertheless, a recent study carried out by [24] found that Verde-lodo clay presented higher affinity and selectivity for copper than silver. So, each clay type have their own selectivity toward specific heavy metals.
Clay sediments interact with heavy metals through a variety of mechanisms. The dominating type of bond depends on the metal, the type of binding sites, the access to these binding sites and a variety of geochemical environment. The main mechanisms for retention of heavy metals in clay minerals inclide adsorption, ion exchange, precipitation and co-precipitation; [5].
The present study has indicated that the ion exchange might be the probable mechanism of Cu and Zn ions uptake by modified kaolinite clays. Besides, a possibility that the metal ions being entrapped into the pores cannot be ruled out.
5 Conclusions
To produce a new modified clay as a sorbent for removing heavy metals and other toxicants from industrial wastewater, two Egyptian natural clay sediments dominated mainly by kaolinite were selected from Aswan and Sinai regions. Unusual treatments depended upon thermal transformation and acid activation were carried out to increase the exchangeability properties of kaolinite and produce modified kaolinite. This was proved by a variety of methods, including XRD analysis, IR spectroscopy, SEM study and C.E.C measurements.
The results of present study have indicated uptake of heavy metals by modified clay samples indicating that the Sinai have rendered maximum ion removing about (27.25% and 18.18%) from (Cu) and (Zn) respectively. Beside, 33.14% (Cu) and 22.18% (Zn) for Aswan modified clay from initial wastewater concentration. It has been indicated that the removing kinetics of Cu are more favored under identical molar concentrations than that of Zn. This may be due to the formation of Cu(OH)+ and Cu(H2O4)2+ which can stabilize copper ions. It was also suggested that Cu2+ ions largely occupy the planar external surface rather than the edges of platelets. Moreover, zinc can form comparatively stable chloride complex in solution which will not get re-adsorbed after getting desorbed into the solution due to the prevailing conditions. This explains the observed lower sorption kinetics for Zn as compared to Cu.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under grant number (G.R.P- 323 -38).
References
[1] Abu-zeid, M., Water in Africa and future challenges. The 18th congress of the international commission an irrigation and drainage, Montreal, Canada, 2002 July 21-28.Suche in Google Scholar
[2] Pierluigi B., Francesca L., Ion Exchange Resins: Catalyst Recovery and Recycle. Chem. Rev., 2009, 109 (2), 515-529 DOI: 10.1021/cr800404j https://pdfs.semanticscholar.org/b196/1f523b5538d5a32698147094c4bc8327a944.pdf10.1021/cr800404jSuche in Google Scholar
[3] Mazzoldi P., Carturan S., Quaranta A., C. Sada S., Sglavo V. M., Ion Exchange Process: History, Evolution and Applications. La Rivista del Nuovo Cimento 2013, 36(9), 397-460 DOI 10.1393/ncr/i2013-10092-110.1393/ncr/i2013-10092-1Suche in Google Scholar
[4] Miranda-Trevino J. C., Coles C. A., Kaolinite properties, structure and influence of metal retention on PH. Appl. Clay Sci. 2003, 23,133-139. https://doi.org/10.1016/S0169-1317(03)00095-410.1016/S0169-1317(03)00095-4Suche in Google Scholar
[5] O’Day P. A., Parks G. A., Brown G. E., Molecular structure and binding sites of cobalt (II) surface complex on kaolinite from X-ray absorption spectroscopy. Clays $#x0026; clay mineral, 1994,42, 337-355. DOI: 10.1346/CCMN.1994.042031210.1346/CCMN.1994.0420312Suche in Google Scholar
[6] Sari A., Tuzen M., Cd (II) adsorption from aqueous solution by raw and modified kaolinite. Appl Clay Sci., 2014, 88, 63-72 https://doi.org/10.1016/j.clay.2013.12.02110.1016/j.clay.2013.12.021Suche in Google Scholar
[7] Kakali G., Perraki T., Tsivilis S., Badogiannis E., Thermal treatment of Kaolin: the effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73-80. https://doi.org/10.1016/S0169-1317(01)00040-010.1016/S0169-1317(01)00040-0Suche in Google Scholar
[8] Murray H. H., Traditional and new applications for kaolin, smectite and playgorskite: a general overview.Appl. Clay sci. 2000, 17, 207-221 https://doi.org/10.1016/S0169-1317(00)00016-810.1016/S0169-1317(00)00016-8Suche in Google Scholar
[9] Coles C. A., Yong R. N., Aspects of kaolinite characterization and retention of Pb and Cd. Appl. Clay Sci. 2002, 22, 39-45. https://pdfs.semanticscholar.org/0a67/90d14853df562568cd6ceaa17689cf08a55d.pdf10.1016/S0169-1317(02)00110-2Suche in Google Scholar
[10] Bhattacharyya K.G., Gupta S. S., Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv. Colloid Interface Sci. 2008, 140(2), 114-31. doi: 10.1016/j.cis.2007.12.10.1016/j.cis.2007.12Suche in Google Scholar
[11] Xiuju Zhang, Huan Liu, Haoxuan Xing, Halyan Li, Hong Jun Hu, Aijun Li, hong Yao, Improved sodium adsorption by modified kaolinite at high temperature using intercalation-exfoliation method. Fuel, 2017, 191, 198-203. https://doi.org/10.1016/j.fuel.2016.11.06710.1016/j.fuel.2016.11.067Suche in Google Scholar
[12] Suraj G., lyer,G. S. P., lalithambika, M., Adsorption of cadmium and copper by modified kaolinites. Appl. Clay Sci., 1998, 13, 293-306 https://doi.org/10.1016/S0169-1317(98)00043-X10.1016/S0169-1317(98)00043-XSuche in Google Scholar
[13] Greenberg A. E., Clesceri L. S., Eaton A. D., Standard methods for the examination of water and wastewater.Published by American public health association, American Water Works Association, Water Environment Federation 1992 https://www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdfSuche in Google Scholar
[14] Black C. A., Evans D. D., Ensminger L. E., White J. L., Clark F. E., Methods of Soil Analysis. American Society of Agronomy, Inc., Madison, Wisconsin, USA Library of Congress Catalog Card Number: 1985, 65-15 8 00, Seventh printing.10.2134/agronmonogr9.1Suche in Google Scholar
[15] Russell J. D., Fraser A. R., Infrared methods. Chapter (2),pp: 11-67. In: Clay mineralogy: spectroscopic and chemical determinative methods. Wilson, M. J. (ed) Chapman $#x0026; Hall, London.UK. Congress Catalog card no. 1994, 93, 74207. DOI: 10.1007/978-94-011-0727-3_710.1007/978-94-011-0727-3_7Suche in Google Scholar
[16] Kittrick J. A., Electron Microscope Techniques. Chapter (47), pp: 1985, 632-651. In : Methods of Soil Analysis. Black et al. (ed.) Am. Soc. Agro., Madison, Wisconsin, U.S.A.10.2134/agronmonogr9.1.c47Suche in Google Scholar
[17] Suraj G., Lyer G. S. P., Rugmini S., Lalithambika M., The effect of micronization on kaolinites and their sorption behavior. Appl. Clay Sci. 1997, 12, 111-130. https://doi.org/10.1016/S0169-1317(96)00044-010.1016/S0169-1317(96)00044-0Suche in Google Scholar
[18] Abd-Allah, S. M., Probability of kaolinization of montmorillonite under certain soil conditions of Egypt. Annals Agric. Sci., Ain Shams Univ., Cairo, Egypt 2000, 45,363-383.Suche in Google Scholar
[19] Sdiri A. T., Higashi T., Jamoussi F., Adsorption of copper and zinc onto natural clay in single and binary systems. Int. J. Environ. Sci. Technol. 2014 11, 1081–1092 http://doi.10.1007/s13762-013-0305-1http://www.bioline.org.br/pdf?st1410710.1007/s13762-013-0305-1Suche in Google Scholar
[20] Vengris T., Brinkiene R., Sveikauskaite A., Nickel, copper and zinc removal from waste water by a modified clay sorbent. Appl. clay Sci. 2001, 18, 183 – 190. https://doi.org/10.1016/S0169-1317(00)00036-310.1016/S0169-1317(00)00036-3Suche in Google Scholar
[21] Susane Gier, William D. J., Heavy metal adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. Appl. Clay Sci. 2000, 16, 289-299. https://doi.org/10.1016/S0169-1317(00)00004-110.1016/S0169-1317(00)00004-1Suche in Google Scholar
[22] Sudha Rani K., Srinivasb B., Gouru Naiduc K., Ramesha K. V., Removal of copper by adsorption on treated laterite. Materials Today: Proceedings 2018, 5, 463–469. https://doi.org/10.1016/j.matpr.2017.11.106https://www.sciencedirect.com/science/article/pii/S221478531732344110.1016/j.matpr.2017.11.106Suche in Google Scholar
[23] Emanuelle Dantas de Freitas, Hewellyn Joacy de Almeida, Gurgel Adeodato Vieira, Binary adsorption of zinc and copper on expanded vermiculite using a fixed bed column, Appl. Clay Sci. 2017, 146, 503-509. https://doi.org/10.1016/j.clay.2017.07.004https://www.sciencedirect.com/science/article/pii/S016913171730297110.1016/j.clay.2017.07.004Suche in Google Scholar
[24] Emanuelle Dantas de Freitas, Hewellyn Joacyde Almeida, Ambrosio Florenciode Almeisa Neto and Melissa Gurgel Adeodato Vieira, Continuous adsorption of silver and copper by Verde-lodo bentonite in a fixed bed flowthrough column. J. Cleaner prod., 2018, 171, 613-621. https://doi.org/10.1016/j.jclepro.2017.10.036https://www.sciencedirect.com/science/article/pii/S095965261732327210.1016/j.jclepro.2017.10.036Suche in Google Scholar
© 2019 S. Abdallah, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Regular Articles
- 2D Seismic Interpretation of the Meyal Area, Northern Potwar Deform Zone, Potwar Basin, Pakistan
- A new method of lithologic identification and distribution characteristics of fine - grained sediments: A case study in southwest of Ordos Basin, China
- Modified Gompertz sigmoidal model removing fine-ending of grain-size distribution
- Diagenesis and its influence on reservoir quality and oil-water relative permeability: A case study in the Yanchang Formation Chang 8 tight sandstone oil reservoir, Ordos Basin, China
- Evaluation of AHRS algorithms for Foot-Mounted Inertial-based Indoor Navigation Systems
- Identification and evaluation of land use vulnerability in a coal mining area under the coupled human-environment
- Hydrocarbon Generation Potential of Chia Gara Formation in Three Selected Wells, Northern Iraq
- Source Analysis of Silicon and Uranium in uranium-rich shale in the Xiuwu Basin, Southern China
- Lithologic heterogeneity of lacustrine shale and its geological significance for shale hydrocarbon-a case study of Zhangjiatan Shale
- Characterization of soil permeability in the former Lake Texcoco, Mexico
- Detrital zircon trace elements from the Mesozoic Jiyuan Basin, central China and its implication on tectonic transition of the Qinling Orogenic Belt
- Turkey OpenStreetMap Dataset - Spatial Analysis of Development and Growth Proxies
- Morphological Changes of the Lower Ping and Chao Phraya Rivers, North and Central Thailand: Flood and Coastal Equilibrium Analyses
- Landscape Transformations in Rapidly Developing Peri-urban Areas of Accra, Ghana: Results of 30 years
- Division of shale sequences and prediction of the favorable shale gas intervals: an example of the Lower Cambrian of Yangtze Region in Xiuwu Basin
- Fractal characteristics of nanopores in lacustrine shales of the Triassic Yanchang Formation, Ordos Basin, NW China
- Selected components of geological structures and numerical modelling of slope stability
- Spatial data quality and uncertainty publication patterns and trends by bibliometric analysis
- Application of microstructure classification for the assessment of the variability of geological-engineering and pore space properties in clay soils
- Shear failure modes and AE characteristics of sandstone and marble fractures
- Ice Age theory: a correspondence between Milutin Milanković and Vojislav Mišković
- Are Serbian tourists worried? The effect of psychological factors on tourists’ behavior based on the perceived risk
- Real-Time Map Matching: A New Algorithm Integrating Spatio-Temporal Proximity and Improved Weighted Circle
- Characteristics and hysteresis of saturated-unsaturated seepage of soil landslides in the Three Gorges Reservoir Area, China
- Petrographical and geophysical investigation of the Ecca Group between Fort Beaufort and Grahamstown, in the Eastern Cape Province, South Africa
- Ecological risk assessment of geohazards in Natural World Heritage Sites: an empirical analysis of Bogda, Tianshan
- Integrated Subsurface Temperature Modeling beneath Mt. Lawu and Mt. Muriah in The Northeast Java Basin, Indonesia
- Go social for your own safety! Review of social networks use on natural disasters – case studies from worldwide
- Forestry Aridity Index in Vojvodina, North Serbia
- Natural Disasters vs Hotel Industry Resilience: An Exploratory Study among Hotel Managers from Europe
- Using Monarch Butterfly Optimization to Solve the Emergency Vehicle Routing Problem with Relief Materials in Sudden Disasters
- Potential influence of meteorological variables on forest fire risk in Serbia during the period 2000-2017
- Controlling factors on the geochemistry of Al-Shuaiba and Al-Mejarma coastal lagoons, Red Sea, Saudi Arabia
- The Influence of Kaolinite - Illite toward mechanical properties of Claystone
- Two critical books in the history of loess investigation: ‘Charakteristik der Felsarten’ by Karl Caesar von Leonhard and ‘Principles of Geology’ by Charles Lyell
- The Mechanism and Control Technology of Strong Strata Behavior in Extra-Thick Coal Seam Mining Influenced by Overlying Coal Pillar
- Shared Aerial Drone Videos — Prospects and Problems for Volunteered Geographic Information Research
- Stable isotopes of C and H in methane fermentation of agriculture substrates at different temperature conditions
- Prediction of Compression and Swelling Index Parameters of Quaternary Sediments from Index Tests at Mersin District
- Detection of old scattered windthrow using low cost resources. The case of Storm Xynthia in the Vosges Mountains, 28 February 2010
- Remediation of Copper and Zinc from wastewater by modified clay in Asir region southwest of Saudi Arabia
- Sedimentary facies of Paleogene lacustrine dolomicrite and implications for petroleum reservoirs in the southern Qianjiang Depression, China
- Correlation between ore particle flow pattern and velocity field through multiple drawpoints under the influence of a flexible barrier
- Atmospheric refractivity estimation from AIS signal power using the quantum-behaved particle swarm optimization algorithm
- A geophysical and hydro physico-chemical study of the contaminant impact of a solid waste landfill (swl) in King Williams’ Town, Eastern Cape, South Africa
- Landscape characterization using photographs from crowdsourced platforms: content analysis of social media photographs
- A Study on Transient Electromagnetic Interpretation Method Based on the Seismic Wave Impedance Inversion Model
- Stratigraphy of Architectural Elements of a Buried Monogenetic Volcanic System
- Variable secondary porosity modeling of carbonate rocks based on μ-CT images
- Traditional versus modern settlement on torrential alluvial fans considering the danger of debris flows: a case study of the Upper Sava Valley (NW Slovenia)
- The Influence of Gangue Particle size and Gangue Feeding Rate on Safety and Service Life of the Suspended Buffer’s Spring
- Research on the Transition Section Length of the Mixed Workface Using Gangue Backfilling Method and Caving Method
- Rainfall erosivity and extreme precipitation in the Pannonian basin
- Structure of the Sediment and Crust in the Northeast North China Craton from Improved Sequential H-k Stacking Method
- Planning Activities Improvements Responding Local Interests Change through Participatory Approach
- GIS-based landslide susceptibility mapping using bivariate statistical methods in North-western Tunisia
- Uncertainty based multi-step seismic analysis for near-surface imaging
- Deformation monitoring and prediction for residential areas in the Panji mining area based on an InSAR time series analysis and the GM-SVR model
- Statistical and expert-based landslide susceptibility modeling on a national scale applied to North Macedonia
- Natural hazards and their impact on rural settlements in NE Romania – A cartographical approach
- Rock fracture initiation and propagation by mechanical and hydraulic impact
- Influence of Rapid Transit on Accessibility Pattern and Economic Linkage at Urban Agglomeration Scale in China
- Near Infrared Spectroscopic Study of Trioctahedral Chlorites and Its Remote Sensing Application
- Problems with collapsible soils: Particle types and inter-particle bonding
- Unification of data from various seismic catalogues to study seismic activity in the Carpathians Mountain arc
- Quality assessment of DEM derived from topographic maps for geomorphometric purposes
- Remote Sensing Monitoring of Soil Moisture in the Daliuta Coal Mine Based on SPOT 5/6 and Worldview-2
- Utilizing Maximum Entropy Spectral Analysis (MESA) to identify Milankovitch cycles in Lower Member of Miocene Zhujiang Formation in north slope of Baiyun Sag, Pearl River Mouth Basin, South China Sea
- Stability Analysis of a Slurry Trench in Cohesive-Frictional Soils
- Integrating Landsat 7 and 8 data to improve basalt formation classification: A case study at Buon Ma Thuot region, Central Highland, Vietnam
- Assessment of the hydrocarbon potentiality of the Late Jurassic formations of NW Iraq: A case study based on TOC and Rock-Eval pyrolysis in selected oil-wells
- Rare earth element geochemistry of sediments from the southern Okinawa Trough since 3 ka: Implications for river-sea processes and sediment source
- Effect of gas adsorption-induced pore radius and effective stress on shale gas permeability in slip flow: New Insights
- Development of the Narva-Jõesuu beach, mineral composition of beach deposits and destruction of the pier, southeastern coast of the Gulf of Finland
- Selecting fracturing interval for the exploitation of tight oil reservoirs from logs: a case study
- A comprehensive scheme for lithological mapping using Sentinel-2A and ASTER GDEM in weathered and vegetated coastal zone, Southern China
- Sedimentary model of K-Successions Sandstones in H21 Area of Huizhou Depression, Pearl River Mouth Basin, South China Sea
- A non-uniform dip slip formula to calculate the coseismic deformation: Case study of Tohoku Mw9.0 Earthquake
- Decision trees in environmental justice research — a case study on the floods of 2001 and 2010 in Hungary
- The Impacts of Climate Change on Maximum Daily Discharge in the Payab Jamash Watershed, Iran
- Mass tourism in protected areas – underestimated threat? Polish National Parks case study
- Decadal variations of total organic carbon production in the inner-shelf of the South China Sea and East China Sea
- Hydrogeothermal potentials of Rogozna mountain and possibility of their valorization
- Postglacial talus slope development imaged by the ERT method: comparison of slopes from SW Spitsbergen, Norway and Tatra Mountains, Poland
- Seismotectonics of Malatya Fault, Eastern Turkey
- Investigating of soil features and landslide risk in Western-Atakent (İstanbul) using resistivity, MASW, Microtremor and boreholes methods
- Assessment of Aquifer Vulnerability Using Integrated Geophysical Approach in Weathered Terrains of South China
- An integrated analysis of mineralogical and microstructural characteristics and petrophysical properties of carbonate rocks in the lower Indus Basin, Pakistan
- Applicability of Hydrological Models for Flash Flood Simulation in Small Catchments of Hilly Area in China
- Heterogeneity analysis of shale reservoir based on multi-stage pumping data
Artikel in diesem Heft
- Regular Articles
- 2D Seismic Interpretation of the Meyal Area, Northern Potwar Deform Zone, Potwar Basin, Pakistan
- A new method of lithologic identification and distribution characteristics of fine - grained sediments: A case study in southwest of Ordos Basin, China
- Modified Gompertz sigmoidal model removing fine-ending of grain-size distribution
- Diagenesis and its influence on reservoir quality and oil-water relative permeability: A case study in the Yanchang Formation Chang 8 tight sandstone oil reservoir, Ordos Basin, China
- Evaluation of AHRS algorithms for Foot-Mounted Inertial-based Indoor Navigation Systems
- Identification and evaluation of land use vulnerability in a coal mining area under the coupled human-environment
- Hydrocarbon Generation Potential of Chia Gara Formation in Three Selected Wells, Northern Iraq
- Source Analysis of Silicon and Uranium in uranium-rich shale in the Xiuwu Basin, Southern China
- Lithologic heterogeneity of lacustrine shale and its geological significance for shale hydrocarbon-a case study of Zhangjiatan Shale
- Characterization of soil permeability in the former Lake Texcoco, Mexico
- Detrital zircon trace elements from the Mesozoic Jiyuan Basin, central China and its implication on tectonic transition of the Qinling Orogenic Belt
- Turkey OpenStreetMap Dataset - Spatial Analysis of Development and Growth Proxies
- Morphological Changes of the Lower Ping and Chao Phraya Rivers, North and Central Thailand: Flood and Coastal Equilibrium Analyses
- Landscape Transformations in Rapidly Developing Peri-urban Areas of Accra, Ghana: Results of 30 years
- Division of shale sequences and prediction of the favorable shale gas intervals: an example of the Lower Cambrian of Yangtze Region in Xiuwu Basin
- Fractal characteristics of nanopores in lacustrine shales of the Triassic Yanchang Formation, Ordos Basin, NW China
- Selected components of geological structures and numerical modelling of slope stability
- Spatial data quality and uncertainty publication patterns and trends by bibliometric analysis
- Application of microstructure classification for the assessment of the variability of geological-engineering and pore space properties in clay soils
- Shear failure modes and AE characteristics of sandstone and marble fractures
- Ice Age theory: a correspondence between Milutin Milanković and Vojislav Mišković
- Are Serbian tourists worried? The effect of psychological factors on tourists’ behavior based on the perceived risk
- Real-Time Map Matching: A New Algorithm Integrating Spatio-Temporal Proximity and Improved Weighted Circle
- Characteristics and hysteresis of saturated-unsaturated seepage of soil landslides in the Three Gorges Reservoir Area, China
- Petrographical and geophysical investigation of the Ecca Group between Fort Beaufort and Grahamstown, in the Eastern Cape Province, South Africa
- Ecological risk assessment of geohazards in Natural World Heritage Sites: an empirical analysis of Bogda, Tianshan
- Integrated Subsurface Temperature Modeling beneath Mt. Lawu and Mt. Muriah in The Northeast Java Basin, Indonesia
- Go social for your own safety! Review of social networks use on natural disasters – case studies from worldwide
- Forestry Aridity Index in Vojvodina, North Serbia
- Natural Disasters vs Hotel Industry Resilience: An Exploratory Study among Hotel Managers from Europe
- Using Monarch Butterfly Optimization to Solve the Emergency Vehicle Routing Problem with Relief Materials in Sudden Disasters
- Potential influence of meteorological variables on forest fire risk in Serbia during the period 2000-2017
- Controlling factors on the geochemistry of Al-Shuaiba and Al-Mejarma coastal lagoons, Red Sea, Saudi Arabia
- The Influence of Kaolinite - Illite toward mechanical properties of Claystone
- Two critical books in the history of loess investigation: ‘Charakteristik der Felsarten’ by Karl Caesar von Leonhard and ‘Principles of Geology’ by Charles Lyell
- The Mechanism and Control Technology of Strong Strata Behavior in Extra-Thick Coal Seam Mining Influenced by Overlying Coal Pillar
- Shared Aerial Drone Videos — Prospects and Problems for Volunteered Geographic Information Research
- Stable isotopes of C and H in methane fermentation of agriculture substrates at different temperature conditions
- Prediction of Compression and Swelling Index Parameters of Quaternary Sediments from Index Tests at Mersin District
- Detection of old scattered windthrow using low cost resources. The case of Storm Xynthia in the Vosges Mountains, 28 February 2010
- Remediation of Copper and Zinc from wastewater by modified clay in Asir region southwest of Saudi Arabia
- Sedimentary facies of Paleogene lacustrine dolomicrite and implications for petroleum reservoirs in the southern Qianjiang Depression, China
- Correlation between ore particle flow pattern and velocity field through multiple drawpoints under the influence of a flexible barrier
- Atmospheric refractivity estimation from AIS signal power using the quantum-behaved particle swarm optimization algorithm
- A geophysical and hydro physico-chemical study of the contaminant impact of a solid waste landfill (swl) in King Williams’ Town, Eastern Cape, South Africa
- Landscape characterization using photographs from crowdsourced platforms: content analysis of social media photographs
- A Study on Transient Electromagnetic Interpretation Method Based on the Seismic Wave Impedance Inversion Model
- Stratigraphy of Architectural Elements of a Buried Monogenetic Volcanic System
- Variable secondary porosity modeling of carbonate rocks based on μ-CT images
- Traditional versus modern settlement on torrential alluvial fans considering the danger of debris flows: a case study of the Upper Sava Valley (NW Slovenia)
- The Influence of Gangue Particle size and Gangue Feeding Rate on Safety and Service Life of the Suspended Buffer’s Spring
- Research on the Transition Section Length of the Mixed Workface Using Gangue Backfilling Method and Caving Method
- Rainfall erosivity and extreme precipitation in the Pannonian basin
- Structure of the Sediment and Crust in the Northeast North China Craton from Improved Sequential H-k Stacking Method
- Planning Activities Improvements Responding Local Interests Change through Participatory Approach
- GIS-based landslide susceptibility mapping using bivariate statistical methods in North-western Tunisia
- Uncertainty based multi-step seismic analysis for near-surface imaging
- Deformation monitoring and prediction for residential areas in the Panji mining area based on an InSAR time series analysis and the GM-SVR model
- Statistical and expert-based landslide susceptibility modeling on a national scale applied to North Macedonia
- Natural hazards and their impact on rural settlements in NE Romania – A cartographical approach
- Rock fracture initiation and propagation by mechanical and hydraulic impact
- Influence of Rapid Transit on Accessibility Pattern and Economic Linkage at Urban Agglomeration Scale in China
- Near Infrared Spectroscopic Study of Trioctahedral Chlorites and Its Remote Sensing Application
- Problems with collapsible soils: Particle types and inter-particle bonding
- Unification of data from various seismic catalogues to study seismic activity in the Carpathians Mountain arc
- Quality assessment of DEM derived from topographic maps for geomorphometric purposes
- Remote Sensing Monitoring of Soil Moisture in the Daliuta Coal Mine Based on SPOT 5/6 and Worldview-2
- Utilizing Maximum Entropy Spectral Analysis (MESA) to identify Milankovitch cycles in Lower Member of Miocene Zhujiang Formation in north slope of Baiyun Sag, Pearl River Mouth Basin, South China Sea
- Stability Analysis of a Slurry Trench in Cohesive-Frictional Soils
- Integrating Landsat 7 and 8 data to improve basalt formation classification: A case study at Buon Ma Thuot region, Central Highland, Vietnam
- Assessment of the hydrocarbon potentiality of the Late Jurassic formations of NW Iraq: A case study based on TOC and Rock-Eval pyrolysis in selected oil-wells
- Rare earth element geochemistry of sediments from the southern Okinawa Trough since 3 ka: Implications for river-sea processes and sediment source
- Effect of gas adsorption-induced pore radius and effective stress on shale gas permeability in slip flow: New Insights
- Development of the Narva-Jõesuu beach, mineral composition of beach deposits and destruction of the pier, southeastern coast of the Gulf of Finland
- Selecting fracturing interval for the exploitation of tight oil reservoirs from logs: a case study
- A comprehensive scheme for lithological mapping using Sentinel-2A and ASTER GDEM in weathered and vegetated coastal zone, Southern China
- Sedimentary model of K-Successions Sandstones in H21 Area of Huizhou Depression, Pearl River Mouth Basin, South China Sea
- A non-uniform dip slip formula to calculate the coseismic deformation: Case study of Tohoku Mw9.0 Earthquake
- Decision trees in environmental justice research — a case study on the floods of 2001 and 2010 in Hungary
- The Impacts of Climate Change on Maximum Daily Discharge in the Payab Jamash Watershed, Iran
- Mass tourism in protected areas – underestimated threat? Polish National Parks case study
- Decadal variations of total organic carbon production in the inner-shelf of the South China Sea and East China Sea
- Hydrogeothermal potentials of Rogozna mountain and possibility of their valorization
- Postglacial talus slope development imaged by the ERT method: comparison of slopes from SW Spitsbergen, Norway and Tatra Mountains, Poland
- Seismotectonics of Malatya Fault, Eastern Turkey
- Investigating of soil features and landslide risk in Western-Atakent (İstanbul) using resistivity, MASW, Microtremor and boreholes methods
- Assessment of Aquifer Vulnerability Using Integrated Geophysical Approach in Weathered Terrains of South China
- An integrated analysis of mineralogical and microstructural characteristics and petrophysical properties of carbonate rocks in the lower Indus Basin, Pakistan
- Applicability of Hydrological Models for Flash Flood Simulation in Small Catchments of Hilly Area in China
- Heterogeneity analysis of shale reservoir based on multi-stage pumping data

