Abstract
C26H22ClFN2O5Sn, triclinic, P1̄ (no. 2), a = 7.1473(6) Å, b = 12.8246(11) Å, c = 13.2899(11) Å, α = 97.541(5)°, β = 94.503(6)°, γ = 96.247(5)°, V = 1195.13(18) Å3, Z = 2, Rgt(F) = 0.0325, wRref(F2) = 0.0756, T = 296(2) K.
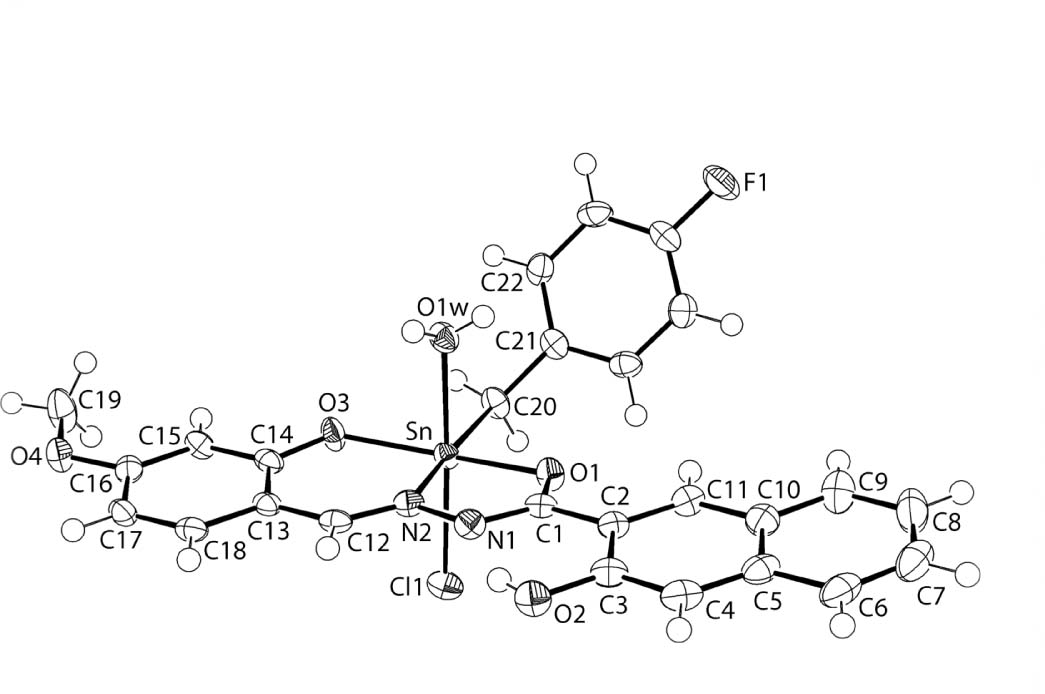
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.32 × 0.28 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.23 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX, ω |
| θmax, completeness: | 28.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12290, 5968, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5146 |
| N(param)refined: | 335 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn | 0.17145(2) | 0.24966(2) | 0.56818(2) | 0.01331(6) |
| Cl1 | −0.13126(9) | 0.29841(5) | 0.62791(5) | 0.02105(14) |
| F1 | 0.8756(2) | 0.03862(14) | 0.82503(14) | 0.0299(4) |
| N1 | 0.3158(3) | 0.48145(17) | 0.57788(17) | 0.0161(5) |
| N2 | 0.2130(3) | 0.39877(17) | 0.51106(16) | 0.0148(4) |
| O1 | 0.3387(3) | 0.35449(14) | 0.68504(14) | 0.0164(4) |
| O2 | 0.4360(3) | 0.67479(15) | 0.64115(15) | 0.0198(4) |
| H2O | 0.384(4) | 0.6208(16) | 0.606(2) | 0.030* |
| O3 | 0.0639(3) | 0.19064(14) | 0.42245(14) | 0.0189(4) |
| O4 | −0.2405(3) | 0.16470(15) | 0.08665(14) | 0.0196(4) |
| O1W | 0.4512(3) | 0.22028(15) | 0.50724(14) | 0.0178(4) |
| H1W | 0.551(3) | 0.232(3) | 0.543(2) | 0.027* |
| H2W | 0.480(4) | 0.250(2) | 0.4592(17) | 0.027* |
| C1 | 0.3754(4) | 0.4510(2) | 0.6643(2) | 0.0146(5) |
| C2 | 0.4864(4) | 0.5323(2) | 0.7409(2) | 0.0148(5) |
| C3 | 0.5101(4) | 0.6420(2) | 0.7280(2) | 0.0172(5) |
| C4 | 0.6047(4) | 0.7162(2) | 0.8032(2) | 0.0206(6) |
| H4 | 0.615232 | 0.787405 | 0.794661 | 0.025* |
| C5 | 0.6865(4) | 0.6866(2) | 0.8935(2) | 0.0196(6) |
| C6 | 0.7898(4) | 0.7614(2) | 0.9728(2) | 0.0253(6) |
| H6 | 0.800641 | 0.833154 | 0.966730 | 0.030* |
| C7 | 0.8730(4) | 0.7291(3) | 1.0573(2) | 0.0282(7) |
| H7 | 0.940992 | 0.779067 | 1.107716 | 0.034* |
| C8 | 0.8575(4) | 0.6213(3) | 1.0693(2) | 0.0306(7) |
| H8 | 0.915255 | 0.600506 | 1.127351 | 0.037* |
| C9 | 0.7574(4) | 0.5468(3) | 0.9956(2) | 0.0269(7) |
| H9 | 0.747240 | 0.475567 | 1.003914 | 0.032* |
| C10 | 0.6692(4) | 0.5778(2) | 0.9067(2) | 0.0199(6) |
| C11 | 0.5662(4) | 0.5025(2) | 0.8291(2) | 0.0171(5) |
| H11 | 0.551836 | 0.431402 | 0.837789 | 0.020* |
| C12 | 0.1439(4) | 0.4228(2) | 0.42441(19) | 0.0143(5) |
| H12 | 0.165175 | 0.493191 | 0.413944 | 0.017* |
| C13 | 0.0390(4) | 0.3501(2) | 0.34483(19) | 0.0143(5) |
| C14 | 0.0048(4) | 0.2392(2) | 0.3451(2) | 0.0154(5) |
| C15 | −0.0908(4) | 0.1751(2) | 0.2588(2) | 0.0155(5) |
| H15 | −0.114278 | 0.102223 | 0.258073 | 0.019* |
| C16 | −0.1505(4) | 0.2206(2) | 0.17452(19) | 0.0156(5) |
| C17 | −0.1188(4) | 0.3304(2) | 0.1734(2) | 0.0173(5) |
| H17 | −0.160563 | 0.359826 | 0.116629 | 0.021* |
| C18 | −0.0258(4) | 0.3929(2) | 0.2569(2) | 0.0162(5) |
| H18 | −0.004030 | 0.465642 | 0.256475 | 0.019* |
| C19 | −0.2692(5) | 0.0518(2) | 0.0802(2) | 0.0287(7) |
| H19A | −0.149482 | 0.025637 | 0.091030 | 0.043* |
| H19B | −0.328514 | 0.021715 | 0.013881 | 0.043* |
| H19C | −0.348819 | 0.032369 | 0.131272 | 0.043* |
| C20 | 0.1733(4) | 0.1078(2) | 0.6372(2) | 0.0177(5) |
| H20A | 0.084865 | 0.109572 | 0.688963 | 0.021* |
| H20B | 0.128522 | 0.047605 | 0.585617 | 0.021* |
| C21 | 0.3647(4) | 0.0911(2) | 0.6855(2) | 0.0154(5) |
| C22 | 0.4631(4) | 0.0114(2) | 0.6426(2) | 0.0189(6) |
| H22 | 0.412135 | −0.030848 | 0.582408 | 0.023* |
| C23 | 0.6378(4) | −0.0063(2) | 0.6885(2) | 0.0212(6) |
| H23 | 0.704526 | −0.058998 | 0.659375 | 0.025* |
| C24 | 0.7070(4) | 0.0569(2) | 0.7781(2) | 0.0199(6) |
| C25 | 0.6152(4) | 0.1371(2) | 0.8232(2) | 0.0202(6) |
| H25 | 0.666600 | 0.178642 | 0.883825 | 0.024* |
| C26 | 0.4434(4) | 0.1542(2) | 0.7755(2) | 0.0182(6) |
| H26 | 0.379905 | 0.208727 | 0.804021 | 0.022* |
Source of material
The melting point of the compound was measured on a Mel-temp II digital melting point apparatus and was uncorrected. The IR spectrum was recorded on a Perkin-Elmer RX1 spectrophotometer as a Nujol mull in a KBr cell from 4000 to 400 cm−1. The 1H NMR spectrum was recorded in DMSO-d6 solution on a Jeol JNM-ECA 400 MHz NMR spectrometer with chemical shifts relative to tetramethylsilane.
(E)-N′-[1-(4-Methoxy-2-hydroxybenzylidene]-3-hydroxy-2-naphthohydrazide was synthesised from 4-methoxy-2-hydroxybenzaldehyde (Merck) and 3-hydroxy-2-napthoic hydrazide (Sigma Aldrich) in a 1:1 molar ratio. Di(4-fluorobenzyl)tin dichloride was synthesised by the direct reaction of 4-fluorobenzyl chloride (Merck) and metallic tin powder (Merck) in toluene according to a literature procedure [5]. Di(4-fluorobenzyl)tin dichloride (0.41 g, 1 mmol) and (E)-N′-[1-(4-methoxy-2-hydroxybenzylidene]-3-hydroxy-2-naphthohydrazide (0.34 g, 1 mmol) were dissolved in methanol (25 mL) and refluxed for 3 h. After filtration, the filtrate was evaporated slowly until yellow crystals formed. The crystals were filtered, washed with a minimum amount of methanol-ethanol and air-dried. Yield: 0.10 g (16%). M.pt: 433–435 K. IR (cm−1): 3391 (br) ν(O—H), 1638 ν(C=N), 1603 ν(C=N—N=C), 1032 ν(C—O), 466 ν(Sn—O). 1H NMR (DMSO-d6, δ (ppm)): δ 2.80 (s, 2H, CH2), 3.45 (s, 2H, OH2), 3.88 (s, 3H, OCH3), 6.70 (d, 1H, J = 8.80 Hz, Ph-H), 7.28–7.45 (m, 4H, Ph—H), 7.50–7.66 (m, 4H, Ph—H), 7.73–7.84 (m, 3H, Ph—H), 8.50 (s, 1H, Ph—H), 8.58 (s, 1H, CH), 11.58 (s, 1H, OH).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.93–0.97 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The O-bound H-atoms were located in a difference Fourier map but were refined with a distance restraint O—H = 0.82 ± 0.01 Å, and with Uiso(H) set to 1.5Ueq(O). Owing to poor agreement, four reflections, i.e. (7 −5 6), (−1 −7 15), (−1 −7 16) and (1 −6 3), were omitted from the final cycles of refinement.
Comment
Organotin compounds have been long-known as potential chemotherapeutic agents for the treatment of a variety of cancers [6]. In this context, molecules related to the title organotin compound with a tridentate hydrazone ligand, have been screened for biological activity [7], [8], [9]. In continuation of these and structural studies of these organotin hydrazone species [10], [11], [12], [13], the title compound, (I), was investigated by X-ray crystallography.
The molecular structure of (I) is shown in the figure (70% displacement ellipsoids). The tin atom is coordinated by the di-negative, tridentate ligand, via the oxide-O1, phenoxide-O3 and imine-N2 atoms, a chlorido ligand, a methylene-carbon atom of the organic substitutent and a water ligand. The resultant coordination geometry is based on an octahedron with the three trans angles deviating significantly from the ideal 180° angle: O1—Sn—O3 = 156.75(7)°, N2—Sn—C20 = 171.06(9)° and Cl1—Sn—O1w = 174.40(5)°. The O,N,O donor atoms of the tridentate ligand occupy meridional positions in the octahedral environment. Deviations are largely related to the acute chelate angles formed in the five-membered, O1—Sn—N2 = 74.21(8)°, and six-membered, O3—Sn—N2 = 84.92(8)°, rings. The Sn—O3(phenoxide) bond length [2.0419(18) Å] is considerably shorter than the Sn—O1(oxide) distance [2.1117(18) Å] and this difference is reflected in the associated C1—O1 [1.306(3) Å] and C14—O3 [1.334(3) Å] bond lengths. Considering the bonding character over the O1—C1—N1—N2—C12 chromophore, the C1—N1 bond [1.315(3) Å] is comparatively short and is only marginally longer than the formally C12—N2 imine bond [1.304(3) Å]. These observations suggest considerable delocalisation of π-electron density over this chromophore; the Sn—Cl1, Sn—N2, Sn—C20 and Sn—O1w bond lengths are 2.4770(7), 2.148(2), 2.143(3) and 2.266(2) Å, respectively. The five-membered chelate ring is planar with the r.m.s. deviation of the five fitted atoms being 0.011 Å. By contrast, the six-membered ring is best described as having an envelope conformation with the O3 atom lying 0.196(3) Å out of the plane defined by the remaining five atoms [r.m.s. deviation = 0.052 Å]; the r.m.s. deviation of the six atoms comprising the chelate ring = 0.075 Å. The dihedral angle between the best planes through the chelate rings is 6.15(8)°, indicating that the rings are almost co-planar. The dihedral angle between the five-membered ring and the least-squares plane through the appended naphthyl ring = 9.30(9)°, a relationship that allows for the formation of an intramolecular hydroxyl-O—H⋯N(hydrazone) hydrogen bond [O2—H2o⋯N1: H2o⋯N1 = 1.78(2) Å, O2⋯N1 = 2.543(3) Å with angle at H2o = 154(2)°]. The dihedral angle between the six-membered ring and attached phenyl ring is 3.55(9)°, also consistent with a co-planar relationship. Finally, the dihedral angle between each of the best planes through the five-and six-membered chelate rings and that through the benzyl ring are 58.57(8) and 62.36(8)°, respectively, indicating a splayed disposition.
The presence of a N,O,O tridentate ligand, with the pendent hydroxy group as in (I), with tin bound to chloride, an organic substituent and a water molecule is a rare structural motif, being observed once previously, namely in aqua-(n-butyl)-chlorido-(2-hydroxy-N-(2-oxy-3-methoxybenzylidene)benzenecarbohydrazonato)tin(IV), isolated as an ethanol solvate [14].
The most prominent supramolecular contacts in the molecular packing are aqua-O—H⋯Cl [O1w—H1w⋯Cl1i: H1w⋯Cl1i = 2.47(2) Å, O1w⋯Cl1i = 3.265(2) Å with angle at H1w = 167(3)° for symmetry operation (i) 1 + x, y, z] and aqua-O—H⋯O(hydroxy) [O1w—H2w⋯O2ii: H2w⋯O2ii = 1.85(2) Å, O1w⋯O2ii = 2.657(3) Å with angle at H2w = 174(2)° for (ii) 1 − x, 1 − y, 1 − z]. These combine to give rise to a double chain, with a linear topology along the a-axis. The phenyl ring of the benzyl substituent is folded towards the water molecule and shields the water-oxygen atom from participating in an additional (acceptor) hydrogen bonding interaction. The chains are linked into a double layer in the ab-plane by very weak methyl-C—H⋯F interactions [C19—H19a⋯F1iii: H19a⋯F1iii = 2.45 Å, C19⋯F1iii = 3.374(4) Å with angle at H19a = 161° for (iii) 1 − x, −y, 1 − z]. While there are π⋯π stacking and C—H⋯π interactions apparent in the packing, these occur within the layers, which stack along the c-axis direction without directional interactions between them.
To analyse the molecular packing further, an analysis of the calculated Hirshfeld surfaces along with two-dimensional (overall and delineated) fingerprint plots ensued, using Crystal Explorer 17 [15] following standard procedures [16]. The most dominant contacts contributing to the surface are those involving hydrogen, accounting for nearly 90% of all contacts. Important contributions come from C⋯H/H⋯C [23.5%], O⋯H/H⋯O [11.4%], Cl⋯H/H⋯Cl [9.8%] and F⋯H/H⋯F [7.8%] contacts but, the prevalent contacts are of the type H⋯H [35.9%]. The next most important contacts are of the type O⋯C/C⋯O [2.6%].
Acknowledgements
Sunway University Sdn Bhd is thanked for financial support of this work through Grant no. STR-RCTR-RCCM-001-2019.
References
1. Rigaku Oxford Diffraction: CrysAlisPRO. Rigaku Corporation, Oxford, UK (2018).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
5. Sisido, K.; Takeda, Y.; Kinugawa, Z.: Direct synthesis of organotin compounds I. di- and tribenzyltin chlorides. J. Am. Chem. Soc. 83 (1961) 538–541.10.1021/ja01464a008Search in Google Scholar
6. Gielen, M.; Tiekink, E. R. T.: Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. John Wiley & Sons Ltd: Chichester, England (2005) p. 421–439.10.1002/0470864052.ch22Search in Google Scholar
7. Lee, S. M.; Ali, H. M.; Sim, K. S.; Malek, S. N. A.; Lo, K. M.: Synthesis, characterization and biological activity of diorganotin complexes with ONO terdentate Schiff base. Inorg. Chim. Acta 406 (2013) 272–278.10.1016/j.ica.2013.04.036Search in Google Scholar
8. Lee, S. M.; Ali, H. M.; Sim, K. S.; Malek, S. N. A.; Nurestri, S.; Lo, K. M.: Synthesis, structural characterization and in vitro cytotoxicity of diorganotin complexes with Schiff base ligands derived from 3-hydroxy-2-naphthoylhydrazide. Appl. Organomet. Chem. 26 (2012) 310–319.10.1002/aoc.2862Search in Google Scholar
9. Hong, M.; Geng, H.; Niu, M.; Wang, F.; Li, D.; Liu, J.; Yin, H.: Organotin(IV) complexes derived from Schiff base N′-[(1E)-(2-hydroxy-3-methoxyphenyl)methylidene]-pyridine-4-carbohydrazone: synthesis, in vitro cytotoxicities and DNA/BSA interaction. Eur. J. Med. Chem. 86 (2014) 550–561.10.1016/j.ejmech.2014.08.070Search in Google Scholar PubMed
10. Rosely, S. N. B. M.; Hussen, R. S. D.; Lee, S. M.; Halcovitch, N. R.; Jotani, M. M.; Tiekink, E. R. T.: [N′-(4-Decyloxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′]dimethyltin(IV): crystal structure and Hirshfeld surface analysis. Acta Crystallogr. E73 (2017) 390–396.10.1107/S2056989017002365Search in Google Scholar PubMed PubMed Central
11. Lee, S. M.; Halcovitch, N. R.; Jotani, M. M.; Tiekink, E. R. T.: N′-[1-(5-Bromo-2-hydroxyphenyl)ethylidene]isonicotinohydrazide monohydrate: crystal structure and Hirshfeld surface analysis. Acta Crystallogr. E73 (2017) 630–636.10.1107/S2056989017004790Search in Google Scholar PubMed PubMed Central
12. Lee, S. M.; Halcovitch, N. R.; Tiekink, E. R. T.: Crystal structure of chlorido-methanol-(N-(2-(oxy)-3-methoxybenzylidene)pyridine-4-carbohydrazonato-κ3O,N,O′)-(4-methylphenyl)methyl-tin(IV), C23H24ClN3O4Sn. Z. Kristallogr. NCS 233 (2018) 519–521.10.1515/ncrs-2017-0410Search in Google Scholar
13. Lee, S. M.; Lo, K. M.; Tiekink, E. R. T.: Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn. Z. Kristallogr. NCS 235 (2019) 121–124.10.1515/ncrs-2019-0530Search in Google Scholar
14. Hong, M.; Chang, G.; Li, R.; Niu, M.: Anti-proliferative activity and DNA/BSA interactions of five mono- or di-organotin(IV) compounds derived from 2-hydroxy-N′-[(2-hydroxy-3-methoxyphenyl)methylidene]-benzohydrazone. New J. Chem. 40 (2016) 7889–7900.10.1039/C6NJ00525JSearch in Google Scholar
15. Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Crystal Explorer v17. The University of Western Australia, Australia (2017).Search in Google Scholar
16. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T.: Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E75 (2019) 308–318.10.1107/S2056989019001129Search in Google Scholar PubMed PubMed Central
©2019 Kong Mun Lo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n
Articles in the same Issue
- 10.1515/ncrs-2020-frontmatter1
- The crystal structure of 3,5-dicarboxybenzenaminium perchlorate monohydrate, C8H8ClNO9
- The crystal structure of poly[(m4-4-bromoisophthalato-κ4O: O′:O′′:O′′′)zinc(II)], C8H3BrO4Zn
- Crystal structure of (E)-2-(2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothioamide, C8H8ClN3O4S
- Crystal structure of 1,1′-methylenebis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(V)), C11H18F12N4P2
- The crystal structure of hexakis(1-isopropyl-1H-imidazole-κ1N)copper(II) dichloride, C36H58Cl2CuN12
- Crystal structure of catena-poly[μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dicobalt(II)], C19H10Cl6CoN3O6
- Crystal structure of (E)-1-(4-(((E)-2-bromo-6-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of ethyl 2-methyl-4-(5-methylthiophen-2-yl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H21NO3S
- The crystal structure of 5-bromo-2-(1-methyl-1H-tetrazol-5-yl)pyridine, C7H6BrN5
- Crystal structure of Bis(acetato-κ2O,O′)-bis[4-(dimethylamino)pyridine-κN]nickel(II), C18H26N4NiO4
- Crystal structure of (E)-3-chloro-2-(((4-chlorophenyl)imino)methyl)phenol, C13H9Cl2NO
- The co-crystal structure of (17b)-estra-1,3,5(10)-triene-3,17diol – acetamide (1/1), a Z′ = 4 structure, C20H29NO3
- Crystal structure of 3-(3-(pyridin-3-yl)ureido)benzoic acid, C13H11N3O3
- The crystal structure of 1,1′-(9-ethyl-9H-carbazole-3,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate(IV)), C24H27N5F12P2
- The crystal structure of 2-bromoisophthalic acid, C8H5BrO4
- The crystal structure of 13-ethoxycarbonyl-9-methyl-4-chlor-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene, C14H15ClN2O3S
- The crystal structure of (E)-4-((4-(diethylamino)benzylidene)amino)-N,N-diphenylaniline, C29H29N3
- Crystal structure of 2-(3-(2-(4-phenylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C27H27N3O2
- Crystal structure of 2-ethoxy-6-((E)-((3-(((E)-3-ethoxy-2-hydroxybenzylidene)amino)-2-hydroxypropyl)iminio)methyl)phenolate, C21H26N2O5
- The crystal structure of catena-poly(μ2-4,4′-bipyridine-κ2N:N′)-tetrakis(μ2-2-((3,5,6-trichloropyridin-2-yl)oxy)acetato-κ2O:O′)dinickel(II)], C19H10Cl6N3NiO6
- Crystal structure of hexakis(μ2-azido-κ2N:N)-diazido-κ1N-tetrakis(phenanthroline-κ2N,N′)tetrazinc(II), C48H32N32Zn4
- Synthesis and crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II)–dichloromethane(1/1), C34H32Br2Cl4CoN4O4
- Crystal structure of (E)-3-chloro-2-(((4-nitrophenyl)imino)methyl)phenol, C13H9ClN2O3
- Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn
- Crystal structure of biaqua(2,2′-bipyridine-4,4′-dicarboxylato-κ2N,N′)(pyridine-2,6-dicarboxylato-κ3O,N,O′)nickel(II) hydrate, C19H15N3NiO10
- Synthesis and crystal structure of bis(2-(((4-(1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-fluorophenolato-κ2N,O)zinc(II) - methanol (1/1), C33H32F2N4O4Zn
- Crystal structure of tetraaqua-bis(μ2-5-aminoisophthalato-κ3N:O,O′)-bis(4,4′-dipyridylsulfide-κ1N)dizinc(II), C36H34N6O12S2Ni2
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)palladium(II) tetracyanonickelate(II), C14H24N8NiPd
- The crystal structure of 3-benzyl-1-((8-(benzyloxy)quinolin-2-yl)methyl)-1H-imidazol-3-ium hexafluorophosphate, C27H24N3OF6P
- Crystal structure of 1-(4-chloro-2-hydroxy-5-iodophenyl)ethan-1-one, C8H6ClIO2
- Crystal structure of hexaaquamagnesium(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MgN6O12S2
- Crystal structure of the coordination polymer catena-poly[(1,2-di(pyridin-4-yl)ethane-κN)-(μ2-2-nitroisophthalato-κ2O:O′)zinc(II)], C20H17N3O7Zn
- Crystal structure of catena-{[tri-aqua-di-sodium bis(2-{[n-butyl(methyl)carbamothioyl]sulfanyl}acetate)]}n, [C16H34N2Na2O7S4]n
- The crystal structure of diaqua-bis(μ2-3-((3-acetyl-5-carboxyphenyl)oxidophosphoryl)-5-carboxybenzoato-κ2O:O′)bis(5,5′-dimethyl-2,2′-bipyridine-k2N,N′)zinc(II), C56H46N4O22P2Zn2
- Crystal structure of N′,2-bis((E)-2-chloro-6-hydroxybenzylidene)hydrazine-1-carbothiohydrazide, C15H12Cl2N4O2S
- Crystal structure of 2-[(1E)-{[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]iminiumyl}methyl]-5-(dodecyloxy)benzen-1-olate, C23H39NO5
- Crystal structure of 12-(2-hydroxybenzoyl)benzo[f]pyrido[1,2-a]indole-6,11-dione, C23H13NO4
- Crystal structure of chlorido-(4-chloro-6-(p-tolyl)pyrimidine-κ2C,N)-(triphenylphosphane-κP)palladium(II), C29H23Cl2N2PPd
- Crystal structure of catena-poly[diaqua-bis(3,4,5,6-tetrabromo-carboxybenzoato-κ1O)-(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C26H14Br8CoN2O10
- Crystal structure of catena-poly[dibenzyl-dichlorido-(μ2-[4,4′-bipyridine]1,1′-dioxide-κ2O:O′)tin(IV)], C24H22Cl2N2O2Sn
- Crystal structure of benzyl-chlorido-(4-chloro-N-[(2-oxidophenyl)methylidene]benzenecarbohydrazonato)-methanol-tin(IV), C22H20Cl2N2O3Sn
- Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(3-nitrophthalato-κ1O)cobalt(II)] — water (2/3), C20H22N5O10.5Co
- Crystal structure of (3R,5R,8R,9R,10R,12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol, C30H52O3
- Crystal structure of 3-(3-(4-carboxyphenyl)ureido)pyridin-1-ium perchlorate, C26H24Cl2N6O14
- Crystal structure of 8-hydroxy-2-methylquinolin-1-ium chloride dihydrate, C10H14ClNO3
- Crystal structure of (dibenzyl sulphoxide-κO)dibromido-bis(4-bromobenzyl-κC)tin(IV), C28H26Br4OSSn
- Crystal structure of bromido-tri(4-chlorophenyl-κ1C)-(ethanol-κ1O)tin(IV) — 4,4′-dimethyl-2,2′-bipyridine (2/1), C52H48Br2Cl6N2O2Sn2
- Crystal structure of 2-butyl-6-(ethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione, C18H20N2O2
- Crystal structure of (4-chloro-N-[(2-oxido-5-chlorophenyl)methylidene] benzene-carbohydrazonato-κ3N,O,O′)bis(2-fluorobenzyl)tin(IV), C28H20Cl2F2N2O2Sn
- Crystal structure of aqua-chlorido-(4-fluorobenzyl-κC)-(N′-(4-methoxy-2-oxidobenzylidene)-3-hydroxy-2-naphthohydrazidato-κ3N,O,O′)tin(IV), C26H22ClFN2O5Sn
- Crystal structure of catena-poly[tri(4-chlorophenyl)-(μ2-hydroxido)tin(IV)] – 2-propanol (1/1), C21H21Cl3O2Sn
- Crystal structure of bromido-dimethyl-4-tolyl-(triphenylphosphine oxide)tin(IV), C27H28BrOPSn
- Crystal structure of 2-(bis(2-hydroxyethyl)ammonio)ethane-1-sulfonate, C6H15NO5S
- Crystal structure of bis[triaqua-(μ2-1,2-di(4-pyridyl)ethylene-κ2N:N′)-(4-sulfonatobenzoato-κ2O,O′)zinc(II)], C13H15NO8SZn
- Crystal structure of 2-((2-(3-hydroxy-7-methylene-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)propan-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol – a marmesin derivative, C20H24O10
- Crystal structure of octa(4-chlorobenzyl)-dichlorido-bis(μ2-methanolato)-bis(μ3-oxo)-tetratin(IV), C58H54Cl10O4Sn4
- Crystal structure of iodido-triphenyl-(triphenylphosphine oxide)tin(IV), C36H30IOPSn
- Crystal structure of dichlorido-bis(4-methylphenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C50H44As2Cl2O2Sn
- Crystal structure of 4-benzyl-1-oxo-N-phenethyl-1H-[1,4]oxazino [4,3-b]indazole-3-carboxamide, C26H21N3O3
- Crystal structure of bis{(N-[(5-chloro-2-oxidophenyl)methylidene]-2-hydroxybenzenecarbohydrazonato)-dioxo-molybdenum(VI)}(μ2-4,4′-bipyridine), C38H26Cl2Mo2N6O10
- Crystal structure of dichlorido-octamethyl-bis(μ3-oxido)-bis(μ2-2-(phenylamino)ethanolato-κ2O:O)tetratin(IV), C24H44Cl2N2O4Sn4
- The crystal structure of 1-(2-(2-(imidazo[1,5-a]pyridine-4-ium)ethoxy)ethyl)-imidazo[1,5-a]pyridine-4-ium bis(hexafluorophosphate) — acetonitrile (1/1), C18H20ON4F12P2
- Crystal structure of cyclo[tetra(μ2-cyanido)-tetracyanido-bis(1,4,7,10-tetraazacyclododecane-κ4N,N′,N′′,N′′′)dinickel(II)dipalladium(II)] hexahydrate, C24H52N16Ni2O6Pd2
- Crystal structure of (dimethyl sulfoxide)-dioxido-[2-hydroxy-N′-(4-oxo-4-phenylbutan-2-ylidene)benzohydrazidato κ3N,O,O′]molybdenum(VI), C19H20MoN2O6S
- Crystal structure of bis(acetylacetonato-κ2O,O′)-(ethanolamine-κ2N,O)copper(II), C14H25CuNO5
- Crystal structure of chlorido-diphenyl-(isopropyl(propyl)carbamodithioato-κ2S,S′)tin(IV), C19H24ClNS2Sn
- The crystal structure of bis(imidazole-1-yl)methane monohydrate, C7H10N4O
- The crystal structure of bis(4-nitroimidazole-1-1yl)methane, C7H6N6O4
- Crystal structure of di(naphthalen-2-yl)sulfane, C20H14S
- Crystal structure of 3-acetyl-6-bromo-4-hydroxy-2H-chromen-2-one, C11H7BrO4
- Crystal structure of N′2,N′6-bis((E)-1-(pyrazin-2-yl)ethylidene)pyridine-2,6-dicarbohydrazide — methanol (1/2), C21H25N9O4
- The crystal structure of 3-nitro-4-(p-tolylamino)-2H-chromen-2-one, C16H12N2O4
- The crystal structure of 1,2-bis((4-methoxyphenyl)ethynyl)benzene, C24H18O2
- Crystal structure of a low-temperature (100 K) polymorph of catena-poly[(μ2-4,4′-bipyridine-κ2N,N′)-bis(O,O′-diethyldithiophosphato-κ1S)zinc(II)], C18H28N2O4P2S4Zn
- The pseudosymmetric low temperature polymorph of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-diethyldithiophosphato-κS)-cadmium(II)], {C18H28CdN2O4P2S4}n
- Crystal structure of 3-iodophthalic acid, C8H5IO4
- The crystal structure of tert-butyl (tert-butoxy(oxo)methyl)(5-bromo-2-fluorophenyl)carbamate, C16H21BrFNO4
- The crystal structure of bis(μ2-5,7-dichloroquinolin-8-olato-κ3N,O:O)-tetrakis(5,7-dichloroquinolin-8-olato-κ2N,O)bis(methanol-κ1O)dieuropium(III) — toluene (1/1), C63H39Cl12Eu2N6O8
- Crystal structure of dichlorido-(N′-(1-(3-ethylpyrazin-2-yl)ethylidene)-4-methoxybenzohydrazide-κ3N,N′,O)cadmium(II), C16H18N4O2Cl2Cd
- A redetermination of the crystal structure of catena-poly[(bis(O,O′-isopropyl dithiophosphato-κ2S,S′)-(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N,N′)cadmium(II)], {C24H38CdN4O4P2S4}n


