Abstract
C13H10ClN3O, monoclinic, P21/c (no. 14), a = 19.0933(2) Å, b = 23.0910(3) Å, c = 10.6831(2) Å, β = 90.064(1)°, V = 4710.00(12) Å3, Z = 16, R gt (F) = 0.0411, wR ref (F 2) = 0.1081, T = 160 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.16 × 0.06 × 0.03 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.80 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy |
| θ max, completeness: | 74.5°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 49,867, 9505, 0.060 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 8033 |
| N(param)refined: | 667 |
| Programs: | CrysAlis PRO [1], Shelx [2, 3], WinGX/Ortep [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cl1 | 0.59359 (4) | 1.04498 (3) | 0.12812 (9) | 0.0508 (2) |
| O1 | 0.81695 (11) | 0.83009 (9) | 0.01032 (17) | 0.0418 (5) |
| N1 | 0.81780 (10) | 0.82111 (9) | 0.22192 (19) | 0.0253 (4) |
| H1N | 0.8052 (16) | 0.8318 (13) | 0.299 (3) | 0.037 (8)* |
| N2 | 0.86948 (9) | 0.77919 (8) | 0.21536 (19) | 0.0258 (4) |
| N3 | 1.02511 (10) | 0.66183 (9) | 0.45775 (19) | 0.0284 (4) |
| C1 | 0.65304 (13) | 0.98756 (11) | 0.1273 (3) | 0.0357 (6) |
| C2 | 0.69210 (13) | 0.97746 (12) | 0.0217 (3) | 0.0370 (6) |
| H2 | 0.687848 | 1.001986 | −0.049335 | 0.044* |
| C3 | 0.73779 (13) | 0.93090 (12) | 0.0205 (2) | 0.0340 (6) |
| H3 | 0.764485 | 0.923116 | −0.052631 | 0.041* |
| C4 | 0.74516 (11) | 0.89532 (10) | 0.1249 (2) | 0.0258 (5) |
| C5 | 0.70582 (12) | 0.90698 (11) | 0.2315 (2) | 0.0298 (5) |
| H5 | 0.710965 | 0.883442 | 0.303896 | 0.036* |
| C6 | 0.65907 (13) | 0.95302 (11) | 0.2322 (3) | 0.0361 (6) |
| H6 | 0.631453 | 0.960677 | 0.304244 | 0.043* |
| C7 | 0.79607 (12) | 0.84577 (11) | 0.1136 (2) | 0.0283 (5) |
| C8 | 0.88926 (12) | 0.75792 (11) | 0.3202 (2) | 0.0270 (5) |
| H8 | 0.867406 | 0.770135 | 0.395525 | 0.032* |
| C9 | 0.94552 (11) | 0.71479 (10) | 0.3244 (2) | 0.0245 (5) |
| C10 | 0.97166 (12) | 0.69866 (11) | 0.4408 (2) | 0.0283 (5) |
| H10 | 0.950236 | 0.714787 | 0.513076 | 0.034* |
| C11 | 1.05365 (12) | 0.63894 (10) | 0.3541 (2) | 0.0277 (5) |
| H11 | 1.091646 | 0.612743 | 0.363559 | 0.033* |
| C12 | 1.03072 (12) | 0.65145 (10) | 0.2344 (2) | 0.0273 (5) |
| H12 | 1.052461 | 0.634029 | 0.163883 | 0.033* |
| C13 | 0.97570 (12) | 0.68966 (10) | 0.2188 (2) | 0.0267 (5) |
| H13 | 0.958728 | 0.698652 | 0.137426 | 0.032* |
| Cl2 | −0.17512 (3) | 1.03769 (3) | 0.29158 (8) | 0.04596 (18) |
| O2 | 0.05856 (11) | 0.83464 (10) | 0.44291 (18) | 0.0454 (5) |
| N4 | 0.06562 (10) | 0.82269 (9) | 0.2330 (2) | 0.0277 (4) |
| H4N | 0.0517 (16) | 0.8318 (14) | 0.154 (3) | 0.040 (8)* |
| N5 | 0.11736 (10) | 0.78179 (9) | 0.24757 (19) | 0.0275 (4) |
| N6 | 0.27438 (11) | 0.65524 (10) | 0.0344 (2) | 0.0318 (5) |
| C14 | −0.11112 (12) | 0.98401 (11) | 0.3011 (3) | 0.0332 (5) |
| C15 | −0.08093 (15) | 0.97208 (12) | 0.4151 (3) | 0.0400 (6) |
| H15 | −0.093630 | 0.993681 | 0.487274 | 0.048* |
| C16 | −0.03174 (14) | 0.92815 (12) | 0.4234 (2) | 0.0354 (6) |
| H16 | −0.011138 | 0.919326 | 0.502082 | 0.042* |
| C17 | −0.01203 (12) | 0.89665 (10) | 0.3175 (2) | 0.0274 (5) |
| C18 | −0.04248 (13) | 0.91051 (11) | 0.2036 (3) | 0.0325 (5) |
| H18 | −0.028996 | 0.889845 | 0.130590 | 0.039* |
| C19 | −0.09222 (13) | 0.95398 (11) | 0.1942 (3) | 0.0340 (5) |
| H19 | −0.113015 | 0.963016 | 0.115805 | 0.041* |
| C20 | 0.04038 (13) | 0.84914 (11) | 0.3376 (2) | 0.0297 (5) |
| C21 | 0.13624 (12) | 0.75560 (11) | 0.1475 (2) | 0.0285 (5) |
| H21 | 0.113513 | 0.763837 | 0.070352 | 0.034* |
| C22 | 0.19281 (12) | 0.71303 (10) | 0.1522 (2) | 0.0263 (5) |
| C23 | 0.21919 (13) | 0.69105 (11) | 0.0409 (2) | 0.0298 (5) |
| H23 | 0.196880 | 0.701990 | −0.035024 | 0.036* |
| C24 | 0.30306 (12) | 0.63875 (11) | 0.1430 (2) | 0.0315 (5) |
| H24 | 0.342417 | 0.613616 | 0.140205 | 0.038* |
| C25 | 0.27879 (13) | 0.65615 (11) | 0.2595 (2) | 0.0305 (5) |
| H25 | 0.299722 | 0.642027 | 0.334233 | 0.037* |
| C26 | 0.22351 (13) | 0.69448 (11) | 0.2638 (2) | 0.0292 (5) |
| H26 | 0.206470 | 0.708119 | 0.341979 | 0.035* |
| Cl3 | 0.08214 (3) | 1.03786 (3) | 0.21099 (8) | 0.04106 (16) |
| O3 | 0.30269 (10) | 0.82449 (9) | 0.05898 (17) | 0.0381 (4) |
| N7 | 0.32103 (10) | 0.82356 (9) | 0.2694 (2) | 0.0271 (4) |
| H7N | 0.3076 (15) | 0.8339 (13) | 0.348 (3) | 0.036 (8)* |
| N8 | 0.37216 (10) | 0.78150 (9) | 0.25922 (19) | 0.0275 (4) |
| N9 | 0.52135 (10) | 0.65395 (9) | 0.4889 (2) | 0.0296 (4) |
| C27 | 0.14266 (12) | 0.98153 (10) | 0.2002 (3) | 0.0305 (5) |
| C28 | 0.14180 (14) | 0.94615 (11) | 0.0960 (3) | 0.0340 (5) |
| H28 | 0.108069 | 0.952010 | 0.031786 | 0.041* |
| C29 | 0.19058 (13) | 0.90211 (11) | 0.0860 (2) | 0.0325 (5) |
| H29 | 0.190897 | 0.878239 | 0.013623 | 0.039* |
| C30 | 0.23923 (12) | 0.89236 (10) | 0.1810 (2) | 0.0261 (5) |
| C31 | 0.23839 (13) | 0.92840 (11) | 0.2849 (2) | 0.0319 (5) |
| H31 | 0.270924 | 0.921952 | 0.350757 | 0.038* |
| C32 | 0.19099 (13) | 0.97365 (12) | 0.2943 (3) | 0.0345 (5) |
| H32 | 0.191854 | 0.998821 | 0.364676 | 0.041* |
| C33 | 0.29055 (12) | 0.84407 (11) | 0.1626 (2) | 0.0269 (5) |
| C34 | 0.38992 (11) | 0.75695 (10) | 0.3616 (2) | 0.0273 (5) |
| H34 | 0.366949 | 0.766978 | 0.437392 | 0.033* |
| C35 | 0.44551 (12) | 0.71345 (10) | 0.3623 (2) | 0.0257 (5) |
| C36 | 0.46772 (12) | 0.69104 (10) | 0.4761 (2) | 0.0283 (5) |
| H36 | 0.443454 | 0.702664 | 0.549461 | 0.034* |
| C37 | 0.55412 (12) | 0.63711 (11) | 0.3841 (2) | 0.0301 (5) |
| H37 | 0.592423 | 0.611049 | 0.391118 | 0.036* |
| C38 | 0.53468 (13) | 0.65593 (11) | 0.2663 (2) | 0.0310 (5) |
| H38 | 0.558770 | 0.642451 | 0.194224 | 0.037* |
| C39 | 0.47991 (12) | 0.69451 (11) | 0.2546 (2) | 0.0279 (5) |
| H39 | 0.465818 | 0.707992 | 0.174426 | 0.033* |
| Cl4 | 0.35255 (4) | 1.04736 (3) | 0.36861 (9) | 0.0527 (2) |
| O4 | 0.55725 (10) | 0.81752 (8) | 0.45932 (17) | 0.0363 (4) |
| N10 | 0.56900 (10) | 0.82025 (9) | 0.2477 (2) | 0.0260 (4) |
| H10N | 0.5583 (17) | 0.8348 (14) | 0.174 (3) | 0.044 (9)* |
| N11 | 0.62016 (10) | 0.77791 (9) | 0.25057 (18) | 0.0263 (4) |
| N12 | 0.77016 (10) | 0.66277 (9) | −0.00942 (19) | 0.0281 (4) |
| C40 | 0.40712 (13) | 0.98701 (11) | 0.3610 (3) | 0.0374 (6) |
| C41 | 0.42203 (19) | 0.95691 (13) | 0.4692 (3) | 0.0533 (9) |
| H41 | 0.402978 | 0.969116 | 0.546921 | 0.064* |
| C42 | 0.46483 (17) | 0.90893 (13) | 0.4635 (3) | 0.0451 (7) |
| H42 | 0.474520 | 0.887993 | 0.538090 | 0.054* |
| C43 | 0.49400 (11) | 0.89049 (10) | 0.3521 (2) | 0.0274 (5) |
| C44 | 0.47856 (14) | 0.92169 (13) | 0.2441 (3) | 0.0380 (6) |
| H44 | 0.497893 | 0.909873 | 0.166278 | 0.046* |
| C45 | 0.43515 (14) | 0.96996 (13) | 0.2489 (3) | 0.0417 (6) |
| H45 | 0.424953 | 0.991047 | 0.174764 | 0.050* |
| C46 | 0.54228 (12) | 0.83948 (11) | 0.3585 (2) | 0.0279 (5) |
| C47 | 0.64068 (12) | 0.75953 (10) | 0.1438 (2) | 0.0279 (5) |
| H47 | 0.619606 | 0.774414 | 0.069887 | 0.033* |
| C48 | 0.69598 (11) | 0.71606 (10) | 0.1334 (2) | 0.0241 (4) |
| C49 | 0.71806 (12) | 0.70012 (10) | 0.0140 (2) | 0.0264 (5) |
| H49 | 0.694571 | 0.716879 | −0.055533 | 0.032* |
| C50 | 0.80208 (12) | 0.63944 (10) | 0.0905 (2) | 0.0279 (5) |
| H50 | 0.839551 | 0.613170 | 0.076552 | 0.033* |
| C51 | 0.78344 (12) | 0.65153 (11) | 0.2122 (2) | 0.0289 (5) |
| H51 | 0.807231 | 0.633411 | 0.279841 | 0.035* |
| C52 | 0.72991 (12) | 0.69020 (11) | 0.2350 (2) | 0.0274 (5) |
| H52 | 0.716338 | 0.699107 | 0.318319 | 0.033* |
1 Source of material
Nicotinaldehyde (1.07 g, 0.01 mol) was added to a stirred suspension of 4-chlorobenzohydrazide (1.71 g, 0.01 mol), in ethanol (10 mL), and the mixture was heated under reflux for 1 h. On cooling, the precipitated crude product was filtered, washed with ethanol, dried and recrystallised from ethanol to yield 2.44 g (94%) of the title compound as colourless plates. Melting point: 491–493 K (uncorrected). 1 H NMR (DMSO-d 6 , 500.13 MHz): d 11.02 (s, 1H, NH), 9.05 (CH=N), 8.99 (s, 1H, Pyridine–H), 8.48–8.62 (m, 2H, Pyridine–H), 7.96 (d, 2H, Ar–H, J = 7.2 Hz) and 7.45–7.59 (m, 2H, Ar–H & 1 Pyridine–H). 13 C NMR (DMSO-d 6 , 125.76 MHz): d 161.24 (C=O), 152.28, 149.20, 134.54, 129.80, 123.90 (Pyridine–C), 144.66 (CH=N), 138.0, 132.24, 130.08, 127.54 (Ar–C). Analysis (%) for C13H10ClN3O (259.69): C, 58.11 (Calc. 58.06); H, 4.14 (Calc. 4.18); N, 15.64 (Calc. 15.57).
2 Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95 Å) and refined as riding with U iso(H) = 1.2U eq(C). The N-bound H atoms were located in a difference map and refined freely. The crystal was refined as a two-component twin with the minor component = 0.2253(9).
3 Comment
Hydrazides and their N′-arylidene derivatives receive considerable interest for their remarkable biological activities [5, 6]. In addition, carbohydrazide-hydrazone derivatives are utilised as efficient intermediates for the synthesis of several biologically-active heterocyclic compounds [7, 8].
The synthesis and the crystal structure of a new N′-arylidene hydrazide derivative (I) are described herein along with an analysis of the molecular packing.
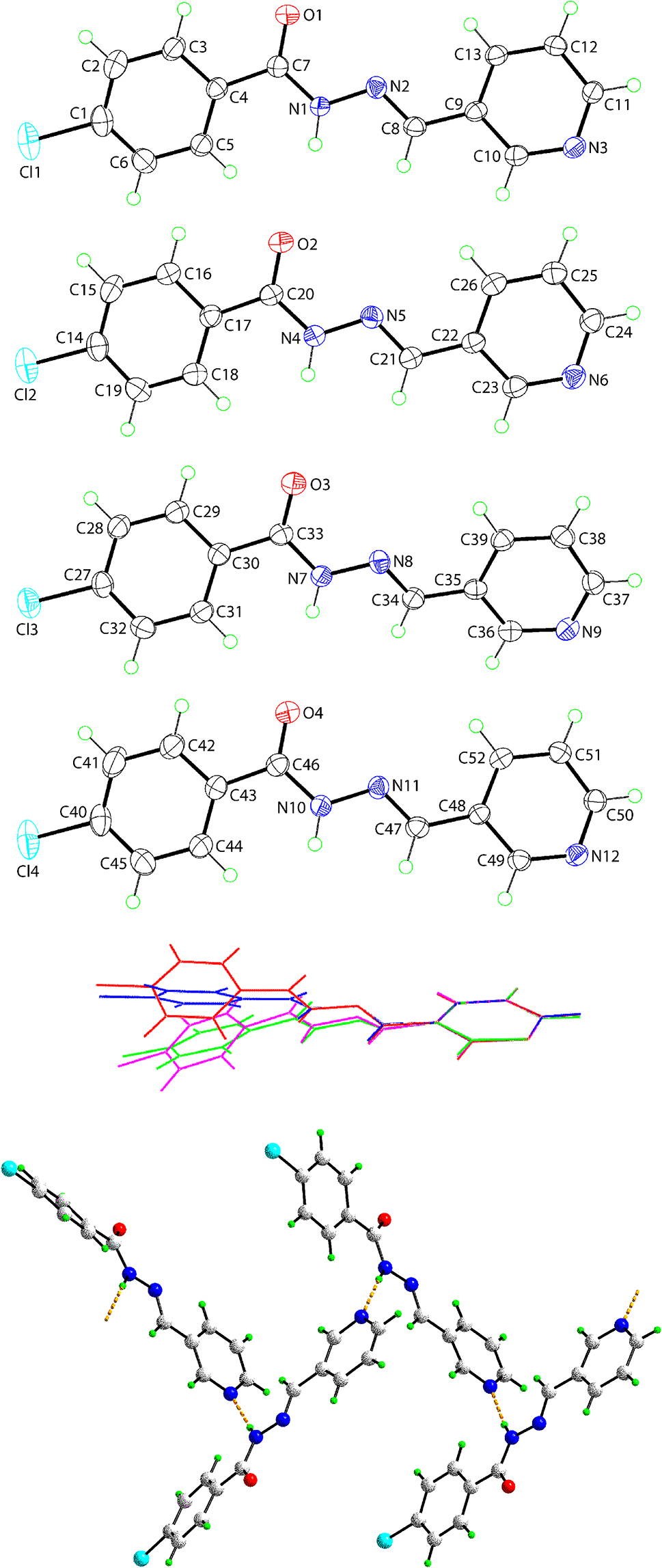
The molecular structures of the four independent molecules comprising the asymmetric-unit of (I) are shown in the upper four images of the figure (50% probability ellipsoids).
While to a first approximation, the molecules are similar to each other, having a strictly planar, central CN2O residue, are C-connected to a 4-chlorophenyl and to a 3-pyridyl residue via the imine bond, conformational differences are evident as shown in the overlay diagram (the color code for the O1- to O4-containing molecules is red, green, blue and pink, respectively; the images were overlaid so the 3-pyridyl rings are coincident). A small twist is noted about the C8–C9 bond as seen in the value of the N2–C8–C9–C10 torsion angle of 171.5(2)°, suggesting this part of the molecule is relatively planar; the comparable values for the O2- to O4-containing molecules are –171.1(2)°, 174.7(2)° and –176.9(2)°, respectively. A greater twist is seen about the C4–C7 bond, i.e. C5–C4–C7–O1 = 163.5(2)°; the values for the O2- to O4-containing molecules are −170.8(3)°, −159.0(2)° and 174.0(3)°, respectively. These differences manifest in a range of dihedral angles between the terminal rings, i.e. 25.04(11)°, 11.79(12)°, 32.82(12)° and 15.16(12)°, respectively. While the differences in conformation confirm the choice of crystal symmetry, these are unlikely to have any chemical significance.
There are three closely related 3-pyridyl derivatives in the literature, which differ only in the nature of the substitution at the 4-position of the phenyl ring, i.e. with H [9], NMe2 [10] and NO2 [11] cf. Cl in (I). For comparison with (I), the values of the dihedral angles between the terminal rings are 47.79(14)°, 5.19(8)° and 28.29(4)°, respectively, highlighting the variability of conformations in this class of compound, at least in the solid-state. The occurrence of a Z' > 1 is not unusual in this class of compounds [12].
A supramolecular chain featuring amide-N–H⋯N(pyridyl) hydrogen bonds is prominent in the molecular packing, as illustrated in the lower view of the figure. The chains involve all four independent molecules [a representative interaction is H1n⋯N12 i [N1–H1n⋯N12 i : H1n⋯N12 i = 2.16(3) Å, N1⋯N12 i = 3.035(3) Å with angle at H1n = 167(3)° for symmetry operation (i): x, 3/2 − y, 1/2 + z].
Molecules assemble into supramolecular layers in the ac-plane. Prominent interactions between the twisted chains along the a-axis include pyridyl-C–H⋯O(carbonyl) [C12–H12⋯O2 ii : H12⋯O2 ii = 2.47 Å, C12⋯O2 ii = 3.176(3) Å with angle at H12 = 131° for (ii): 1 + x, 3/2 − y, −1/2 + z] and pyridyl-C–H⋯N(imine) [C49–H49⋯N11 i : H49⋯N11 i = 2.51 Å, C49⋯N11 i = 3.415(3) Å with angle at H49 = 158°] interactions. The 4-chlorophenyl rings project alternatively to either side of the layer and these inter-digitate with adjacent layers along the b-axis with close phenyl-C–Cl⋯π(phenyl, pyridyl) contacts [closest contact: C1–Cl1⋯Cg(N9-pyridyl) iii : Cl1⋯Cg(N9-pyridyl) iii = 3.4860(12) Å with angle at Cl1 = 170.47(9)° for (iii): 1 − x, 1/2 + y, 1/2 − z].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2023R3), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Rigaku Corporation: Oxford, UK, 2021.Suche in Google Scholar
2. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Farrugia, L. J. WinGX and Ortep for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
5. Popiołek, Ł. Updated information on antimicrobial activity of hydrazide-hydrazones. Int. J. Mol. Sci. 2021, 22, 9389; https://doi.org/10.3390/ijms22179389.Suche in Google Scholar PubMed PubMed Central
6. Onyeyilim, E. L., Ezeokonkwo, M. A., Ugwu, D. I., Uzoewulu, C. P., Eze, F. U., Okonkwo, V. I., Eze, C. C., Ezugwu, J. A. Carbohydrazide analogues: a review of synthesis and biological activities. Mini Rev. Med. Chem. 2022, 22, 661–682; https://doi.org/10.2174/1389557521666210831154935.Suche in Google Scholar PubMed
7. El-Emam, A. A., Alrashood, K. A., Al-Omar, M. A., Al-Tamimi, A. M. S. Synthesis and antimicrobial activity of N′-heteroarylidene-1-adamantylcarbohydrazides and (+-)-2-(1-adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475–3483; https://doi.org/10.3390/molecules17033475.Suche in Google Scholar PubMed PubMed Central
8. Ishii, M., Jorge, S. D., de Oliveira, A. A., Palace-Berl, F., Sonehara, I. Y., Pasqualoto, K. F., Tavares, L. C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-trypanosoma cruzi and antifungal agents. Bioorg. Med. Chem. 2011, 19, 6292–6301; https://doi.org/10.1016/j.bmc.2011.09.009.Suche in Google Scholar PubMed
9. Wen, L., Yin, H., Li, W., Li, W. N′-[(E)-3-pyridylmethylidene]benzohydrazide. Acta Crystallogr. 2009, E65, o2623; https://doi.org/10.1107/s160053680903894x.Suche in Google Scholar
10. Ding, Y.-W., Ni, L.-L. 4-Dimethylamino-N′-(3-pyridylmethylidene)benzohydrazide. Acta Crystallogr. 2010, E66, o2636; https://doi.org/10.1107/s1600536810037670.Suche in Google Scholar
11. Ahmad, T., Zia-ur-Rehman, M., Siddiqui, H. L., Mahmud, S., Parvez, M. 4-Nitro-N′-[(E)-3-pyridylmethylidene]benzohydrazide. Acta Crystallogr. 2010, E66, o976; https://doi.org/10.1107/s1600536810011244.Suche in Google Scholar
12. Lee, S. M., LoK. M., Tiekink, E. R. T. Crystal structure of 4-chloro-N′-[(1E)-3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3. Z. Kristallogr. – N. Cryst. Struct. 2019, 234, 1341–1344; https://doi.org/10.1515/ncrs-2019-0528.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

