Abstract
Eu2Pd3.37Zn13.63, trigonal,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
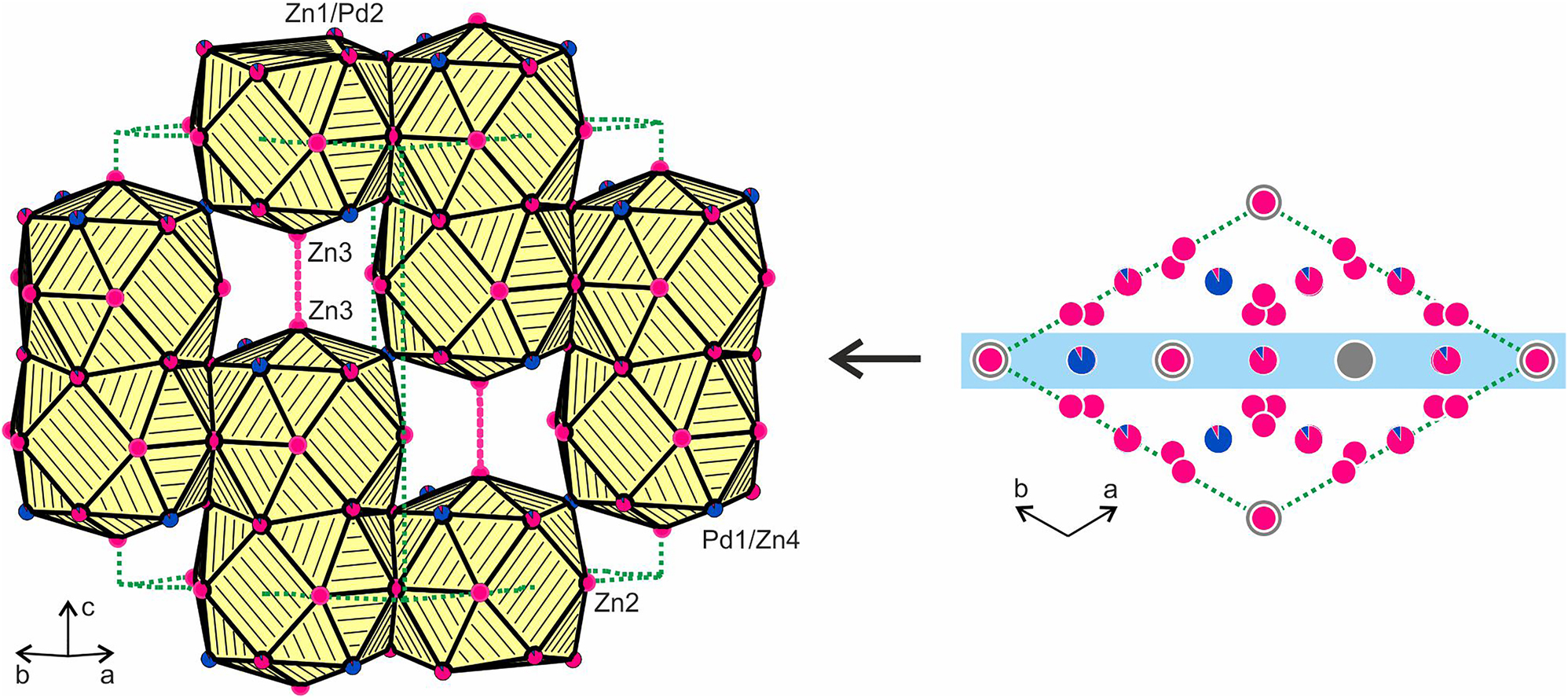
Projection of the Eu2Pd3.37Zn13.63 structure onto the xy plane (right). Europium, palladium and zinc atoms are drawn as medium grey, blue and magenta circles, respectively. Mixed occupied sites are indicated by segments. A cutout with one row of condensed Eu@(Pd/Zn)19 polyhedra is shown left.
Data collection and handling.
| Crystal: | Grey block |
| Size: | 0.030 × 0.040 × 0.075 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 39.0 mm−1 |
| Diffractometer, scan mode: | IPDS Stoe, ω scans |
| θ max, completeness: | 33.3°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 9175, 488, 0.037 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 417 |
| N(param)refined: | 26 |
| Programs: | X-Area [1], JANA2006 [2], SUPERFLIP [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | Y | z | U iso*/U eq |
|---|---|---|---|---|
| Eu1 | 0 | 0 | 0.34506(2) | 0.01304(7) |
| Pd1a | 0.5 | 0 | 0.5 | 0.01283(13) |
| Zn1b | 0.5 | 0 | 0.5 | 0.01283(13) |
| Zn2c | 0.50154(2) | 0.49846(2) | 0.15132(4) | 0.01548(15) |
| Pd2d | 0.50154(2) | 0.49846(2) | 0.15132(4) | 0.01548(15) |
| Zn3 | 0.29377(5) | 0 | 0 | 0.01484(12) |
| Zn4 | 0 | 0 | 0.10343(5) | 0.01629(16) |
-
aOccupancy: 0.917(10), bOccupancy: 0.083(10), cOccupancy: 0.897(8), dOccupancy: 0.103(8).
Source of material
Crystals of the title compound Eu2Pd3.37Zn13.63 were obtained by induction melting of the elements (3:4:12 atomic ratio) in a sealed tantalum ampoule in a water-cooled sample chamber. Starting materials were europium ingots (American Elements, 99.99%), palladium sheets (Agosi, 99.99%) and zinc granules (99.9%, Merck). The sample was first heated to 1473 K, kept for 5 min and quenched. The ampoule was then sealed in an evacuated silica tube, heated to 1223 K with a heating rate of 100 K h−1. The sample was kept at 1223 K for 24 h followed by slow cooling (1 K h−1) to room temperature. Eu2Pd3.37Zn13.63 is light grey with metallic luster and stable in air.
Experimental details
The Eu2Pd3.37Zn13.63 crystals were selected from the mechanically fragmented sample of the nominal composition 3Eu:4Pd:12Zn. The crystal quality was first tested through Laue photographs (Buerger camera, image plate detection system). Single crystal X-ray diffraction was performed at room temperature on a Stoe IPDS–II diffractometer (graphite monochromatized MoKα radiation; oscillation mode). A numerical absorption correction was applied. The starting atomic parameters were deduced with the charge-flipping algorithm [3] implemented in Superflip [4] and the structure was refined on F 2 with the Jana2006 software package [2], with anisotropic displacement parameters for all atoms. Separate refinement of the occupancy parameters indicated small degrees of Pd/Zn mixing for the 9d and 18h sites, which were refined as least-squares variables in the final cycles. The Eu2Pd3.37Zn13.63 crystal is a reverse/obverse twin with a domain ratio of 0.971(4):0.029(4).
Comment
Eu2Pd3.37Zn13.63 crystallizes with the Th2Zn17 type structure [5], space group
Examples of coloring variants for Th2Zn17 type representatives (space group
| Compound | 6c | 6c | 9d | 18f | 18h | Ref. |
|---|---|---|---|---|---|---|
| Th2Zn17 | Th | Zn | Zn | Zn | Zn | [5] |
| Ce2Fe17 | Ce | Fe | Fe | Fe | Fe | [7] |
| Zr2Be17 | Zr | Be | Be | Be | Be | [8] |
| Ba2Mg17 | Ba | Mg | Mg | Mg | Mg | [9] |
| Ce2Co15Al2 | Ce | Al | Co | Co | Co | [7] |
| Gd2Co3Zn14 | Gd | Zn | Co | Zn | Zn | [6] |
Acknowledgements
We thank Dipl.–Ing. J. Kösters for the intensity data collection.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. X–Area. The Stoe Single Crystal Diffraction Software Package; STOE & Cie GmbH: Darmstadt, Germany.Suche in Google Scholar
2. Petříček, V., Dušek, M., Palatinus, L. Crystallographic computing system Jana2006: general features. Z. Kristallogr. 2014, 229, 345–352.10.1515/zkri-2014-1737Suche in Google Scholar
3. Palatinus, L. The charge-flipping algorithm in crystallography. Acta Crystallogr. 2013, B69, 1–16; https://doi.org/10.1107/s0108768112051361.Suche in Google Scholar
4. Palatinus, L., Chapuis, G. Superflip – a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Suche in Google Scholar
5. Makarov, E. S., Vinogradov, S. I. Crystal structure of Th2Zn17 and U2Zn17. Sov. Phys. Crystallogr. 1956, 1, 499–505.Suche in Google Scholar
6. Gross, N., Block, G., Jeitschko, W. Ternary rare earth transition metal zinc compounds Ln2T3Zn14 (T = Fe, Co, Rh, Ni, Pd, Pt) with ordered Th2Zn17-type structure and structure refinement of the corresponding binary compound La2Zn17. Chem. Mater. 2002, 14, 2725–2731; https://doi.org/10.1021/cm021112n.Suche in Google Scholar
7. Zarechnyuk, O. S., Krypyakevych, P. I. Crystal structures of ternary compounds in the systems cerium-transition metal-aluminum. Sov. Phys. Crystallogr. 1963, 7, 436–446.Suche in Google Scholar
8. Zalkin, A., Bedford, R. G., Sands, D. E. Crystal structures of ZrBe5 and Zr2Be17. Acta Crystallogr. 1959, 12, 700; https://doi.org/10.1107/s0365110x59002055.Suche in Google Scholar
9. Gladyshevskii, E. I., Krypyakevych, P. I., Teslyuk, M. Y., Zarechnyuk, O. S., Kuz’ma, Y. B. Crystal structures of some intermetallic compounds. Sov. Phys. Crystallogr. 1961, 6, 207–208.Suche in Google Scholar
10. Villars, P., Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (release 2022/23); ASM International ®: Materials Park, Ohio (USA), 2022.Suche in Google Scholar
11. Nowotny, H. Die Kristallstrukturen von Ni5Ce, Ni5La, Ni5Ca, Cu5La, Cu5Ca, Zn5La, Zn5Ca, Ni2Ce, MgCe, MgLa und MgSr. Z. Metallkd. 1942, 34, 247–252; https://doi.org/10.1515/ijmr-1942-341101.Suche in Google Scholar
12. Hilleke, K. P., Fredrickson, R. T., Vinokur, A. I., Fredrickson, D. C. Substitution patterns understood through chemical pressure analysis: atom/dumbbell and Ru/Co ordering in derivatives of YCo5. Cryst. Growth Des. 2017, 17, 1610–1619; https://doi.org/10.1021/acs.cgd.6b01606.Suche in Google Scholar
13. Donohue, J. The Structures of the Elements; Wiley: New York, 1974.Suche in Google Scholar
14. Emsley, J. The Elements; Oxford University Press: Oxford, 1999.Suche in Google Scholar
15. Mishra, T., Heymann, G., Huppertz, H., Pöttgen, R. Dimorphism in the REPdZn series. Intermetallics 2012, 20, 110–114; https://doi.org/10.1016/j.intermet.2011.08.012.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

