Abstract
C17H13F4NOS, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.14 × 0.11 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.26 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 4888, 2652, 0.031 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2036 |
| N(param)refined: | 218 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | 0.18420 (12) | 0.45943 (8) | 0.88482 (6) | 0.0299 (2) |
| F1 | 1.0196 (3) | 0.50923 (15) | 0.60674 (12) | 0.0295 (4) |
| F2 | 0.1815 (3) | 0.18028 (19) | 1.02542 (13) | 0.0454 (5) |
| F3 | 0.1931 (4) | 0.05626 (19) | 0.90021 (15) | 0.0564 (6) |
| F4 | 0.4719 (3) | 0.14536 (17) | 0.90564 (13) | 0.0410 (4) |
| O1 | 1.2038 (3) | 0.21223 (18) | 0.61478 (13) | 0.0235 (4) |
| N1 | 0.8326 (3) | 0.2969 (2) | 0.57365 (15) | 0.0181 (5) |
| C1 | 0.8841 (4) | 0.1918 (2) | 0.42068 (19) | 0.0184 (6) |
| C2 | 0.8680 (5) | 0.1430 (3) | 0.3288 (2) | 0.0236 (6) |
| H2 | 0.728041 | 0.165367 | 0.303476 | 0.028* |
| C3 | 1.0672 (5) | 0.0595 (3) | 0.2756 (2) | 0.0259 (6) |
| H3 | 1.059601 | 0.024370 | 0.214316 | 0.031* |
| C4 | 1.2765 (5) | 0.0280 (3) | 0.3122 (2) | 0.0256 (6) |
| H4 | 1.408173 | −0.026069 | 0.274375 | 0.031* |
| C5 | 1.2925 (4) | 0.0757 (3) | 0.4041 (2) | 0.0228 (6) |
| H5 | 1.432562 | 0.053408 | 0.429404 | 0.027* |
| C6 | 1.0931 (4) | 0.1579 (2) | 0.45783 (19) | 0.0174 (6) |
| C7 | 1.0608 (4) | 0.2224 (2) | 0.55712 (19) | 0.0189 (6) |
| C8 | 0.7021 (4) | 0.2852 (3) | 0.49284 (19) | 0.0202 (6) |
| H8A | 0.637340 | 0.376686 | 0.458536 | 0.024* |
| H8B | 0.579140 | 0.242068 | 0.520800 | 0.024* |
| C9 | 0.7342 (4) | 0.3878 (2) | 0.65465 (19) | 0.0182 (6) |
| C10 | 0.5348 (4) | 0.3750 (3) | 0.71954 (19) | 0.0209 (6) |
| H10 | 0.467375 | 0.305299 | 0.710621 | 0.025* |
| C11 | 0.4366 (4) | 0.4661 (3) | 0.7973 (2) | 0.0225 (6) |
| C12 | 0.5317 (5) | 0.5750 (3) | 0.8103 (2) | 0.0233 (6) |
| C13 | 0.7278 (5) | 0.5868 (3) | 0.7444 (2) | 0.0240 (6) |
| H13 | 0.793895 | 0.658067 | 0.751200 | 0.029* |
| C14 | 0.8259 (4) | 0.4944 (3) | 0.6691 (2) | 0.0216 (6) |
| C15 | 0.4248 (5) | 0.6771 (3) | 0.8931 (2) | 0.0316 (7) |
| H15A | 0.265369 | 0.719900 | 0.886532 | 0.047* |
| H15B | 0.504101 | 0.748972 | 0.885453 | 0.047* |
| H15C | 0.437121 | 0.627302 | 0.959923 | 0.047* |
| C16 | 0.1204 (5) | 0.3001 (3) | 0.8621 (2) | 0.0306 (7) |
| H16A | −0.044331 | 0.312885 | 0.878949 | 0.037* |
| H16B | 0.162634 | 0.284809 | 0.789203 | 0.037* |
| C17 | 0.2417 (5) | 0.1721 (3) | 0.9229 (2) | 0.0364 (8) |
1 Source of materials
The 2-(chloromethyl)-N-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)benzamide (CTPB) was synthesized according to the literature method [4]. Sodium hydride (60% in oil, 0.15 g, 3.75 mmol) was washed with hexane three times and the washed sodium hydride was added to a stirred solution of CTPB (1.00 g, 2.56 mmol) in dry tetrahydrofuran (20 mL) at room temperature. The reaction mixture was continuously stirred at room temperature for 6 h and the reaction was monitored by TLC. After the reaction was complete, the reaction solution was poured into 0.1 N hydrochloric acid, which was then brought to basic using aqueous sodium bicarbonate solution, and extracted with ethyl acetate. The organic layer was collected, and distilled under reduced pressure. The remaining substance was purified by flash column chromatography using petroleum ether: ethyl acetate (20:1, v/v) to give a white solid (0.69 g, 75.9% yield), M.p.: 85.1–86.5 °C. 5.80 mg of white solid was taken and dissolved in 10 mL of dichloromethane and 5 mL of ethanol was added. After slow volatilization for three days, crystals of the title compound were obtained.
2 Experimental details
The hydrogen atom positions were fixed geometrically at the calculated distances and allowed to ride on the parent atoms. The U iso of the H-atoms were set to 1.2 and 1.5 times U eq of the parent atoms with C–H = 0.93 Å (aromatic) and C–H = 0.97 Å (aliphatic).
3 Comment
Isoindolin-1-ones can be found in various natural and synthetic products that show a wide range of biological activities [5], [6], [7], [8], [9], [10]. Many references showed crystal structures of the derivatives with the isoindolin-1-one group [11], [12], [13], [14], [15], [16], [17], [18], [19], [20].
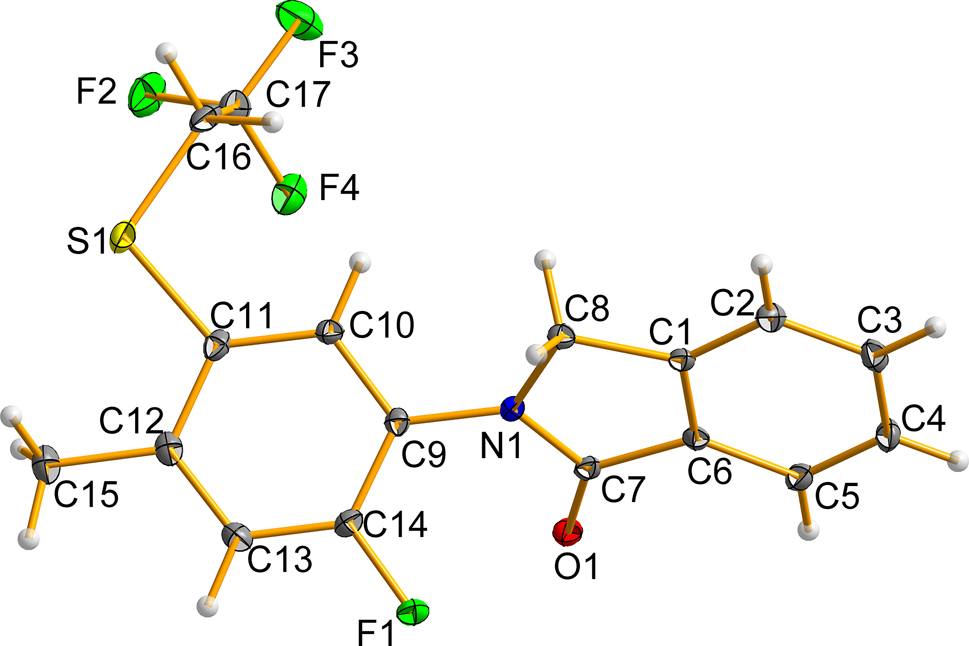
As shown in the figure, in the main moiety of title compound, the nitrogen atom on isoindole-1-one connects a phenyl group, and the dihedral angle between isoindole-1-one plane and phenyl plane (C9 → C14) is 55.04°. All C–C bond lengths in aromatic rings range from 1.373 to 1.407 Å. All of C–C single bond lengths are between 1.484 and 1.513 Å. N–C bonds are 1.374 and 1.468 Å. The C–S bonds are 1.779 and 1.804 Å. All bond lengths and angles are within a reasonable range [11], [12], [13], [14], [15], [16], [17], [18]. In the crystal, title molecules are linked by hydrogen bonds, which are the major intermolecular force to maintain the stability of the crystal structure. The angle of hydrogen bond C(8)–H(8B)⋯O(1) is 160°, and the length is 2.50 Å. The angle of hydrogen bond C(16)–H(16B)⋯O(1) is 163°, and the length is 2.44 Å.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies: CrysAlisPRO Software System (Version 171.38.43f); Agilent Technologies UK Ltd: Oxford, UK, 2015.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Kalugin, V. E., Shestopalov, A. M. The synthesis of 3-cyano-2-(organylamino)thieno[3,2-c]isoquinoline derivatives. Russ. Chem. Bull. 2019, 68, 588–596; https://doi.org/10.1007/s11172-019-2459-6.Suche in Google Scholar
5. Ghosh, S., Cho, S. J. Structural insights from molecular modeling of isoindolin-1-one derivatives as PI3Kγ inhibitors against gastric carcinoma. Biomedicines 2022, 10, 813; https://doi.org/10.3390/biomedicines10040813.Suche in Google Scholar PubMed PubMed Central
6. Kumar, J. S., Naimisha, R., Thirupataiah, B., Reddy, G. S., Bung, N., Roy, A., Bulusu, G., Mishra, A., Yadav, P. N., Misra, P., Pal, M. Sonochemical synthesis and biological evaluation of isoquinolin- 1(2H)-one/isoindolin-1-one derivatives: discovery of a positive ago-allosteric modulator (PAAM) of 5HT2CR. Bioorg. Chem. 2022, 129, 106202; https://doi.org/10.1016/j.bioorg.2022.106202.Suche in Google Scholar PubMed
7. Bhatia, R. K. Isoindole derivatives: propitious anticancer structural motifs. Curr. Top. Med. Chem. 2017, 17, 189–207; https://doi.org/10.2174/1568026616666160530154100.Suche in Google Scholar PubMed
8. Zhao, J., Liu, J., Shen, Y., Tan, Z., Zhang, M., Chen, R., Zhao, J., Zhang, D., Yu, L., Dai, J., Stachybotrysams, A.-E. prenylated isoindolinone derivatives with anti-HIV activity from the fungus stachybotrys chartarum. Phytochem. Lett. 2017, 20, 289–294; https://doi.org/10.1016/j.phytol.2017.04.031.Suche in Google Scholar
9. Wang, K., Bao, L., Qi, Q., Zhao, F., Ma, K., Pei, Y., Liu, H. Erinacerins C–L, isoindolin-1-ones with α-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J. Nat. Prod. 2015, 78, 146–154; https://doi.org/10.1021/np5004388.Suche in Google Scholar PubMed
10. El-Tamany, E. S. H., Sowellim, S. Z., Hamed, A. A., Radwan, A. S. Synthesis and antimicrobial activity of some isoindole derivatives. Res. Chem. Intermed. 2015, 41, 2675–2685; https://doi.org/10.1007/s11164-013-1378-7.Suche in Google Scholar
11. Mukherjee, A. K., Guha, S., Khan, M. W., Kundu, N. G., Helliwell, M. Two (Z)-N-aryl-3-benzylideneisoindolin-1-ones. Acta Crystallogr. 2000, C56, 85–87; https://doi.org/10.1107/s0108270199012445.Suche in Google Scholar PubMed
12. Zeng, D., Xing, A. P., Wei, J. J., Chu, Y. X., Zhang, S. L. Crystal structure of 3-((1H-benzo[d]imidazol-2-yl)amino)-2-(1H-benzo[d] imidazol-2-yl) isoindolin-1-one, C22H16N6O. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 249–251; https://doi.org/10.1515/ncrs-2018-0286.Suche in Google Scholar
13. Ha, K. Crystal structure of 2-(tricyclo [3.3.1.1(3, 7)] decan-1-yl)-3-(tricyclo [3.3.1.1(3, 7)]-decan-1-ylimino) isoindolin-1-one, C28H34N2O. Z. Kristallogr. N. Cryst. Struct. 2013, 228, 107–108; https://doi.org/10.1524/ncrs.2013.0070.Suche in Google Scholar
14. Zheng, Y., Wu, J. L. 2-(4-Hydroxybiphenyl-3-yl) isoindolin-1-one. Acta Crystallogr. 2010, E66, o448; https://doi.org/10.1107/s1600536810002370.Suche in Google Scholar
15. Liu, S., Zhang, X. L., Zhang, W. H., Zhu, H. J. 3-Hydroxy-3-phenylisoindolin-1-one 0.33-hydrate. Acta Crystallogr. 2009, E65, o3011; https://doi.org/10.1107/s160053680904608x.Suche in Google Scholar
16. Li, W., Yin, H., Wen, L., Cui, J., Wang, D. 3-Ethoxy-2-(1,3-thiazol-2-yl) isoindolin-1-one. Acta Crystallogr. 2009, E65, o2594; https://doi.org/10.1107/s1600536809038884.Suche in Google Scholar
17. Urban, J., Ludvík, J., Fábry, J., Dušek, M., Fejfarová, K. 2-(2-Hydroxyethyl)-3-[(2-hydroxyethyl) imino] isoindolin-1-one. Acta Crystallogr. 2009, E65, o2092–o2093; https://doi.org/10.1107/s1600536809030487.Suche in Google Scholar
18. Wang, J., Johnson, D. M., Tiekink, E. R. Crystal structure of 2-(3-oxo-1, 3-dihydro-2-benzofuran-5-yl)-3-(2-oxopropyl) isoindolin-1-one, C19H15NO4. Z. Kristallogr. N. Cryst. Struct. 2008, 223, 27–28; https://doi.org/10.1524/ncrs.2008.0013.Suche in Google Scholar
19. Fun, H. K., Shen, Y. M., Xu, J. H., Chantrapromma, S. 3-Benzyl-4,5,6,7-tetrachloro-3-hydroxy-2-(3-hydroxypropyl) isoindolin-1-one. Acta Crystallogr. 2007, E63, o944–o945; https://doi.org/10.1107/s1600536807002838.Suche in Google Scholar
20. Chowdhury, K., Mukhopadhyay, R., Mukherjee, M., Broder, C. K., Kundu, N. G. (Z)-3-Acetonylidene-2,3-dihydro-1H-isoindolin-1-one. Acta Crystallogr. 2001, E57, o421–o423; https://doi.org/10.1107/s1600536801005864.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

