Abstract
C8H4INS3, monoclinic, P21/c (no. 14), a = 11.8060(19) Å, b = 7.8961(14) Å, c = 11.972(2) Å, β =
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.23 × 0.15 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.62 mm−1 |
| Diffractometer, scan mode: | Bruker Apex-II, φ and ω |
| θ max, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 6284, 2398, 0.027 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2070 |
| N(param)refined: | 118 |
| Programs: | Bruker [1], Shelx [2, 3], WinGX/Ortep [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| I1 | 0.17125 (2) | 0.55094 (3) | 0.56857 (2) | 0.05658 (11) |
| S1 | 0.95419 (7) | 0.75629 (11) | 0.72613 (7) | 0.04593 (19) |
| S2 | 0.86658 (9) | 0.93158 (12) | 0.59960 (9) | 0.0596 (2) |
| S3 | 0.83166 (8) | 0.48844(11) | 0.80685 (8) | 0.0494 (2) |
| N1 | 0.7290 (2) | 0.8764 (4) | 0.5848 (3) | 0.0526 (7) |
| C1 | 0.3502 (3) | 0.6211 (4) | 0.5958 (3) | 0.0386 (6) |
| C2 | 0.4385 (3) | 0.5974 (4) | 0.7077 (3) | 0.0438 (7) |
| H2 | 0.417662 | 0.554721 | 0.770198 | 0.053* |
| C3 | 0.5577 (3) | 0.6374 (4) | 0.7259 (3) | 0.0405 (6) |
| H3 | 0.617351 | 0.621527 | 0.800938 | 0.049* |
| C4 | 0.5890 (2) | 0.7016 (3) | 0.6325 (2) | 0.0360(6) |
| C5 | 0.7148 (3) | 0.7493 (4) | 0.6480 (2) | 0.0380 (6) |
| C6 | 0.8234 (3) | 0.6616 (4) | 0.7271 (2) | 0.0367 (6) |
| C8 | 0.4985 (3) | 0.7251 (4) | 0.5219 (3) | 0.0452 (7) |
| H8 | 0.518449 | 0.768256 | 0.459050 | 0.054* |
| C9 | 0.3794(3) | 0.6856 (4) | 0.5033 (3) | 0.0467 (7) |
| H9 | 0.319408 | 0.702511 | 0.428621 | 0.056* |
1 Source of material
All chemicals were purchased from commercial sources and used as received without further purification. A mixture of 1-(4-iodophenyl)ethanone O-acetyl oxime (15.1 g, 0.05 mol), S8 (9.6 g, 0.3 mol), CuBr (0.72 g, 0.005 mol), Li2CO3 (1.85 g, 0.025 mol), and DMSO (100 mL) were added successfully to a 250 mL oven-dried reaction flask. The sealed reaction flask was stirred at 130° C for 8 h. After cooling to room temperature, the reaction was diluted with ethyl acetate and water. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (100 mL) for three times. The combined organic layer was brine and dried over magnesium sulfate and the volatiles were removed under reduced pressure. The residue was purified by column chromatography on silica gel (PE/EA: 20/1) to yield the desired product (7.2 g, 46%) as a brown solid. Subsequently, dissolve 1 g of the target compound in 30 ml of dichloromethane, heat to reflux until the solid is completely dissolved, filtered. Finally, the title crystal was precipitated by controlling solvent volatilization.
2 Experimental details
All H-atoms bonded to C atoms were placed geometrically and refined using a riding model with common isotropic displacement factors U iso(H) = 1.2 or 1.5U eq (parent C-atom).
3 Comment
The 1,2,3-dithiazoles are important fused heterocycles because they show to have a broad biological activity profile, including antibacterial [5], anticancer [6], antifungal/herbicidal [7], and anti-melanin activities [8]. The 1,2,3-dithiazole compounds plays an important role in the mechanism of 1,2,3-dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism [9].
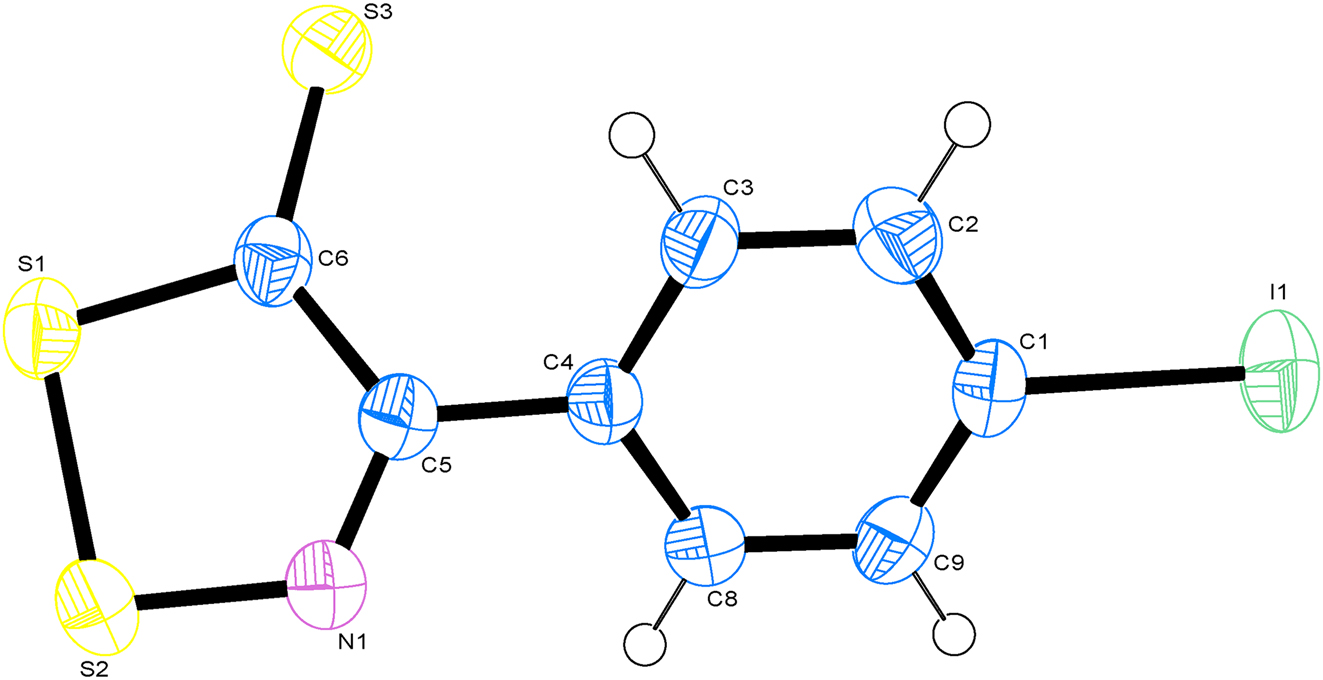
Single-crystal structure analysis revealed that the title compound crystallized in the monoclinic space group P21/c. The Ortep diagram is presented in Figure. The bond lengths of S1–S2 in the title molecule is 2.0393(13) Å, it is similar to the literature [10], [11], [12]. The bond lengths of C6–S3, C6–S1 and N1–S2 in the title molecule are 1.651(3) Å, 1.719(3) Å and 1.628(3) Å, respectively. They are similar to the literature [13, 14]. The bond length of C1–I1 is 2.094(3) Å, which is similar to those reported in the literature [15], [16].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Scientific & Technological Projects of Hengyang city (No. 202150063426), Scientific Research Fund of Hunan Provincial Education Department of China (No. 21B0634), Science Foundation of Hengyang Normal University of China (No. 2020QD07) and Key Laboratory of Functional Metal–Organic Compounds of Hunan Province (2022HSKFJJ024).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Shelxtl – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WinGX and Ortep for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Konstantinova, L. S., Bol’shakov, O. I., Obruchnikova, N. V., Laborie, H., Tanga, A., Sopena, V., Lanneluc, I., Picot, L., Sable, S., Thiery, V., Rakitin, O. A. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141; https://doi.org/10.1016/j.bmcl.2008.11.010.Search in Google Scholar PubMed
6. Oppedisano, F., Catto, M., Koutentis, P. A., Nicolotti, O., Pochini, L., Koyioni, M., Introcaso, A., Michaelidou, S. S., Carotti, A., Indiveri, C. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102; https://doi.org/10.1016/j.taap.2012.09.011.Search in Google Scholar PubMed
7. Konstantinova, L. S., Bol’shakov, O. A., Obruchnikova, N. V., Golova, S. P., Nelyubina, Y. V., Lyssenko, K. A., Rakitin, O. A. Synthesis of 1,2,5-thiadiazole-3(2H)-thiones and 1,2,5-thiadiazol-3(2H)-ones from 1,2,3-dithiazoles. Mendeleev Commun. 2009, 19, 84–86; https://doi.org/10.1016/j.mencom.2009.03.010.Search in Google Scholar
8. Charalambous, A., Koyioni, M., Antoniades, I., Pegeioti, D., Eleftheriou, I., Michaelidou, S. S., Amelichev, S. A., Konstantinova, L. S., Rakitin, O. A., Koutentis, P. A., Skourides, P. A. 1,2,3–dithiazoles – new reversible melanin synthesis inhibitors: a chemical genomics study. MedChemComm 2015, 6, 935–946; https://doi.org/10.1039/c5md00052a.Search in Google Scholar
9. Asquith, C. R., Konstantinova, L. S., Laitinen, T., Meli, M. L., Poso, A., Rakitin, O. A., Hofmann–Lehmann, R., Hilton, S. T. Evaluation of substituted 1,2,3-dithiazoles as inhibitors of the Feline Immunodeficiency Virus (FIV) nucleocapsid protein via a proposed zinc ejection mechanism. ChemMedChem 2016, 11, 2119–2126; https://doi.org/10.1002/cmdc.201600260.Search in Google Scholar PubMed
10. Mayo, R. A., Morgan, I. S., Soldatov, D. V., Clérac, R., Preuss, K. E. Heisenberg spin chains via chalcogen bonding: noncovalent S, O contacts enable long-range magnetic order. Inorg. Chem. 2021, 60, 11338–11346; https://doi.org/10.1021/acs.inorgchem.1c01287.Search in Google Scholar PubMed
11. Konstantinova, L. S., Baranovsky, I. V., Pritchina, E. A., Mikhailov, M. S., Bagryanskaya, I. Y., Semenov, N. A., Irtegova, I. G., Salnikov, G. E., Lyssenko, K. A., Gritsan, N. P., Zibarev, A. V., Rakitin, O. A. Fused 1,2,3-thiaselenazoles synthesized from 1,2,3-dithiazoles through selective chalcogen exchange. Chem. Eur J. 2017, 23, 17037–17047; https://doi.org/10.1002/chem.201703182.Search in Google Scholar PubMed
12. Konstantinova, L. S., Baranovsky, I. V., Pritchina, E. A., Mikhailov, M. S., Bagryanskaya, I. Y., Semenov, N. A., Irtegova, I. G., Salnikov, G. E., Lyssenko, K. A., Gritsan, N. P., Zibarev, A. V., Rakitin, O. A. Fused 1,2,3-thiaselenazoles synthesized from 1,2,3-dithiazoles through selective chalcogen exchange. Chem. Eur J. 2017, 23, 17037–17047; https://doi.org/10.1002/chem.201703182.Search in Google Scholar PubMed
13. Koyioni, M., Manoli, M., Koutentis, P. A. The reaction of DABCO with 4-chloro-5H-1,2,3-dithiazoles: synthesis and chemistry of 4-[N-(2-chloroethyl)piperazin-1-yl]-5H-1,2,3-dithiazoles. J. Org. Chem. 2016, 81, 615–631; https://doi.org/10.1021/acs.joc.5b02497.Search in Google Scholar PubMed
14. Bo’shakov, O. I., Yushina, I. D., Stash, A. I., Aysin, R. R., Bartashevich, E. V., Rakitin, O. A. Structure and properties of 4-phenyl-5H-1,2,3-dithiazole-5-thione polyiodide with S–I+–S bridged complex. Struct. Chem. 2020, 31, 1729–1737; https://doi.org/10.1007/s11224-020-01584-y.Search in Google Scholar
15. Ranjan, S., Takamizawa, S. Two-dimensional organoferroelasticity in a single crystal of 4-iodoaniline. Cryst. Growth Des. 2022, 22, 1831–1836; https://doi.org/10.1021/acs.cgd.1c01394.Search in Google Scholar
16. Dishman, S. N., Laconsay, C. J., Fettinger, J. C., Tantillo, D. J., Shaw, J. T. Divergent stereochemical outcomes in the insertion of donor/donor carbenes into the C–H bonds of stereogenic centers. Chem. Sci. 2022, 13, 1030–1036; https://doi.org/10.1039/d1sc04622e.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

