Abstract
C18H14CuN8, triclinic, P1̄ (no. 2), a = 7.2431(5) Å, b = 7.3783(5) Å, c = 9.8740(6) Å, α = 110.174(6)°, β = 105.036(5)°, γ = 92.219(5)°, V = 473.55(6) Å3, Z = 1, R gt (F) = 0.0409, wR ref (F 2) = 0.1272, T = 293(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green flaky |
| Size: | 0.48 × 0.30 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.79 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θ max, completeness: | 78.1°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 4781, 1910, 0.031 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1707 |
| N(param)refined: | 124 |
| Programs: | CrysAlisPRO [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | X | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.6756 (5) | 0.6146 (4) | 0.8808 (3) | 0.0539 (7) |
| C2 | 0.7038 (5) | 0.3042 (5) | 0.8647 (3) | 0.0569 (7) |
| C3 | 0.0990 (5) | 0.8996 (5) | 0.8057 (4) | 0.0630 (8) |
| H3 | 0.073409 | 0.944071 | 0.898388 | 0.076* |
| C4 | −0.0544 (5) | 0.8346 (6) | 0.6765 (4) | 0.0671 (9) |
| H4 | −0.179890 | 0.836007 | 0.683699 | 0.081* |
| C5 | 0.3136 (5) | 0.8367 (5) | 0.6697 (3) | 0.0553 (7) |
| H5 | 0.440650 | 0.836950 | 0.666057 | 0.066* |
| C6 | 0.1672 (5) | 0.7691 (5) | 0.5358 (3) | 0.0590 (8) |
| H6 | 0.196614 | 0.724446 | 0.444629 | 0.071* |
| C7 | −0.0237 (5) | 0.7676 (5) | 0.5366 (3) | 0.0563 (7) |
| C8 | −0.1879 (5) | 0.6990 (6) | 0.3983 (4) | 0.0717 (9) |
| H8 | −0.310322 | 0.706631 | 0.411502 | 0.086* |
| C9 | −0.1812 (6) | 0.6299 (7) | 0.2617 (4) | 0.0822 (11) |
| H9A | −0.062582 | 0.618937 | 0.241866 | 0.099* |
| H9B | −0.294868 | 0.590820 | 0.182522 | 0.099* |
| Cu01 | 0.500000 | 1.000000 | 1.000000 | 0.0494 (2) |
| N1 | 0.6281 (4) | 0.7643 (4) | 0.9217 (3) | 0.0596 (7) |
| N2 | 0.7320 (6) | 0.4499 (5) | 0.8229 (5) | 0.0967 (13) |
| N3 | 0.6832 (5) | 0.1683 (4) | 0.8900 (4) | 0.0750 (8) |
| N4 | 0.2815 (4) | 0.9019 (3) | 0.8043 (3) | 0.0498 (5) |
1 Source of material
The 10 mL of Cu(ClO4)2·6H2O (116 mg, 0.5 mmol) and 4-vinylpyridine (105 mg, 1.0 mmol) ethanol solution was added to a 10 mL aqueous solution of sodium dicyanamide (89 mg, 1 mmol) with severely stirring at 80 °C. The mixture was stirred for 10 min and then cooled to room temperature. After few minutes, green flaky crystals were collected, washed by ethanol, and dried in air, yield 60% (based on 4-vinylpyridine).
2 Experimental details
The structure was solved by Direct Methods and refined by full-matrix least-squares method on F 2 with anisotropic thermal parameters for all non-hydrogen atoms. The hydrogen atom positions were determined by theoretical calculations and refined with an isotropic displacement factor.
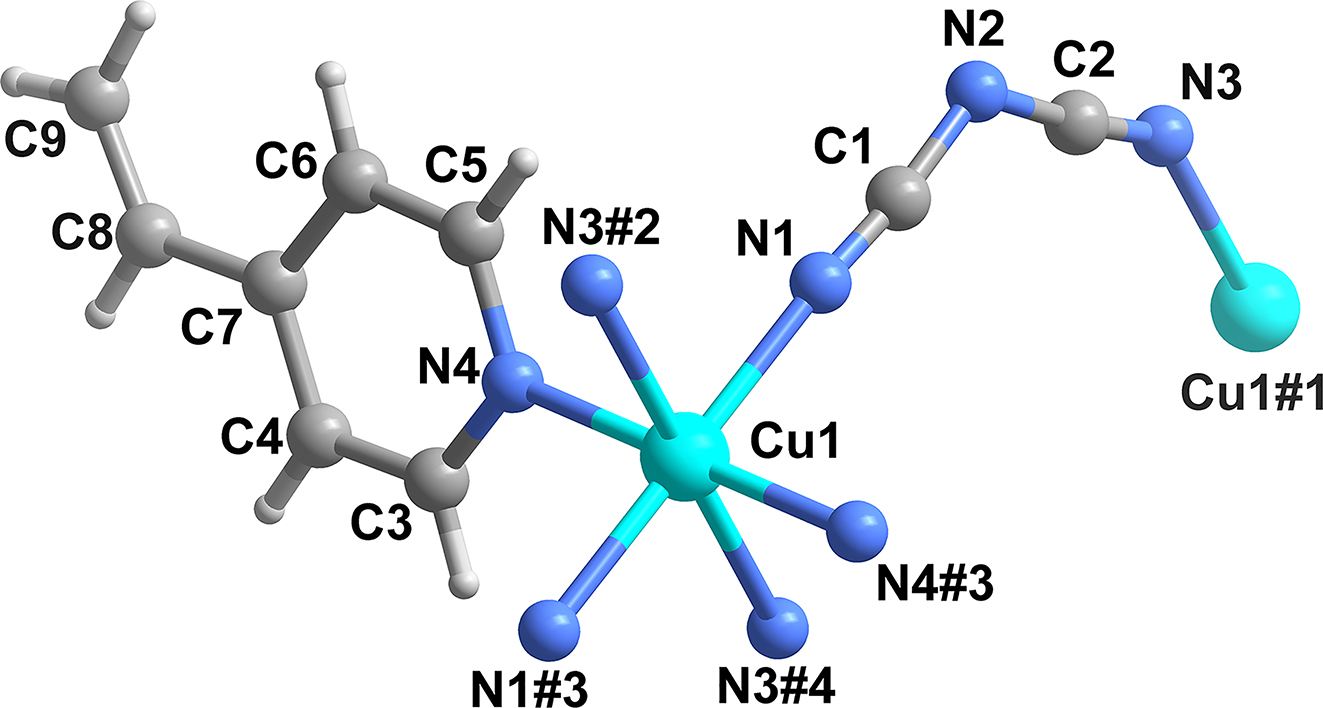
3 Comment
Metal-based coordination polymers have attracted much attention in the fields of coordination chemistry and crystal engineering. Nitrogen-containing organic ligands containing unsaturated groups have been used to assemble hypergolic fuels due to their unique activities. Moreover, high-energy dicandiamine ions (DCA) also have great advantages in regulating the structure and properties of compounds. In this work, we present a 1D coordination polymer based on 4-vinylpyridine and DCA [3], [4], [5], [6], [7], [8], [9], [10], [11].
The title crystal structure belongs to the triclinic space group
Acknowledgements
We thank the Center of Testing and Analysis, Fujian Institute of Research on the Structure of Matter, for support.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO. Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Jin, Y., Shi, Y., Zhang, W., Qi, X., Xia, H., Zhang, Q. Cationic effect on properties related to thermal stability and ignition delay for hypergolic ionic liquids. J. Mol. Liq. 2021, 336, 116572; https://doi.org/10.1016/j.molliq.2021.116572.Search in Google Scholar
4. Li, Z., Zhong, Y., Liang, L., Feng, Y., Zhang, J., Zhang, T., Zhang, Y. Hypergolic coordination compounds as modifiers for ionic liquid propulsion. Chem. Eng. J. 2021, 423, 130187; https://doi.org/10.1016/j.cej.2021.130187.Search in Google Scholar
5. Liu, F.-Q., Li, R.-X., Li, S.-X., Sun, L.-S., Liu, G.-Y., Tetra-μ-acetato-κ8O:O′-bis[(4-vinylpyridine-κN)copper(II)](Cu–Cu). Acta Crystallogr. 2007, E63, m2455.Search in Google Scholar
6. Liu, T., Qi, X., Huang, S., Jiang, L., Li, J., Tang, C., Zhang, Q. Exploiting hydrophobic borohydride-rich ionic liquids as faster-igniting rocket fuels. Chem. Commun. 2016, 52, 2031–2034; https://doi.org/10.1039/c5cc09737a.Search in Google Scholar
7. Taş, M., Karabağ, E., Titiz, A., Kaya, M., Ataseven, M., Dal, H. Synthesis and characterization of tetrakis-μ-[N-phenylanthranilato](O,O′)-bis[(4-vinylpyridine copper(II)] complex. Z. für Kristallogr. Cryst. Mater. 2013, 228, 92–99, https://doi.org/10.1524/zkri.2013.1583.Search in Google Scholar
8. Volz, D., Nieger, M., Friedrichs, J., Baumann, T., Bräse, S. Small change, big red shift: syntheses, structure and photoluminescence of Cu2Br2(Ph3P)2py2 (py=pyridine, 4-vinylpyridine). Inorg. Chem. Commun. 2013, 37, 106–109; https://doi.org/10.1016/j.inoche.2013.09.023.Search in Google Scholar
9. Xu, Y., Wang, Y., Zhong, Y., Lei, G., Li, Z., Zhang, J., Zhang, T. High-energy metal-organic frameworks with a dicyanamide linker for hypergolic fuels. Inorg. Chem. 2021, 60, 5100–5106; https://doi.org/10.1021/acs.inorgchem.1c00109.Search in Google Scholar
10. Yang, R., Sun, Y., Zhao, D., Guo, L. Synthesis of asymmetric binuclear copper(I) complexes containing 4-vinylpyridine and bis(diphenylphosphino) methane. J. Coord. Chem. 2010, 56, 1169–1177; https://doi.org/10.1080/00958970310001624465.Search in Google Scholar
11. Zhao, J. Tetrakis(μ-2-chloro-benzoato-κO:O′)bis-[(4-vinyl-pyridine- κN)copper(II)]. Acta Crystallogr. 2008, E64, m1336.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

