Abstract
C18H40N5O4S, monoclinic, P21/n (no. 14), a = 8.4254(9) Å, b = 8.4254(9) Å, c = 16.1470(17) Å, β = 98.302(2)°, V = 2411.9(4) Å3, Z = 4, R gt(F) = 0.0409, wR ref(F 2) = 0.1162, T = 296(2) K.
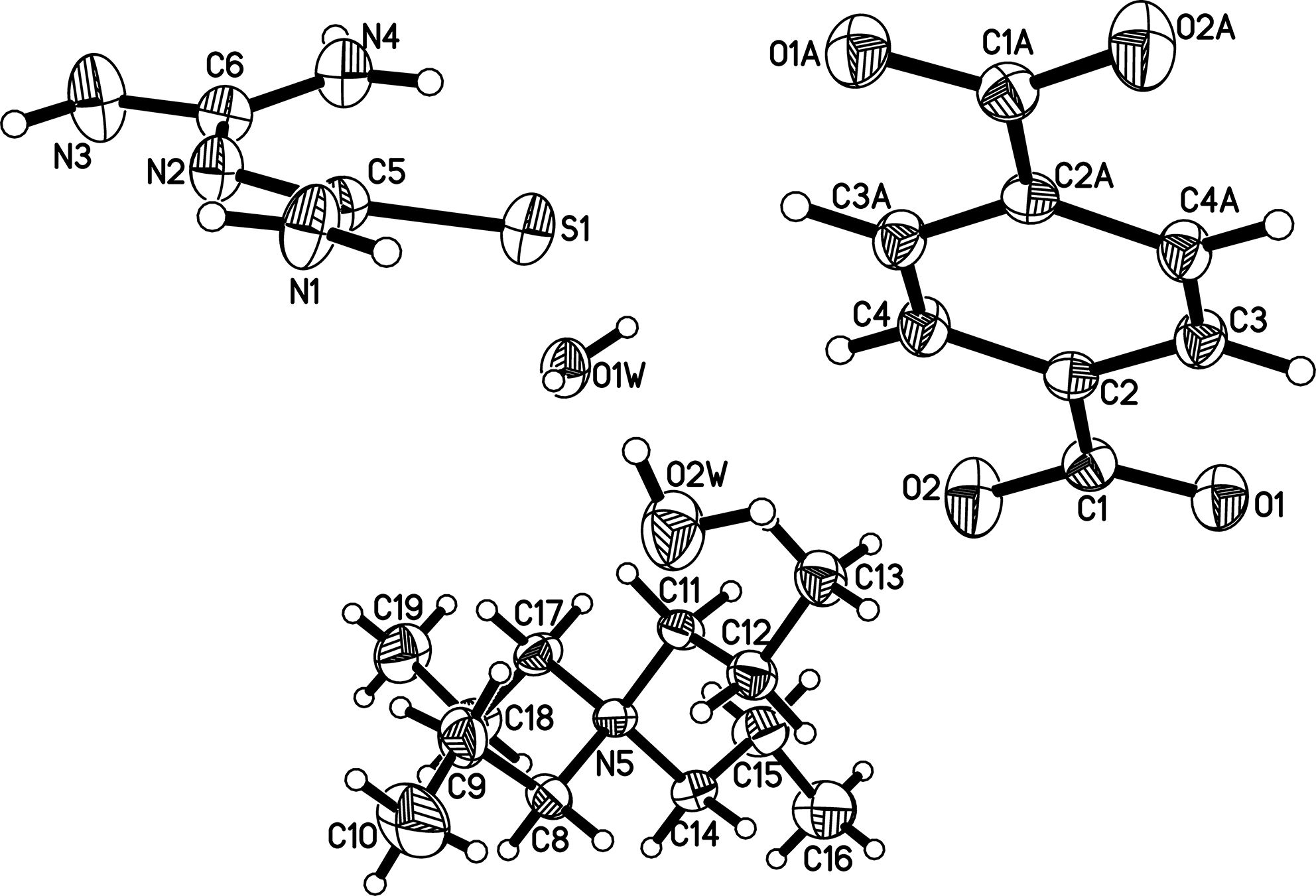
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.40 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.17 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 12,101, 4245, 0.021 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3591 |
| N(param)refined: | 253 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.34682 (19) | 0.85885 (9) | 0.53201 (10) | 0.0377 (4) |
| N1 | 0.4709 (2) | 1.01283 (8) | 0.11194 (10) | 0.0543 (4) |
| H1A | 0.4659 | 1.0325 | 0.0630 | 0.065* |
| H1B | 0.4503 | 1.0394 | 0.1534 | 0.065* |
| O1 | 0.32977 (15) | 0.84318 (6) | 0.60614 (7) | 0.0491 (3) |
| S1 | 0.51596 (6) | 0.90591 (3) | 0.22298 (3) | 0.05203 (16) |
| O1W | 0.47721 (18) | 0.15818 (9) | 0.22663 (9) | 0.0674 (4) |
| H1WA | 0.4518 | 0.2044 | 0.2190 | 0.101* |
| H1WB | 0.5438 | 0.1569 | 0.2720 | 0.101* |
| C2 | 0.42669 (17) | 0.93211 (8) | 0.51590 (9) | 0.0328 (3) |
| N2 | 0.53983 (18) | 0.90651 (7) | 0.05306 (8) | 0.0421 (3) |
| O2 | 0.30074 (18) | 0.81831 (7) | 0.46993 (8) | 0.0618 (4) |
| O2W | 0.2278 (2) | 0.79113 (9) | 0.29901 (9) | 0.0752 (4) |
| H2WA | 0.3080 | 0.8084 | 0.2790 | 0.113* |
| H2WB | 0.2592 | 0.8023 | 0.3499 | 0.113* |
| C3 | 0.47187 (19) | 0.98258 (9) | 0.57998 (10) | 0.0382 (4) |
| H3A | 0.4538 | 0.9713 | 0.6341 | 0.046* |
| N3 | 0.6209 (2) | 0.81132 (9) | −0.02399 (10) | 0.0659 (5) |
| H3B | 0.6705 | 0.7699 | −0.0287 | 0.079* |
| H3C | 0.5766 | 0.8345 | −0.0680 | 0.079* |
| C4 | 0.45618 (19) | 0.95030 (9) | 0.43571 (10) | 0.0388 (4) |
| H4A | 0.4272 | 0.9169 | 0.3920 | 0.047* |
| N4 | 0.68277 (18) | 0.80151 (8) | 0.11661 (9) | 0.0485 (4) |
| H4B | 0.7312 | 0.7603 | 0.1093 | 0.058* |
| H4C | 0.6800 | 0.8180 | 0.1664 | 0.058* |
| C5 | 0.51118 (19) | 0.94056 (9) | 0.12356 (10) | 0.0392 (4) |
| N5 | 0.00279 (15) | 0.05238 (8) | 0.25538 (9) | 0.0419 (3) |
| C6 | 0.6126 (2) | 0.83952 (9) | 0.05139 (10) | 0.0404 (4) |
| C8 | −0.1172 (2) | 0.10458 (11) | 0.20593 (12) | 0.0510 (4) |
| H8A | −0.1671 | 0.1345 | 0.2451 | 0.061* |
| H8B | −0.2007 | 0.0748 | 0.1741 | 0.061* |
| C9 | −0.0480 (3) | 0.15670 (13) | 0.14604 (14) | 0.0720 (6) |
| H9A | 0.0477 | 0.1804 | 0.1750 | 0.086* |
| H9B | −0.0174 | 0.1279 | 0.1000 | 0.086* |
| C10 | −0.1652 (4) | 0.2150 (2) | 0.1125 (2) | 0.1264 (13) |
| H10A | −0.1178 | 0.2469 | 0.0750 | 0.190* |
| H10B | −0.2592 | 0.1917 | 0.0829 | 0.190* |
| H10C | −0.1942 | 0.2441 | 0.1579 | 0.190* |
| C11 | 0.1380 (2) | 0.09682 (10) | 0.30580 (11) | 0.0461 (4) |
| H11A | 0.2018 | 0.0629 | 0.3439 | 0.055* |
| H11B | 0.2068 | 0.1161 | 0.2675 | 0.055* |
| C12 | 0.0861 (2) | 0.16127 (12) | 0.35598 (13) | 0.0590 (5) |
| H12A | 0.0270 | 0.1971 | 0.3184 | 0.071* |
| H12B | 0.0150 | 0.1430 | 0.3937 | 0.071* |
| C13 | 0.2278 (3) | 0.19921 (12) | 0.40571 (13) | 0.0638 (5) |
| H13A | 0.1914 | 0.2398 | 0.4369 | 0.096* |
| H13B | 0.2854 | 0.1640 | 0.4436 | 0.096* |
| H13C | 0.2973 | 0.2180 | 0.3684 | 0.096* |
| C14 | −0.0891 (2) | 0.00804 (10) | 0.31340 (11) | 0.0470 (4) |
| H14A | −0.1317 | 0.0427 | 0.3507 | 0.056* |
| H14B | −0.1795 | −0.0159 | 0.2798 | 0.056* |
| C15 | 0.0067 (3) | −0.05105 (13) | 0.36585 (16) | 0.0713 (6) |
| H15A | 0.0561 | −0.0843 | 0.3296 | 0.086* |
| H15B | 0.0915 | −0.0274 | 0.4039 | 0.086* |
| C16 | −0.0984 (3) | −0.09537 (15) | 0.41519 (19) | 0.0928 (9) |
| H16A | −0.0350 | −0.1324 | 0.4479 | 0.139* |
| H16B | −0.1460 | −0.0626 | 0.4517 | 0.139* |
| H16C | −0.1813 | −0.1195 | 0.3776 | 0.139* |
| C17 | 0.0802 (2) | 0.00133 (11) | 0.19762 (12) | 0.0537 (5) |
| H17A | 0.1340 | 0.0321 | 0.1607 | 0.064* |
| H17B | 0.1618 | −0.0282 | 0.2314 | 0.064* |
| C18 | −0.0324 (3) | −0.05121 (14) | 0.14445 (16) | 0.0775 (7) |
| H18A | −0.0841 | −0.0838 | 0.1804 | 0.093* |
| H18B | −0.1151 | −0.0226 | 0.1103 | 0.093* |
| C19 | 0.0577 (3) | −0.09743 (16) | 0.08885 (17) | 0.0900 (8) |
| H19A | −0.0155 | −0.1304 | 0.0556 | 0.135* |
| H19B | 0.1076 | −0.0651 | 0.0527 | 0.135* |
| H19C | 0.1385 | −0.1262 | 0.1227 | 0.135* |
Source of materials
Amidinothiourea, terephthalic acid and tetrapropylammonium hydroxide (25% aqueous solution), which are commercially available, were mixed in a molar ratio of 1:1:2. The mixture was dissolved in a minimum amount of ethanol and water, then stirred for about 35 min. Subsequently the clean solution was set aside to allow slow evaporation at room temperature. Colorless block crystals were obtained about seven days.
Comment
Amidinothiourea, a derivative of thiourea, can be utilized to synthesize the related organic crystals to explore supramolecular architecture [4, 5]. The amidinothiourea as an approximately planar molecule is composed of two triangular motifs, and it would form various hydrogen bonds by the NH2 groups if the appropriate hydrogen bond acceptor were provided. Searching in the related literatures, it can be seen the crystal structures of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea [6] and terephthalate-1-(diaminomethylene)thiourea [7] are reported.
In the asymmetric unit of the title compound, there exist one independent 1-(diaminomethylene)thiourea, one half terephthalate positioned on the symmetry center, one tetrapropylammonium cation and two water molecules. Analyzing the crystal structure, the neutral amidinothiourea molecules firstly form a dimer by a pair of N–H···N hydrogen bonds, then the dimer links with the terephthalate by N–H···O hydrogen bonds to generate a zigzag chain extended along the b axis. Consecutively, the hydrogen-bonded chains interact with two independent water molecules by varied O–H···O contacts to regularly arrange to yield a 3-dimensional hydrogen-bonded framework with channels.
Funding source: Doctoral Fund of Henan University of Chinese Medicine
Award Identifier / Grant number: BSJJ2022-15
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was funded by Doctoral Fund of Henan University of Chinese Medicine. (BSJJ2022-15).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Janczak, J. Supramolecular architecture formed between amidinothiourea and 2-pyridinecarboxylic acid. J. Mol. Struct. 2021, 1242, 130736; https://doi.org/10.1016/j.molstruc.2021.130736.Suche in Google Scholar
5. Janczak, J. Supramolecular architecture and SHG activity of organic crystals formed between the amidinothiourea and nicotinic acid. J. Mol. Struct. 2023, 1273, 134385; https://doi.org/10.1016/j.molstruc.2022.134385.Suche in Google Scholar
6. Xu, D., Wang, B., Zhu, X. Crystal structure of tetrabutylammonium sulfanilate – 1-(diaminomethylene)thiourea (1/2). Z. Kristallogr. N. Cryst. Struct. 2022, 237, 59–60; https://doi.org/10.1515/ncrs-2021-0361.Suche in Google Scholar
7. Hou, Y., Zhang, Z., Zhu, X. Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 879–880; https://doi.org/10.1515/ncrs-2021-0107.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

