Abstract
C20H32O3, monoclinic, P21 (no. 4), a = 7.8981(17) Å, b = 10.302(2) Å, c = 11.028(3) Å, β = 90.020(2)°, V = 897.3(3) Å3, Z = 2, R gt (F) = 0.0603, wR ref (F 2) = 0.1342, T = 273(2) K.
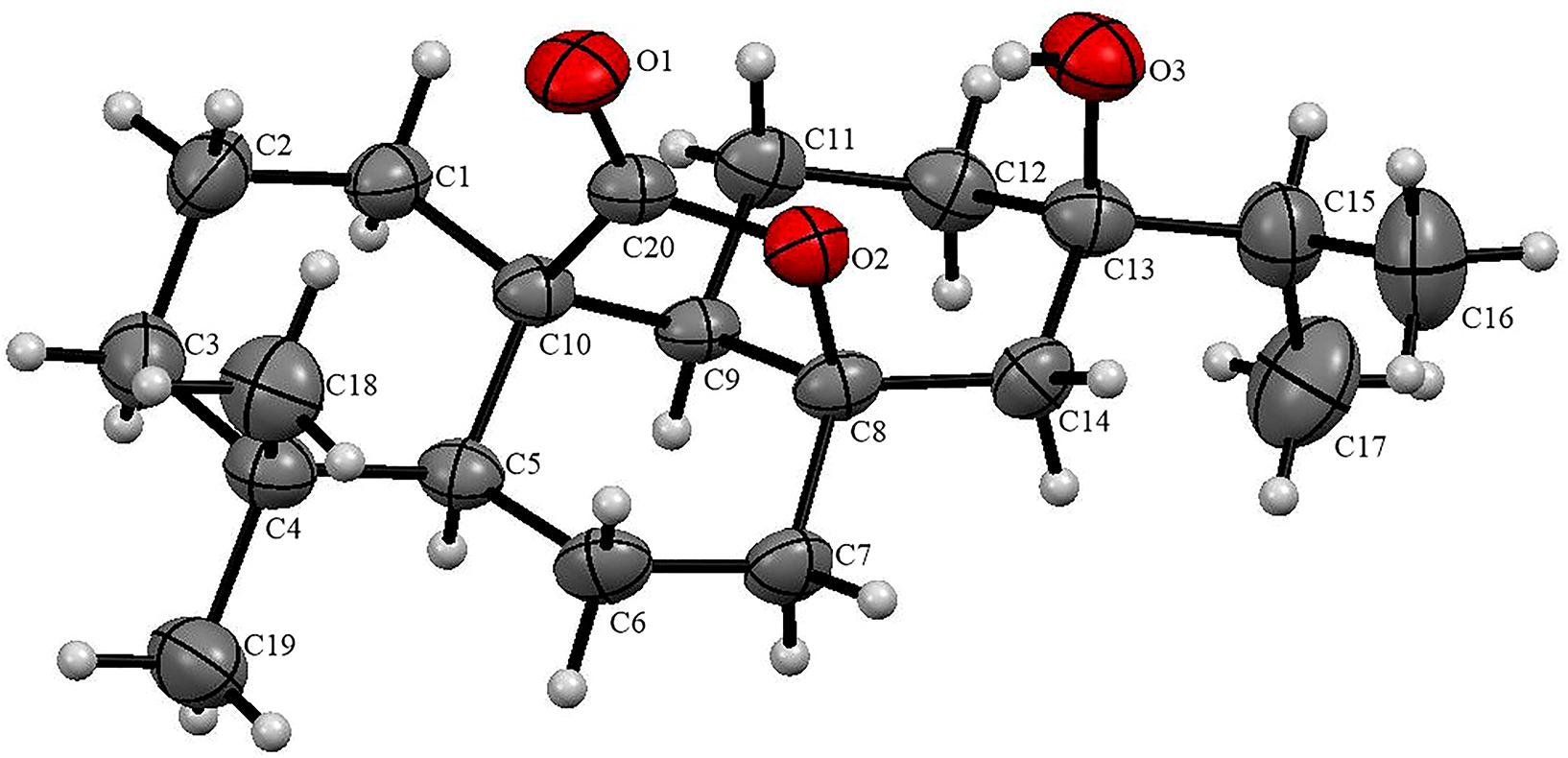
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.24 × 0.21 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 31.0°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 5323, 4825, 0.019 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2583 |
| N(param) refined: | 213 |
| Programs: | Olex2 [1], Bruker [2], SHELX [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| O1 | 0.6734 (3) | 0.2919 (2) | 0.3986 (2) | 0.0530 (7) |
| C1 | 0.3929 (4) | 0.4837 (3) | 0.4476 (3) | 0.0411 (9) |
| H1A | 0.380995 | 0.412992 | 0.504986 | 0.049* |
| H1AB | 0.333720 | 0.558234 | 0.480651 | 0.049* |
| C2 | 0.3081 (5) | 0.4447 (4) | 0.3289 (3) | 0.0510 (9) |
| H2A | 0.356020 | 0.363558 | 0.300538 | 0.061* |
| H2AB | 0.188054 | 0.431422 | 0.342639 | 0.061* |
| O2 | 0.8512 (3) | 0.4320 (2) | 0.4890 (2) | 0.0384 (6) |
| O3 | 0.9047 (4) | 0.3847 (2) | 0.7534 (2) | 0.0559 (7) |
| H3 | 0.882811 | 0.361071 | 0.684114 | 0.084* |
| C3 | 0.3325 (5) | 0.5482 (4) | 0.2333 (4) | 0.0515 (10) |
| H3A | 0.280874 | 0.628338 | 0.261039 | 0.062* |
| H3AB | 0.274820 | 0.521785 | 0.159642 | 0.062* |
| C4 | 0.5202 (5) | 0.5734 (4) | 0.2045 (3) | 0.0441 (9) |
| C6 | 0.8089 (4) | 0.6237 (4) | 0.3073 (3) | 0.0449 (9) |
| H6A | 0.855041 | 0.544152 | 0.273758 | 0.054* |
| H6AB | 0.829594 | 0.692786 | 0.249438 | 0.054* |
| C5 | 0.6175 (4) | 0.6076 (3) | 0.3242 (3) | 0.0355 (8) |
| H5 | 0.576065 | 0.693593 | 0.347961 | 0.043* |
| C8 | 0.8441 (4) | 0.5697 (3) | 0.5310 (3) | 0.0351 (8) |
| C7 | 0.9012 (4) | 0.6552 (4) | 0.4258 (3) | 0.0417 (9) |
| H7A | 1.021935 | 0.643993 | 0.413791 | 0.050* |
| H7AB | 0.881165 | 0.745389 | 0.446456 | 0.050* |
| C9 | 0.6529 (4) | 0.5857 (3) | 0.5513 (3) | 0.0338 (7) |
| H9 | 0.622414 | 0.677940 | 0.551002 | 0.041* |
| C10 | 0.5819 (4) | 0.5177 (3) | 0.4360 (3) | 0.0336 (7) |
| C11 | 0.5965 (4) | 0.5222 (4) | 0.6700 (3) | 0.0420 (9) |
| H11A | 0.480102 | 0.546565 | 0.686523 | 0.050* |
| H11B | 0.600275 | 0.428615 | 0.660794 | 0.050* |
| C12 | 0.7069 (4) | 0.5615 (4) | 0.7772 (3) | 0.0440 (9) |
| H12A | 0.664368 | 0.519595 | 0.849889 | 0.053* |
| H12B | 0.698090 | 0.654564 | 0.788960 | 0.053* |
| C13 | 0.8931 (4) | 0.5255 (3) | 0.7605 (3) | 0.0412 (8) |
| C14 | 0.9573 (4) | 0.5852 (3) | 0.6417 (3) | 0.0416 (8) |
| H14A | 0.975277 | 0.677280 | 0.655101 | 0.050* |
| H14B | 1.066516 | 0.547096 | 0.623234 | 0.050* |
| C15 | 1.0019 (5) | 0.5633 (4) | 0.8717 (3) | 0.0563 (10) |
| H15 | 0.958460 | 0.513253 | 0.940575 | 0.068* |
| C16 | 1.1877 (5) | 0.5248 (6) | 0.8562 (5) | 0.0846 (17) |
| H16A | 1.194572 | 0.435086 | 0.833299 | 0.127* |
| H16B | 1.246856 | 0.537841 | 0.931250 | 0.127* |
| H16C | 1.238324 | 0.577467 | 0.794132 | 0.127* |
| C17 | 0.9914 (6) | 0.7060 (5) | 0.9077 (5) | 0.0847 (16) |
| H17A | 1.033624 | 0.758815 | 0.842748 | 0.127* |
| H17B | 1.058302 | 0.720423 | 0.979171 | 0.127* |
| H17C | 0.875658 | 0.728469 | 0.923976 | 0.127* |
| C18 | 0.5942 (5) | 0.4546 (4) | 0.1382 (4) | 0.0594 (11) |
| H18A | 0.527554 | 0.436426 | 0.067410 | 0.089* |
| H18B | 0.592320 | 0.380856 | 0.191370 | 0.089* |
| H18C | 0.708762 | 0.472645 | 0.114584 | 0.089* |
| C19 | 0.5287 (5) | 0.6915 (4) | 0.1180 (4) | 0.0611 (12) |
| H19A | 0.644044 | 0.706320 | 0.094445 | 0.092* |
| H19B | 0.485736 | 0.766999 | 0.158745 | 0.092* |
| H19C | 0.461467 | 0.674460 | 0.047236 | 0.092* |
| C20 | 0.6995 (4) | 0.4006 (3) | 0.4358 (3) | 0.0361 (8) |
Source of material
Lophanic acid (100 mg, 0.3125 mmol), triphenylphosphine (PPh3, 164 mg, 0.625 mmol), I2 (40 mg, 0.1562 mmol), K2CO3 (86 mg, 0.625 mmol) were dissolved in methylene chloride (DCM, 2 mL). The resulting mixture was stirred at room temperature for 0.5 h until the starting materials were completely transformed. When the reaction was complete, checked by thin-layer chromatography (TLC) analysis, after termination by pure water (30 mL), the reaction was extracted with methylene chloride (3*90 mL). The combined organic phase was dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography eluting with petroleum ether/ethyl acetate (10:1) to afford compound (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H, 6H-8a,4a-(epoxymethano) phenanthren-12-one in 85% yield. And the obtained solid was recrystallized with methanol to obtain the colorless needle crystal.
Experimental details
Comment
Lophanic acid is an abietane diterpenoid compound widely seen in medicinal plants I. flavidus [5] and I. lophanthoides [6]. Studies have revealed that this type of compound has significant antibacterial, anti-inflammatory, antitumor, anti-cancer, and other activities [7], [8], [9], [10], [11], [12]. The research group started from medicinal plants I. flavidus isolated rich in lophanic acid. Nevertheless, the structural modification of the active functional groups and targets of lophanic acid has not been reported in the relevant literature. Hence, to investigate the active site of lophanic acid and synthesize a highly active derivative, we used lophanic acid as a raw material. Under the action of triphenylphosphine/iodine/potassium carbonate, the C8–C9 double bond of corinoic acid was oxidized to a hydroxyl group, where the C-8 hydroxyl group formed a lactone with C-20 carboxylic acid. Finally, the target compound is obtained. The title compound (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano) phenanthren-12-one was obtained by Lophanic acid oxidation, esterification reaction.
The title compound is shown in the figure. The bond lengths and angles which were derived from the title structure are within normal ranges. In the molecule, the carbonyl group was confirmed by the distance d(C20–O1) = 1.210(4) Å. The compound contains three six membered carbon-based rings, one hydroxyl, five methyl and one carbonyl.
Funding source: National Natural Science Foundation of China
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The Technology Fund of Guizhou Administration ofTraditional Chinese Medicine [grant number QZYY-2022-019] and National Natural Science Foundation of China (82204605).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
2. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Jiang, B., Lu, Z. Q., Zhang, J. H., Zhao, Q. S., Sun, H. D. Diterpenoids from Isodon Lophanthoides. Fitoterapia 2000, 71, 360–364; https://doi.org/10.1016/s0367-326x(00)00126-x.Suche in Google Scholar PubMed
6. Zhao, M. Z., Li, J. Q., Zhang, Y., Zhang, X. J., Jiang, B. Study on chemical constituents from rhizome of Rabdosia flavida. Zhong Yao Cai 2014, 37, 1193–1196.Suche in Google Scholar
7. Zou, J., Pan, L. T., Li, Q. J., Pu, J. X., Yao, P., Zhu, M., Banas, J. B., Zhang, H. J., Sun, H. D. Rubesanolides C–E abietane diterpenoids isolated from Isodon rubescens and evaluation of their anti-biofilm activity. Org. Biomol. Chem. 2012, 26, 5039–5044; https://doi.org/10.1039/c2ob25192b.Suche in Google Scholar PubMed
8. Li, J. X., Li, Q. J., Guan, Y. F., Song, X., Liu, Y. H., Zhang, J. J., Li, W. F., Du, J., Zhu, M., Banas, J. A., Li, X. N., Pan, L. T., Zhang, H. J. Discovery of antifungal constituents from the miao medicinal plant Isodon Flavidus. J. Ethnopharmacol. 2016, 191, 372–378; https://doi.org/10.1016/j.jep.2016.06.046.Suche in Google Scholar PubMed
9. Yang, L. B., Li, L., Huang, S. X., Pu, J. X., Zhao, Y., Ma, Y. B., Chen, J. J., Leng, C. H., Tao, Z. M., Sun, H. D. Anti-hepatitis B virus and cytotoxic diterpenoids from Isodon lophanthoides var. gerardianus. Chem. Pharm. Bull. 2011, 9, 1102–1105; https://doi.org/10.1248/cpb.59.1102.Suche in Google Scholar PubMed
10. Wan, J., Jiang, H. Y., Tang, J. W., Li, X. R., Du, X., Li, Y., Sun, H. D., Pu, J. X. Ent-abietanoids isolated from Isodon serra. Molecules 2017, 22, 309–319; https://doi.org/10.3390/molecules22020309.Suche in Google Scholar PubMed PubMed Central
11. Zhao, W., Pu, J. X., Du, X., Su, J., Li, X. N., Yang, J. H., Xue, Y. B., Li, Y., Xiao, W. L., Sun, H. D. Structure and cytotoxicity of diterpenoids from Isodon adenolomus. Chin. J. Nat. Med. 2021, 4, 253–258.10.1016/S1875-5364(11)60060-5Suche in Google Scholar
12. Qin, S., Chen, S. H., Guo, Y. W., Gu, Y. C. Diterpenoids of Isodon macrophylla. Helv. Chim. Acta 2007, 10, 2041–2046; https://doi.org/10.1002/hlca.200790212.Suche in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

