Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
-
Svetlana Belošević
, Vukadin M. Leovac
, Mirjana M. Radanović
, Milica G. Bogdanović
, Nikola D. Radnović
und Marko V. Rodić
Abstract
C6H23I2N6NiO2.50S2, monoclinic, C2/c (no. 15), a = 8.2282(4) Å, b = 21.9200(7) Å, c = 11.4906(4) Å, β = 109.451(4)°, V = 1954.19(14) Å3, Z = 4, Rgt (F) = 0.0322, wRref (F 2) = 0.1113, T = 295.
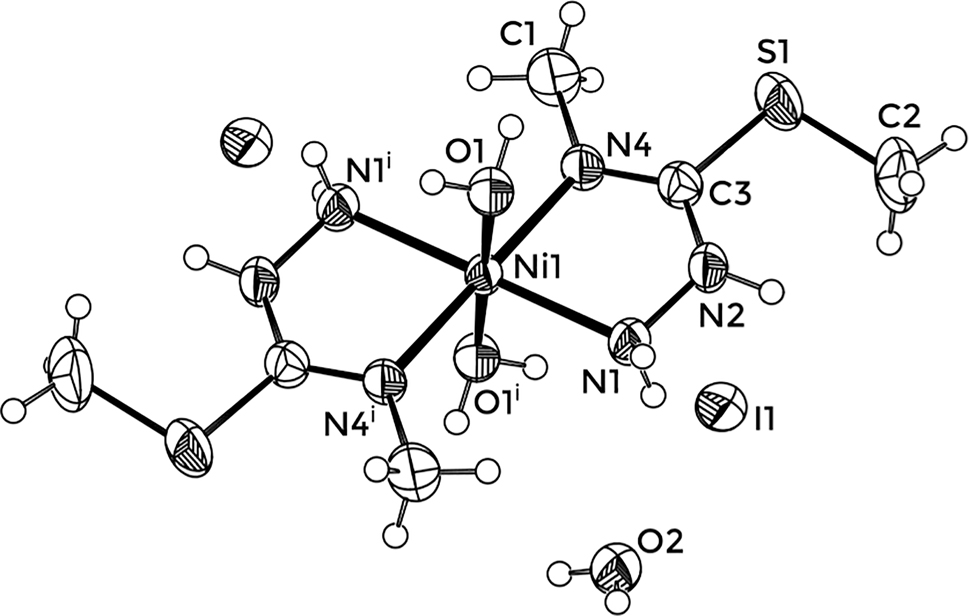
The title crystal structure is shown in the figure. Table 1 contains crystallographic data, Table 2 contains the list of the atoms including atomic coordinates and displacement parameters, and Table 3 contains selected structural parameters of title complex and closely related structures.
Data collection and handling.
| Crystal: | Purple prism |
| Size: | 0.49 × 0.18 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 4.38 mm−1 |
| Diffractometer, scan mode: | Gemini S, ω |
| θ max, completeness: | 29.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 4196, 4196, 0.034 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2990 |
| N(param)refined: | 114 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], PLATON [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| I1 | 0.72095(5) | 0.86708(2) | 0.46855(4) | 0.05177(17) |
| S1 | 0.2711(2) | 0.54413(7) | 0.54313(17) | 0.0626(4) |
| Ni1 | 0.250000 | 0.750000 | 0.500000 | 0.0323(2) |
| O1 | 0.5261(4) | 0.75062(17) | 0.5790(3) | 0.0423(8) |
| H1O1 | 0.578(7) | 0.7801(15) | 0.558(6) | 0.063* |
| H2O1 | 0.585(6) | 0.7216(16) | 0.561(6) | 0.063* |
| C1 | 0.2467(10) | 0.6310(3) | 0.3458(6) | 0.0684(19) |
| H1D | 0.227781 | 0.662027 | 0.283760 | 0.103* |
| H1E | 0.153729 | 0.602085 | 0.321218 | 0.103* |
| H1C | 0.353405 | 0.610437 | 0.355339 | 0.103* |
| N1 | 0.2386(5) | 0.7123(2) | 0.6647(4) | 0.0383(9) |
| H1A | 0.315(4) | 0.724(3) | 0.733(3) | 0.046* |
| H1B | 0.141(3) | 0.711(2) | 0.680(4) | 0.046* |
| C2 | 0.2285(11) | 0.5171(3) | 0.6771(8) | 0.088(2) |
| H2A | 0.123997 | 0.535209 | 0.680524 | 0.132* |
| H2B | 0.322427 | 0.528086 | 0.749695 | 0.132* |
| H2C | 0.216366 | 0.473530 | 0.672892 | 0.132* |
| N2 | 0.2738(6) | 0.6494(2) | 0.6647(4) | 0.0413(10) |
| H2 | 0.255(7) | 0.629(2) | 0.723(4) | 0.050* |
| C3 | 0.2626(6) | 0.6239(2) | 0.5540(5) | 0.0369(10) |
| N4 | 0.2544(5) | 0.65887(19) | 0.4628(4) | 0.0383(9) |
| O2a | 0.000000 | 0.7838(5) | 0.750000 | 0.047(2) |
| H2O2a | 0.046721 | 0.804361 | 0.811687 | 0.071* |
-
aOccupancy: 0.5.
Comparison of selected structural parameters of Ni(II) complexes with N 4-methyl-S-methylisothiosemicarbazide (L), and S-methylisothiosemicarbazide (L1) ligands. Atom labels used correspond to the title complex.
| [NiL2(H2O)2]I2·0.5H2O | [Ni(L1)2(H2O)2](tph)·2H2O | [Ni(L1)2]I2 | [Ni(L1)2]I2 | |
|---|---|---|---|---|
| CSD refcode | N.A. | FERLAQ | MCBNIA01 | MCBNIA |
| CCDC No. | N.A. | 262776 | 680517 | 1210100 |
| Reference | This work | [13] | [12] | [11] |
| d(Ni1–N1)/Å | 2.095(4) | 2.094(3) | 1.9119(16) | 1.923(10) |
| d(Ni1–N4)/Å | 2.046(4) | 2.027(3) | 1.8624(17) | 1.873(11) |
| d(Ni1–O1)/Å | 2.147(3) | 2.136(3) | – | – |
| d(N1–N2)/Å | 1.410(6) | 1.425(3) | 1.427(2) | 1.429(12) |
| d(N2–C3)/Å | 1.363(7) | 1.359(4) | 1.350(3) | 1.315(12) |
| d(C3–N4)/Å | 1.282(6) | 1.274(5) | 1.300(2) | 1.284(12) |
| ∠(N4–Ni1–N1)/° | 79.23(16) | 80.15(12) | 84.24(7) | 84.1(6) |
| ∠(N1–Ni1–O1)/° | 91.14(14) | 91.85(12) | – | – |
| ∠(N4–Ni1–O1)/° | 90.26(15) | 92.68(11) | – | – |
Source of material
The mixture of Ni(OAc)2·4H2O (0.1 mmol) and N 4-methyl-S-methylisothiosemicarbazide-hydrogeniodide, L·HI, (0.2 mmol) is disolved in warm EtOH (5 mL). The solution is spontaneously evaporated to a small volume. Bluish-purple single crystals are filtered and washed with EtOH. Non-commercial ligand L·HI is obtained in the reaction of N 4-methyl-thiosemicarbazide and methyl-iodide in absolute ethanol during reflux for 45 min.
Experimental details
The used crystal specimen was a non-merohedral twin. Upon determination of the twin law, simultaneous integration of reflecton intensities was performed. Deconvoluted intensity data (SHELX HKLF 4 format) were used for structure solution, while refinement was performed against 4152 merged reflections in SHELX HKLF 5 format.
Coordinates of carbon-bonded hydrogen atoms were introduced in idealized positions and refined using riding model. Their U iso values are approximated as U iso = kU eq of the parent atom (k = 1.2 for sp 2 and 1.5 for sp 3 hybridized atoms). Locations of hydrogen atoms bonded to heteroatoms were determined from residual electron density maps and were refined with distance restraints. Their U iso values are approximated as U iso = kU eq of the parent atom (k = 1.2 for nitrogen- and 1.5 for oxygen-bonded atoms). Non-coordinated water molecule was refined as a rigid group.
Comment
Introduction
Numerous complexes of transition metals with derivatives of S-alkylisothiodemicarbazide are synthesized and characterized by single crystal X-ray diffraction [5]. Besides the interesting metal-induced reactions of ligands and unusual open chain and macrocyclic structures, some of these tetra- and octadentate ligands can stabilize Cu(III), Ni(III), and Fe(IV) ions [6], [7], [8], [9], [10].
In Refs. [11, 12] the structure of trans-square-planar Ni(II) complex with NN bidentate S-methylisothiosemicarbazide (L1), i.e. [Ni(L1)2]I2 is described. This complex is obtained in the reaction of EtOH solutions of Ni(OAc)2·4H2O and L1·HI in a 1:2 M ratio. In this paper, it is shown that under the same synthetic conditions, the reaction of Ni(OAc)2·4H2O and L·HI results in the formation of the trans-octahedral complex of the formula [NiL2(H2O)2]I2·0.5H2O.
Structural comment
The asymmetric unit of [NiL2(H2O)2]I2·0.5H2O consists of half of the complex cation situated at a special position of site symmetry
As already mentioned, the constitution of complex cation in [NiL2(H2O)2]I2·0.5H2O is different from that found in [Ni(L1)2]I2 [11, 12], even though syntheses were conducted under equivalent conditions. Significant differences are observed when metal–ligand bond lengths are compared to [Ni(L1)2]I2, where nickel(II) is in a square-planar environment composed of two S-methylisothiosemicarbazide ligands. Namely, in [Ni(L1)2]I2, both metal ligand bonds are shorter, and the chelate bite angle is larger. On the other hand, intraligand bond lengths of the common type in all mentioned complexes have similar values. This indicates that methylation of N4 did not significantly change the ligand structure.
A complex network of hydrogen bonds is present in the crystal structure, where all potential hydrogen bond donors are involved in bonding.
Funding source: Ministry of Science, Technological Development and Innovation of the Republic of Serbia N.A.
Award Identifier / Grant number: 451-03-47/2023-01/200125
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The authors acknowledge the financial support of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant No. 451-03-47/2023-01/200125).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO Software System; Rigaku Corporation: Wroclaw, Poland, 2022.Suche in Google Scholar
2. Sheldrick, G. M. SHELXT – integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXT. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. https://doi.org/10.1107/S090744490804362X.Suche in Google Scholar PubMed PubMed Central
5. Arion, V. B. Coordination chemistry of S-substituted isothiosemicarbazides and isothiosemicarbazones. Coord. Chem. Rev. 2019, 387, 348–397. https://doi.org/10.1016/j.ccr.2019.02.013.Suche in Google Scholar
6. Knof, U., Weyhermüller, T., Wolter, T., Wieghardt, K. Synthesis of low spin [MnII(L2)2]I2·2MeOH and [CuIII(L1)] via condensation of S-methylisothiosemicarbazide and pentane-2,4-dione in the presence of air. J. Chem. Soc., Chem. Commun. 1993, 726–728. https://doi.org/10.1039/C39930000726.Suche in Google Scholar
7. Arion, V., Wieghardt, K., Weyhermueller, T., Bill, E., Leovac, V., Rufinska, A. Synthesis, structure, magnetism, and spectroscopic properties of some mono and dinuclear nickel complexes containing noninnocent pentane-2,4-dione bis(S-alkylisothiosemicarbazonate)-derived ligands. Inorg. Chem. 1997, 36, 661–669. https://doi.org/10.1021/ic960802o.Suche in Google Scholar
8. Gerbeleu, N. V., Simonov, Y. A., Arion, V. B., Leovac, V. M., Turta, K. I., Indrichan, K. M., Gradinaru, D. I., Zavodnik, V. E., Malinovskii, T. I. Transition metal complexes with thiosemicarbazide-based ligands. 14. Iron(IV) complexes with 2,4-pentanedione bis(S-alkylisothiosemicarbazone). Crystal and molecular structure of iodo{2,4-pentanedione bis(S-ethylisothiosemicarbazonato)(3–)}iron(IV). Inorg. Chem. 1992, 31, 3264–3268. https://doi.org/10.1021/ic00041a019.Suche in Google Scholar
9. Leovac, V. M., Jovanović, L. S., Češljević, V. I., Bjelica, L. J., Ević, N. J. Transition metal complexes with the thiosemicarbazide-based ligand – XVI. Novel iron(IV) complexes with pentane-2,4-dione bis(S-methylisothiosemicarbazone). Polyhedron 1992, 11, 1029–1035. https://doi.org/10.1016/S0277–5387(00)84470–8.10.1016/S0277-5387(00)84470-8Suche in Google Scholar
10. Leovac, V. M., Herak, R., Prelesnik, B., Niketic, S. R. Transition metal complexes with thiosemicarbazide-based ligands. Part 13. Synthesis and structure of μ-oxo-bis-{[pentane-2,4-dione bis(S-methylisothiosemicarbazonato-κ2N,N″) (3–)] iron(IV)}. J. Chem. Soc., Dalton Trans. 1991, 2295–2299. https://doi.org/10.1039/DT9910002295.Suche in Google Scholar
11. Obadović, D. Ž., Divjaković, V., Leovac, V. M. Transition metal complexes with the thiosemicarbazide-based ligands.—XXIX. Crystal and molecular structure of β-bis(S-methylisothiosemicarbazide)nickel(II) bromide and the energies of electronic states of NiII in similar structures. Polyhredon 1997, 16, 695–699. https://doi.org/10.1016/0277–5387(96)00283–5.10.1016/0277-5387(96)00283-5Suche in Google Scholar
12. Dobrov, A., Arion, V. B., Shova, S., Roller, A., Rentschler, E., Keppler, B. K. Spontaneous resolution of a triple-stranded dinickel(II) helicate generated via intermolecular transamination reaction of S-methylisothiocarbohydrazide in the presence of Ni2+. Eur. J. Inorg. Chem. 2008, 2008, 4140–4145. https://doi.org/10.1002/ejic.200800605.Suche in Google Scholar
13. Novaković, S. B., Bogdanović, G. A., Leovac, V. M. Transition metal complexes with thiosemicarbazide-based ligands. XLIV. The supramolecular arrangement in the Ni(II) complexes of S-methylisothiosemicarbazide. Inorg. Chem. Commun. 2005, 8, 9–13. https://doi.org/10.1016/j.inoche.2004.10.001.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

