Abstract
C22H16O5Pb, orthorhombic, P212121 (no. 19), a = 7.3395(8) Å, b = 10.7768(11) Å, c = 23.034(3) Å, V = 1821.9(3) Å3, Z = 4, R gt(F) = 0.0327, wR ref(F 2) = 0.0735, T = 296(2) K.
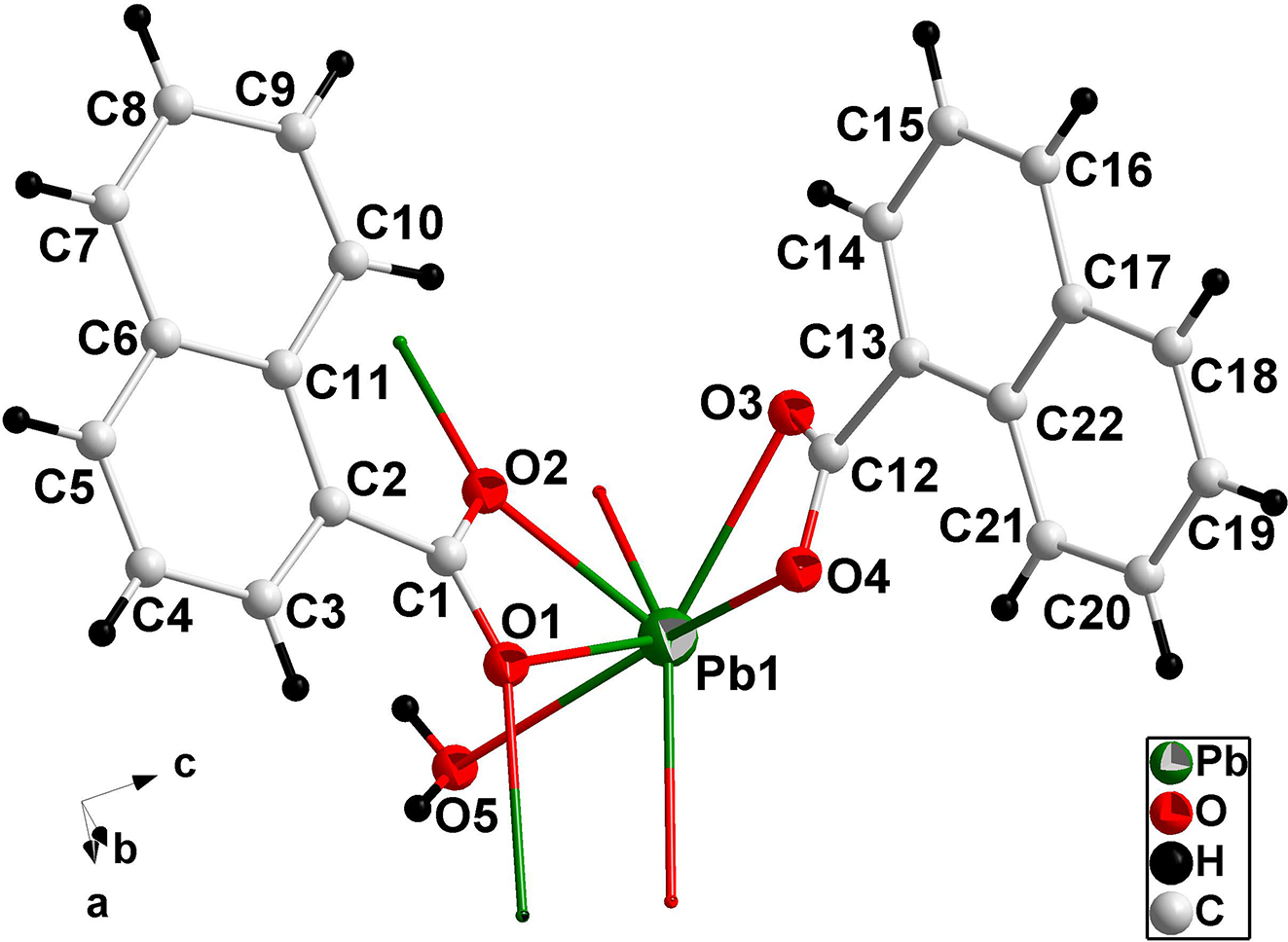
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size | 0.24 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 9.29 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 26.0°, >99% |
| N(hkl) measured, N(hkl) unique, R int: | 9946, 3545, 0.056 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 3423 |
| N(param) refined: | 255 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Pb1 | 0.73035 (4) | 0.80457 (3) | 0.03859 (2) | 0.02730 (12) |
| O1 | 0.8849 (9) | 0.6349 (7) | −0.0172 (3) | 0.0349 (17) |
| O2 | 0.5902 (9) | 0.6350 (6) | −0.0105 (3) | 0.0363 (17) |
| O3 | 0.5682 (9) | 0.6904 (9) | 0.1218 (3) | 0.0489 (18) |
| O4 | 0.8631 (9) | 0.6604 (7) | 0.1136 (3) | 0.0429 (19) |
| O5 | 0.7246 (11) | 0.8886 (7) | −0.0714 (3) | 0.0540 (19) |
| H5A | 0.807426 | 0.857132 | −0.088028 | 0.065* |
| H5B | 0.608026 | 0.882350 | −0.086578 | 0.065* |
| C1 | 0.7344 (11) | 0.5847 (8) | −0.0281 (3) | 0.0246 (17) |
| C2 | 0.7265 (14) | 0.4679 (7) | −0.0627 (4) | 0.0283 (18) |
| C3 | 0.8715 (14) | 0.4408 (9) | −0.0975 (5) | 0.038 (2) |
| H3 | 0.973597 | 0.491835 | −0.095887 | 0.046* |
| C4 | 0.8731 (17) | 0.3401 (11) | −0.1350 (5) | 0.052 (3) |
| H4 | 0.974594 | 0.324197 | −0.158033 | 0.063* |
| C5 | 0.7264 (18) | 0.2660 (9) | −0.1378 (5) | 0.049 (3) |
| H5 | 0.726359 | 0.199977 | −0.163848 | 0.058* |
| C6 | 0.5732 (14) | 0.2854 (9) | −0.1026 (5) | 0.037 (2) |
| C7 | 0.4220 (17) | 0.2047 (11) | −0.1052 (5) | 0.055 (3) |
| H7 | 0.422440 | 0.139519 | −0.131649 | 0.066* |
| C8 | 0.2777 (19) | 0.2204 (10) | −0.0703 (6) | 0.061 (3) |
| H8 | 0.178946 | 0.166566 | −0.072784 | 0.074* |
| C9 | 0.2754 (15) | 0.3184 (9) | −0.0297 (5) | 0.046 (2) |
| H9 | 0.175260 | 0.329211 | −0.005481 | 0.056* |
| C10 | 0.4197 (13) | 0.3972 (9) | −0.0260 (4) | 0.033 (2) |
| H10 | 0.417855 | 0.460002 | 0.001665 | 0.040* |
| C11 | 0.5726 (13) | 0.3865 (8) | −0.0631 (4) | 0.030 (2) |
| C12 | 0.7147 (14) | 0.6400 (9) | 0.1386 (4) | 0.033 (2) |
| C13 | 0.7076 (14) | 0.5518 (8) | 0.1896 (4) | 0.033 (2) |
| C14 | 0.5507 (16) | 0.4857 (10) | 0.1966 (5) | 0.045 (3) |
| H14 | 0.452933 | 0.499507 | 0.171710 | 0.054* |
| C15 | 0.5354 (17) | 0.3962 (10) | 0.2412 (6) | 0.050 (3) |
| H15 | 0.429416 | 0.349459 | 0.244642 | 0.060* |
| C16 | 0.6722 (17) | 0.3786 (11) | 0.2785 (5) | 0.047 (3) |
| H16 | 0.659402 | 0.320919 | 0.308289 | 0.057* |
| C17 | 0.8351 (15) | 0.4461 (8) | 0.2734 (4) | 0.036 (2) |
| C18 | 0.9783 (18) | 0.4313 (10) | 0.3134 (5) | 0.049 (3) |
| H18 | 0.965086 | 0.373330 | 0.342974 | 0.059* |
| C19 | 1.1332 (18) | 0.4973 (12) | 0.3106 (6) | 0.057 (3) |
| H19 | 1.225637 | 0.484550 | 0.337564 | 0.068* |
| C20 | 1.1533 (16) | 0.5850 (12) | 0.2668 (5) | 0.051 (3) |
| H20 | 1.259481 | 0.631894 | 0.264884 | 0.061* |
| C21 | 1.0203 (15) | 0.6033 (10) | 0.2268 (5) | 0.041 (3) |
| H21 | 1.038048 | 0.661518 | 0.197568 | 0.049* |
| C22 | 0.8547 (15) | 0.5354 (9) | 0.2286 (4) | 0.034 (2) |
1 Source of material
All chemicals were purchased from commercial sources and used as received. In a typical experiment 2 mmol 1-naphthoic acid and 1 mmol lead acetate were added in CH3OH (50 mL) and refluxed with agitating for 11 h. Then, the reaction solution was filtered. Finally, the title crystal was precipitated by controlling solvent volatilization.
2 Experimental details
All H-atoms bonded to C atoms were placed geometrically and refined using a riding model with common isotropic displacement factors U iso(H) = 1.2 or 1.5 U eq (parent C-atom). The absolute structure was established by refinement of the Flack parameter (−0.018(16) from 1350 selected quotients) using Parsons’ method [5].
3 Comment
In recent years, rational designs and syntheses of coordination polymer of lead have attracted wide attention, owing to their rich structural aesthetics and functionalities [6], [7], [8], [9]. Aromatic organic carboxylic acids are widely used as ligands to construct inorganic and organic hybrid materials [10–11]. The different coordination modes make the expected structures much more robust. Deprotonated carboxylate groups can form hydrogen bonds to participate in supermolecular self-assembly with coordination bonds as acceptors [12]. So, the synthesis and crystal structure of the title compound are of great significance to studying the structure and function.
Single-crystal structure analysis reveals that the title compound crystallizes in the orthorhombic space group P212121 and exhibits a chain structure (see the Figure). The Pb(II) ion is seven-coordinated by six carboxylate oxygen atoms from four different 1-naphthalene carboxyl and one water molecule. The Pb–O bond lengths and O–Pb–O angles, all of which are within the range of those observed for other analogical Pb complexes [13, 14], are ranging from 2.382(7) to 2.796(7) Å and 51.1(2)° to 149.2(2)°, respectively.
Intramolecular and intermolecular hydrogen bonds exist in the crystal structure of the title compound. In an infinite chain, the oxygen atom O5 provides one intramolecular hydrogen bond to O4′ and O3″ (O5···O4′ = 2.874(1) Å; O5···O3″ = 2.903(1) Å; ′ = x − 0.5, −y + 1.5, −z; ″ = x + 0.5, −y + 1.5, −z).
It should not be unmentioned that the water free analogous structure has been already reported [15].
Funding source: Hunan Provincial Natural Science Foundation of China
Award Identifier / Grant number: 2021JJ40010
Funding source: College Students Innovation and Entrepreneurship Training Program of Hunan Province General Project
Award Identifier / Grant number: cxcy2022011
Funding source: Science and Technology Plan Project of Hengyang City
Award Identifier / Grant number: 202002042081
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Hunan Provincial Natural Science Foundation of China (No. 2021JJ40010), and College Students Innovation and Entrepreneurship Training Program of Hunan Province General Project (No. cxcy2022011), Science and Technology Plan Project of Hengyang City (No. 202002042081).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Parsons, S., Flack, H. D., Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, B69, 249–259; https://doi.org/10.1107/s2052519213010014.Search in Google Scholar PubMed PubMed Central
6. Martínez-Casado, F. J., Ramos-Riesco, M., Rodríguez-Cheda, J. A., Cucinotta, F., Matesanz, E., Miletto, I., Gianotti, E., Marchese, L., Mateěj, Z. Unraveling the decomposition process of lead(II) acetate: anhydrous polymorphs, hydrates, and byproducts and room temperature phosphorescence. Inorg. Chem. 2016, 55, 8576–8586; https://doi.org/10.1021/acs.inorgchem.6b01116.Search in Google Scholar PubMed
7. Kowalik, M., Masternak, J., Kazimierczuk, K., Kupcewicz, B., Khavryuchenkod, O. V., Barszcza, B. Exploring thiophene-2-acetate and thiophene-3-acetate binding modes towards the molecular, supramolecular structures and photoluminescence properties of Pb(II) polymers. CrystEngComm 2020, 22, 7025–7035; https://doi.org/10.1039/d0ce01224f.Search in Google Scholar
8. Chen, Y., Hu, C. L., Fang, Z., Li, Y. L., Mao, J. G. From Pb(H2C3N3O3)(OH) to Pb(H2C3N3O3)F: homovalent anion substitution-induced band gap enlargement and birefringence enhancement. Inorg. Chem. 2022, 61, 1778–1786; https://doi.org/10.1021/acs.inorgchem.1c03711.Search in Google Scholar PubMed
9. Kamakura, Y., Chinapang, P., Masaoka, S., Saeki, A., Ogasawara, K., Nishitani, S. R., Yoshikawa, H., Katayama, T., Tamai, N., Sugimoto, K., Tanaka, D. Semiconductive nature of lead-based metal-organic frameworks with three-dimensionally extended sulfur secondary building units. J. Am. Chem. Soc. 2020, 142, 27–32; https://doi.org/10.1021/jacs.9b10436.Search in Google Scholar PubMed
10. Fu, F., Chen, S.-P., Ren, Y.-X., Gao, S.-L. Single crystal structures and fluorescence property of a series of lanthanide coordination polymers incorporating isophthalate. Acta Chim. Sinica 2008, 66, 1663–1668.Search in Google Scholar
11. Yang, J., Miao, J., Dai, J., Yang, L., Zhang, L.-N. Syntheses, crystal structures and properties of two lead(II) complexes with para-substituted benzoic acid and 2,2′-bipyridine co-ligand. Chinese J. Inorg. Chem. 2010, 26, 1992–2000.Search in Google Scholar
12. Shen, W., Hu, W.-J., Wu, X.-Y., Zhao, G.-L. Syntheses, structures and DNA interaction of Zn(II) and Pb(II) complexes based on imidazo-phenanthrolin-phenoxy acetic acid. Chinese J. Inorg. Chem. 2016, 32, 1101–1110.Search in Google Scholar
13. Sarma, R., Jubaraj, B., Baruah, J. B. Solvent coordination in changing dimensionality of lead benzoate coordination polymers. Inorg. Chim. Acta 2009, 362, 4977–4984; https://doi.org/10.1016/j.ica.2009.07.032.Search in Google Scholar
14. Xue, J.-L., Tian, C.-H. Lead(II) coordination polymer with 4-chlorophenoxyacetate as ligand: structure, luminescence and biological bctivity. Chinese J. Inorg. Chem. 2014, 30, 907–912.Search in Google Scholar
15. Hammer, A. C., Jia, X., Zeller, M., Coughlin, E. J., Zhang, W., Oertel, C. M. Ligand geometry directs the packing and symmetry of one-dimensional helical motifs in lead oxide naphthoates and biphenylcarboxylates. CrystEngComm 2020, 22, 6465–6477; https://doi.org/10.1039/d0ce01150a.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

