Abstract
C14H19ClN2O, monoclinic, P21/c (no. 14), a = 12.8823(9) Å, b = 9.1617(6) Å, c = 11.7421(9) Å, β = 98.377(7)°, V = 1371.06(17) Å3, Z = 4, R gt (F) = 0.0678, wR ref (F2) = 0.1912, T = 293 K.
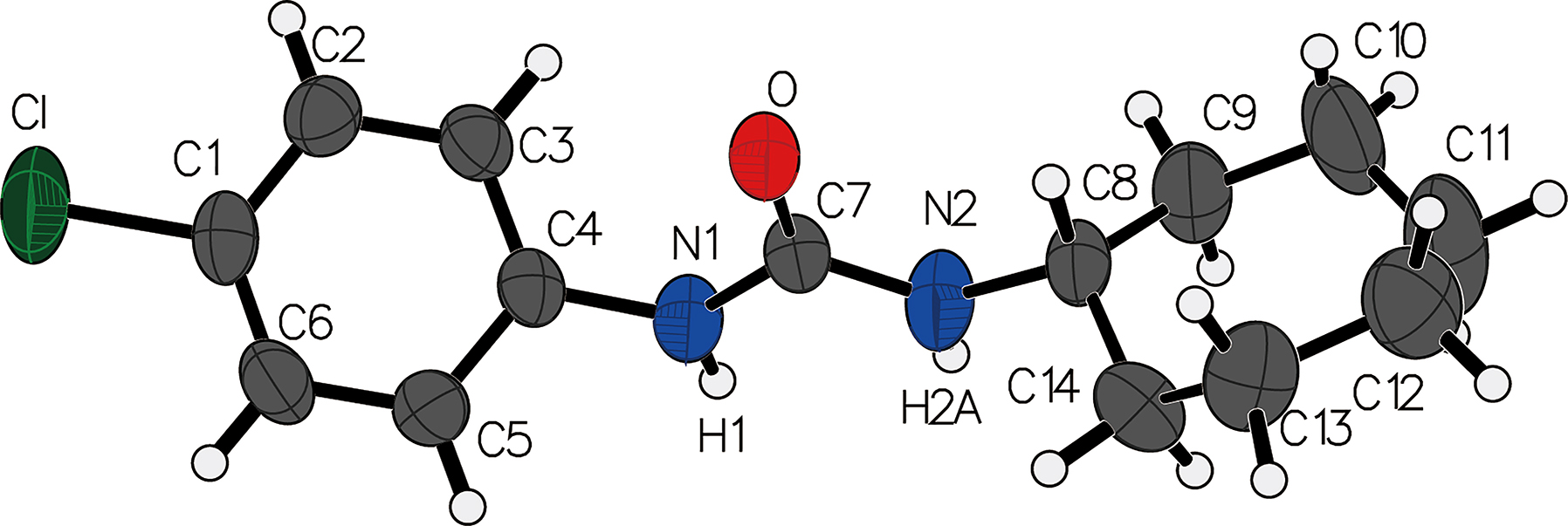
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.43 × 0.37 × 0.33 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θ max, completeness: | 29.3°, >99% |
| N(hkl)measured , N(hkl)unique, R int: | 6456, 3150, 0.028 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2039 |
| N(param)refined: | 164 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.1781 (2) | 0.4030 (3) | 0.4453 (3) | 0.0486 (7) |
| C2 | 0.2794 (2) | 0.4315 (3) | 0.4254 (3) | 0.0486 (7) |
| H2 | 0.291555 | 0.476607 | 0.357692 | 0.058* |
| C3 | 0.3621 (2) | 0.3917 (3) | 0.5081 (2) | 0.0446 (7) |
| H3 | 0.430601 | 0.408966 | 0.495354 | 0.053* |
| C4 | 0.34414 (19) | 0.3265 (3) | 0.6092 (2) | 0.0379 (6) |
| C5 | 0.2420 (2) | 0.3018 (3) | 0.6283 (2) | 0.0479 (7) |
| H5 | 0.229353 | 0.258863 | 0.696696 | 0.057* |
| C6 | 0.1587 (2) | 0.3408 (3) | 0.5457 (3) | 0.0532 (8) |
| H6 | 0.090075 | 0.324678 | 0.558506 | 0.064* |
| C7 | 0.50912 (18) | 0.3610 (3) | 0.7407 (2) | 0.0370 (6) |
| C8 | 0.6651 (2) | 0.3622 (3) | 0.8919 (2) | 0.0451 (7) |
| H8 | 0.669120 | 0.463511 | 0.866093 | 0.054* |
| C9 | 0.7669 (2) | 0.2875 (4) | 0.8757 (3) | 0.0643 (9) |
| H9A | 0.773082 | 0.288147 | 0.794352 | 0.077* |
| H9B | 0.763358 | 0.186385 | 0.899297 | 0.077* |
| C10 | 0.8661 (3) | 0.3563 (6) | 0.9422 (4) | 0.1024 (15) |
| H10A | 0.925935 | 0.303451 | 0.921673 | 0.123* |
| H10B | 0.870803 | 0.455419 | 0.914420 | 0.123* |
| C11 | 0.8777 (3) | 0.3624 (6) | 1.0671 (4) | 0.1075 (16) |
| H11A | 0.859929 | 0.266700 | 1.093934 | 0.129* |
| H11B | 0.951504 | 0.378125 | 1.095035 | 0.129* |
| C12 | 0.8192 (3) | 0.4684 (5) | 1.1226 (4) | 0.0837 (12) |
| H12A | 0.848163 | 0.563538 | 1.109187 | 0.100* |
| H12B | 0.834174 | 0.450403 | 1.204743 | 0.100* |
| C13 | 0.7024 (3) | 0.4804 (4) | 1.0927 (3) | 0.0719 (10) |
| H13A | 0.687135 | 0.574357 | 1.055995 | 0.086* |
| H13B | 0.673207 | 0.481949 | 1.164285 | 0.086* |
| C14 | 0.6436 (2) | 0.3654 (4) | 1.0160 (3) | 0.0612 (9) |
| H14A | 0.568988 | 0.380620 | 1.015417 | 0.073* |
| H14B | 0.660849 | 0.270429 | 1.050067 | 0.073* |
| Cl | 0.07362 (7) | 0.44725 (11) | 0.33836 (8) | 0.0803 (4) |
| N1 | 0.42754 (17) | 0.2769 (2) | 0.6918 (2) | 0.0471 (6) |
| H1 | 0.426672 | 0.186938 | 0.712617 | 0.057* |
| N2 | 0.57934 (18) | 0.2915 (2) | 0.8172 (2) | 0.0553 (7) |
| H2A | 0.573433 | 0.198385 | 0.822662 | 0.066* |
| O | 0.51665 (14) | 0.49159 (18) | 0.71584 (17) | 0.0465 (5) |
Source of materials
1-Chloro-4-isocyanatobenzene and cycloheptylamine were purchased from Aladdin Co. Ltd. and used as received. The 1-(4-chlorophenyl)-3-cycloheptylurea was synthesized using a modified literature procedure [5]. In a dry flask, a mixture of 1-chloro-4-isocyanatobenzene (0.15 g, 1 mmol) and cycloheptylamine (0.11 g, 1 mmol) was stirred in dichloromethane (15 mL). The reaction was monitored by thin-layer chromatography (TLC) on silica gel plates. The resulting precipitate was dried under vacuum at room temperature. The solution was prepared by dissolving 1-(4-chlorophenyl)-3-cycloheptylurea (0.05 g) in ethanol (10 mL) and stirring the solution at room temperature for 20 minutes. The solution was then placed in a vial and left to evaporate at room temperature. The crystals were obtained and collected after three days.
Experimental details
The structure was solved by Direct Methods and refined using the SHELXL program [3]. The structure refinement, and the graphical representation of the structure were performed using the OLEX2 software package [2].
Comment
Urea derivatives have been used as a starting material in the synthesis of a variety of heterocyclic compounds [6]. Many urea derivatives crystals have been reported [7], [8], [9], [10], [11], [12]. In these crystal structures, the hydrogen bond between molecules is the main force for the stacking of molecules into crystals.
1-(4-Chlorophenyl)-3-cycloheptylurea crystallizes in the monoclinic space group P21/c with a unit cell containing four molecules. The structure is stabilized by intermolecular hydrogen bonds. The CPCU molecule consists of a cycloheptylurea ring, with a 4-chlorophenyl group attached to the nitrogen atom. The 4-chlorophenyl group is twisted away from the ring plane with a dihedral angle of 55.4°.
When compared to the isostructural bromocompound of the title compound, 1-(4-bromophenyl)-3-cycloheptylurea (BPBU) [13], the crystal structure of CPCU is similar in many respects. Both molecules contain a cycloheptylurea ring with a phenyl group attached to the nitrogen atom. In CPCU and BPBU, the 4-chlorophenyl group is twisted away from the ring plane with a similar angle. However, the crystal structures of the two molecules differ in the C–X (X=Cl or Br) bonds. The C–Cl bond (1.749(3) Å) is shorter than that of the C–Br bond (1.905(4) Å).
The crystal structure is stabilized by strong intermolecular interactions from hydrogen bonds (N1–H1–O) and (N2–H2A–O). The hydrogen bonds are formed between the hydrogen atoms attached to the nitrogen atoms of the cycloheptylurea ring. The angles of these two hydrogen bonds are 158.07(15)° and 151.13(16)°, and the length are 2.0648(17) Å and 2.2333(17) Å.
Funding source: National College Students’ Innovation and Entrepreneurship Training Program http://dx.doi.org/10.13039/501100013254
Award Identifier / Grant number: 202213277014
Funding source: K. C. Wong Magna Fund in Ningbo University http://dx.doi.org/10.13039/501100020738
Funding source: HuLan Outstanding Doctor Fund in Ningbo University http://dx.doi.org/10.13039/501100004387
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by the National College Students’ Innovation and Entrepreneurship Training Program, China (No. 202213277014). Contract grant sponsors: K. C. Wong Magna Fund and HuLan Outstanding Doctor Fund in Ningbo University.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton: Oxfordshire, England, 2017.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. SHELXTL – Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
5. Zhu, Z., Zhang, L. The crystal structure of 1-(4-bromophenyl)-3-(2-chlorobenzyl)urea, C14H12BrClN2O. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 13–14; https://doi.org/10.1515/ncrs-2022-0456.Suche in Google Scholar
6. Ghosh, A. K., Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2019, 63, 2751–2788; https://doi.org/10.1021/acs.jmedchem.9b01541.Suche in Google Scholar PubMed PubMed Central
7. Ali, H., Halim, S. N. A., Khamis, N. A., Yusof, M. S., Yamin, B. M. N-(p–Methoxybenzoyl)-N’-(o-methoxyphenyl) thiourea. Acta. Crystallogr. 2004, E60, o1497–o1498; https://doi.org/10.1107/s1600536804018823.Suche in Google Scholar
8. Xu, M., Jupp, A. R., Ong, M. S. E., Burton, K. I., Chitnis, S. S., Stephan, D. W. Synthesis of urea derivatives from CO2 and silylamines. Angew. Chem. Int. Ed. 2019, 58, 5707–5711; https://doi.org/10.1002/ange.201900058.Suche in Google Scholar
9. Capacci–Daniel, C. A., Bertke, J. A., Dehghan, S., Hiremath–Darji, R., Swift, J. A. Concomitant polymorphs of 1,3-bis(3-fluorophenyl)urea. Acta. Crystallogr. 2016, C72, 692–696; https://doi.org/10.1107/s2053229616013565.Suche in Google Scholar
10. Koshti, V. S., Thorat, S. H., Gote, R. P., Chikkali, S. H., Gonnade, R. G. The impact of modular substitution on crystal packing: the tale of two ureas. CrystEngComm 2016, 18, 7078–7094; https://doi.org/10.1039/c6ce01324d.Suche in Google Scholar
11. Choi, H., Shim, Y. S., Lee, S. C., Kang, S. K., Sung, C. K. 1-(2-Hydroxy-2-phenylethyl)-3-(4-methoxyphenyl)urea. Acta. Crystallogr. 2011, E67, o2632; https://doi.org/10.1107/s1600536811036464.Suche in Google Scholar
12. Capacci–Daniel, C. A., Mohammadi, C., Urbelis, J. H., Heyrana, K., Khatri, N. M., Solomos, M. A., Swift, J. A. Structural diversity in 1,3-bis(m-cyanophenyl)urea. Cryst. Growth Des. 2015, 15, 2373–2379; https://doi.org/10.1021/acs.cgd.5b00168.Suche in Google Scholar
13. Gao, K., Zha, S.-E., Liu, C.-Y., Wang, Q.-J., Wang, E.-Q. The crystal structure of 1-(4-bromophenyl)-3-cycloheptylurea, C14H19BrN2O. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 117–118; https://doi.org/10.1515/ncrs-2022-0486.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

