Abstract
K2PdSe10, orthorhombic, Pcma (no. 55), a = 8.293(3) Å, b = 10.987(3) Å, c = 16.432(5) Å, V = 1497.1(8) Å3, Z = 4, R gt(F) = 0.0349, wR ref (F 2) = 0.0688, T = 173(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Black block |
| Size: | 0.17 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.0 mm−1 |
| Diffractometer, scan mode: | Bruker APEXII, φ and ω |
| θ max, completeness: | 56.6°, >99% |
| N(hkl) measured , N(hkl) unique, R int: | 13239, 9148, 0.025 |
| Criterion for I obs, N(hkl) gt: | I obs > 2σ(I obs), 7183 |
| N(param) refined: | 686 |
| Programs: | Bruker [1], SHELX [2], WinGX [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| Atom | X | y | z | U iso*/U eq |
|---|---|---|---|---|
| Pd1 | 0.000000 | 0.24848 (7) | 0.000000 | 0.01540 (18) |
| Se1 | −0.29166 (9) | 0.23077 (7) | 0.01754 (4) | 0.01795 (18) |
| Se2 | −0.34455 (9) | 0.16713 (7) | 0.15235 (4) | 0.01867 (18) |
| Se3 | −0.17289 (13) | 0.000000 | 0.17631 (6) | 0.0205 (3) |
| Se4 | 0.03532 (9) | 0.26779 (7) | 0.14648 (4) | 0.01865 (18) |
| Se5 | 0.30080 (9) | 0.33339 (7) | 0.17514 (4) | 0.01978 (18) |
| Se6 | 0.35360 (13) | 0.500000 | 0.08944 (6) | 0.0212 (3) |
| K1 | −0.2224 (3) | 0.500000 | 0.12557 (15) | 0.0303 (6) |
| K2 | 0.2671 (3) | 0.000000 | 0.11187 (15) | 0.0318 (7) |
1 Source of material
K2PdCl4 (0.040 g, 0.12 mmol), K2Se4 (0.190 g, 0.48 mmol) and Et3NHCl (0.033 g, 0.24 mmol) were charged to a Pyrex tube with a diameter of 9 mm under an argon atmosphere and about 0.5 mL MeOH was added as a solvent. While the solvent being frozen, the Pyrex tube was evacuated under vacuum and sealed with the use of a flame. The sealed tube was placed in an oven and heated at 80 °C for 3 days, then cooled to room temperature. Black platelet crystals were isolated by filtration and washed with MeOH and diethyl ether several times. Crystals of K2[Pd(Se5)2] were obtained in 26% yield, based on the Pd metal used.
2 Experimental details
2.1 Comment
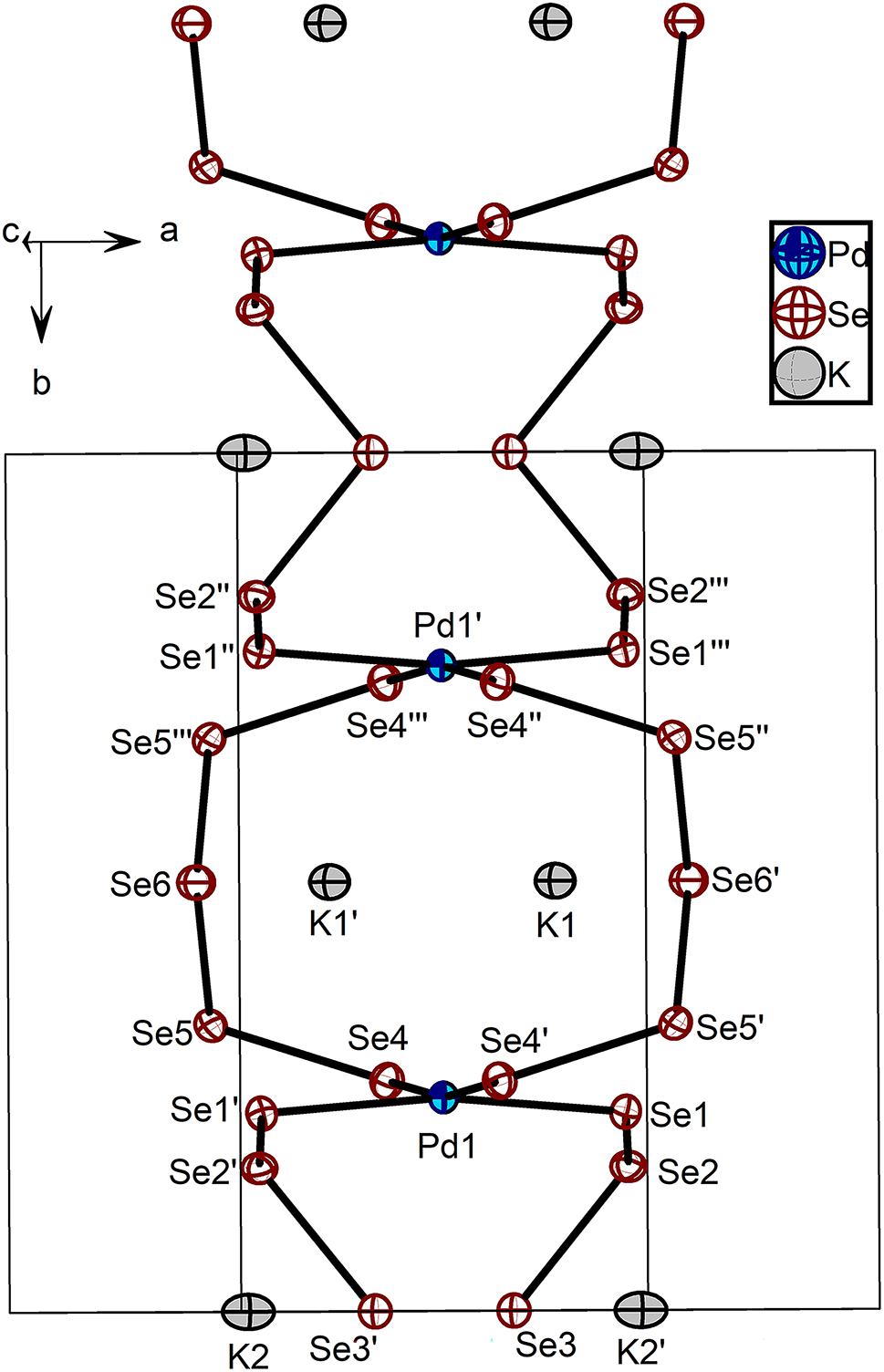
The title compound, K2[Pd(Se5)2], which has been prepared by the solvothermal reaction of K2PdCl4, K2Se4 and Et3NHCl with methanol as a solvent, is composed of a 1D polymeric [Pd(Se5)2]2− anion and charge-balancing K+ cations. The 1D structure of [Pd(Se5)2]2− is the first example among the members of Pd polyselenide family [Pd(Se
x
)2]2− (x = 2 − 7) [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Actually the chain structure of [Pd(Se5)2]2− is quite unprecedented in the metal polyselenide chemistry [15, 16]. Two independent pairs of symmetrically equivalent Se5
2− bridging ligands connect adjacent Pd(II) atoms alternatively in a helical screw mode to form a chain. So far, the bridging pentaselenide, Se5
2−
The other two compounds containing [Pd(Se5)2]2− anions are (H3NCH2CH2NH2)2[Pd(Se5)2], which has a layered structure of [Pd(Se5)2]2−, and (Et4N)5[2Pd(Se4)2·0.5Pd(Se5)2], which has a 0D molecular [Pd(Se5)2]2− [6, 7]. Compared to the synthetic condition of K2[Pd(Se5)2], use of PdCl2 and water instead of K2 PdCl4 and methanol, respectively, had been reported to yield another K2PdSe10, which is better described as K4[Pd(Se4)2][Pd(Se6)2] [5]. The K4[Pd(Se4)2][Pd(Se6)2] is composed of interpenetrating 3D [Pd(Se4)2]2− and [Pd(Se6)2]2− frameworks. In addition, the methanothermal reaction of K2PdCl4 and K2Se5 in a 1:6 M ratio at 80 °C had been reported to produce K6[Pd(Se5)4] containing a molecular [Pd(Se5)4]4− anion, composed of a Pd(II) metal center and four dangling Se5 2− ligands [7]. When K2Se4 was used in less amount instead of K2Se5, the title compound, K2[Pd(Se5)2] instead of K6[Pd(Se5)4] was produced. Dangling Se5 2− ligands of molecular [Pd(Se5)4]6− anions are supposed to be connected additionally by Pd(II) atoms in excess to form a [Pd(Se5)2]2− chain. For the synthesis of K2[Pd(Se5)2], Et3NH+ organic cations seemed to be necessary at least to yield good enough crystals for single crystal X-ray diffraction study.
For the structure of [Pd(Se5)2]2− in K2[Pd(Se5)2], the square planar geometry around Pd(II), coordinated by four bridging pentaselenide ligands, is quite ideal as Se–Pd–Se angles are in the range of 90.26(2)–90.54(2)°. The Pd–Se and Se–Se distances are also typical, ranging from 2.4339(10) to 2.4435(10) Å, and from 2.3507(11) to 2.3640(12) Å, respectively, similar to those found in the other [Pd(Se
x
)2]2− complexes [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. The distances between the adjacent Pd atom centers in the 1D chain structure of [Pd(Se5)2]2− are 5.4601(18) Å and 5.5269(19) Å, respectively. Considering the distances, 8.507 Å, between the Pd atom centers in the 2D layered structure of
Acknowledgements
This work was supported by the Incheon National University Research Grant in 2018.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Incheon National University Research Grant in 2018.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854, https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Kim, K.–W., Kanatzidis, M. G. Hydrothermal synthesis of K2PdSe10 coexistence of two large interpenetrating three-dimensional frameworks of [Pd(Se4)2]2− and [Pd(Se6)2]2−. J. Am. Chem. Soc. 1992, 114, 4878–4883, https://doi.org/10.1021/ja00038a063.Search in Google Scholar
6. McConnachie, J. M., Ansari, M. A., Ibers, J. A. Monomeric selenopalladates and selenoplatinates. Inorg. Chem. 1993, 32, 3250–3255; https://doi.org/10.1021/ic00067a010.Search in Google Scholar
7. Kim, K.–W., Kanatzidis, M. G. Counterion effects in Pd polyselenides: evolution from molecular to three-dimensional framework structures. J. Am. Chem. Soc. 1998, 120, 8124–8135; https://doi.org/10.1021/ja981297s.Search in Google Scholar
8. Li, J., Chen, Z., Wang, R.–J., Lu, J. Y. Cs2PdSe8: a unique open framework structure with double helical assemblies of [Pd(Se4)2]2−. J. Solid State Chem. 1998, 140, 149–153, https://doi.org/10.1006/jssc.1998.7950.Search in Google Scholar
9. Wachhold, M., Kanatzidis, M. G. Powerful templating effect in Rb/Pd/Sex promoted by crown ether-like [Rb(Se8)]+ coordination. Formation of Rb2[Pd(Se4)2]Se8: a layered Pd polyselenide with “encapsulated” eight-membered selenium rings. J. Am. Chem. Soc. 1999, 121, 4189–4195, https://doi.org/10.1021/ja984053g.Search in Google Scholar
10. Chen, Z., Wang, R.–J., Li, J. Solvothermal synthesis of alkaline metal selenides Cs2PdSe16 and studies of thermal stabilities. Chem. J. Chinese Univ. 2001, 22, 1091–1094.Search in Google Scholar
11. Kim, K.–W., Kim, J. Crystal structure of bis(N,N,N-triethylammonium) bis(tetraselenido-κ2Se1,Se4)palladate(II), C12H32N2PdSe8. Z. Kristallogr. N. Cryst. Struct. 2015, 230, 269–270, https://doi.org/10.1515/ncrs-2014-9015.Search in Google Scholar
12. Kim, K.–W., Wang, D. Crystal structure of bis(ethanaminium) poly[bis(hexaselenido-κ2Se1,Se6)palladate(II)], C4H16N2PdSe12. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 933–934, https://doi.org/10.1515/ncrs-2016-0011.Search in Google Scholar
13. Kim, K.–W., Wang, D. Crystal structure of bis(N,N,N-ethyldimethylethanaminium) bis(heptaselenido-κ2Se1,Se7)palladate(II), C12H32N2PdSe14. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 965–967, https://doi.org/10.1515/ncrs-2017-0101.Search in Google Scholar
14. Wang, D., Yun, H., Kim, K.–W. Crystal structure of bis(N,N,N-trimethylethanaminium) poly[μ2-bis(heptaselenido-κ2Se1,Se7)palladate(II)], C10H28N2PdSe14. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 995–997, https://doi.org/10.1515/ncrs-2017-0126.Search in Google Scholar
15. Ruck, M., Locherer, F. Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium. Coord. Chem. Rev. 2015, 285, 1–10, https://doi.org/10.1016/j.ccr.2014.10.010.Search in Google Scholar
16. Sheldrick, W. S. Polychalcogenide anions: structural diversity and ligand versatility. Z. Anorg. Allg. Chem. 2012, 638, 2401–2424; https://doi.org/10.1002/zaac.201200241.Search in Google Scholar
17. Müller, U., Ha–Eierdanz, M.–L., Kräuter, G., Dehnicke, K. Synthesis and crystal structure of (PPh4)4[Cu2Se14]. Z. Naturforsch. 1990, 45B, 1128–1132.10.1515/znb-1990-0804Search in Google Scholar
18. Dhingra, S. S., Kanatzidis, M. G. Polyselenide chemistry of indium and thallium in dimethylformamide, acetonitrile, and water. Syntheses, structures, and properties of the new complexes [In2(Se4)2(Se5)]4−, [In2Se2(Se4)2]2−, [In3Se3(Se4)3]3−, and [Tl3Se3(Se4)3]3−. Inorg. Chem. 1993, 32, 1350–1362, https://doi.org/10.1021/ic00060a007.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

