Abstract
C18H10N2, monoclinic, P21/c (no. 14), a = 7.9297(9) Å, b = 11.4021(14) Å, c = 13.3572(15) Å, β = 105.363(8)°, V = 1164.5(2) Å3, Z = 4, Rgt(F) = 0.0325, wRref(F2) = 0.0774, T = 210(2) K.
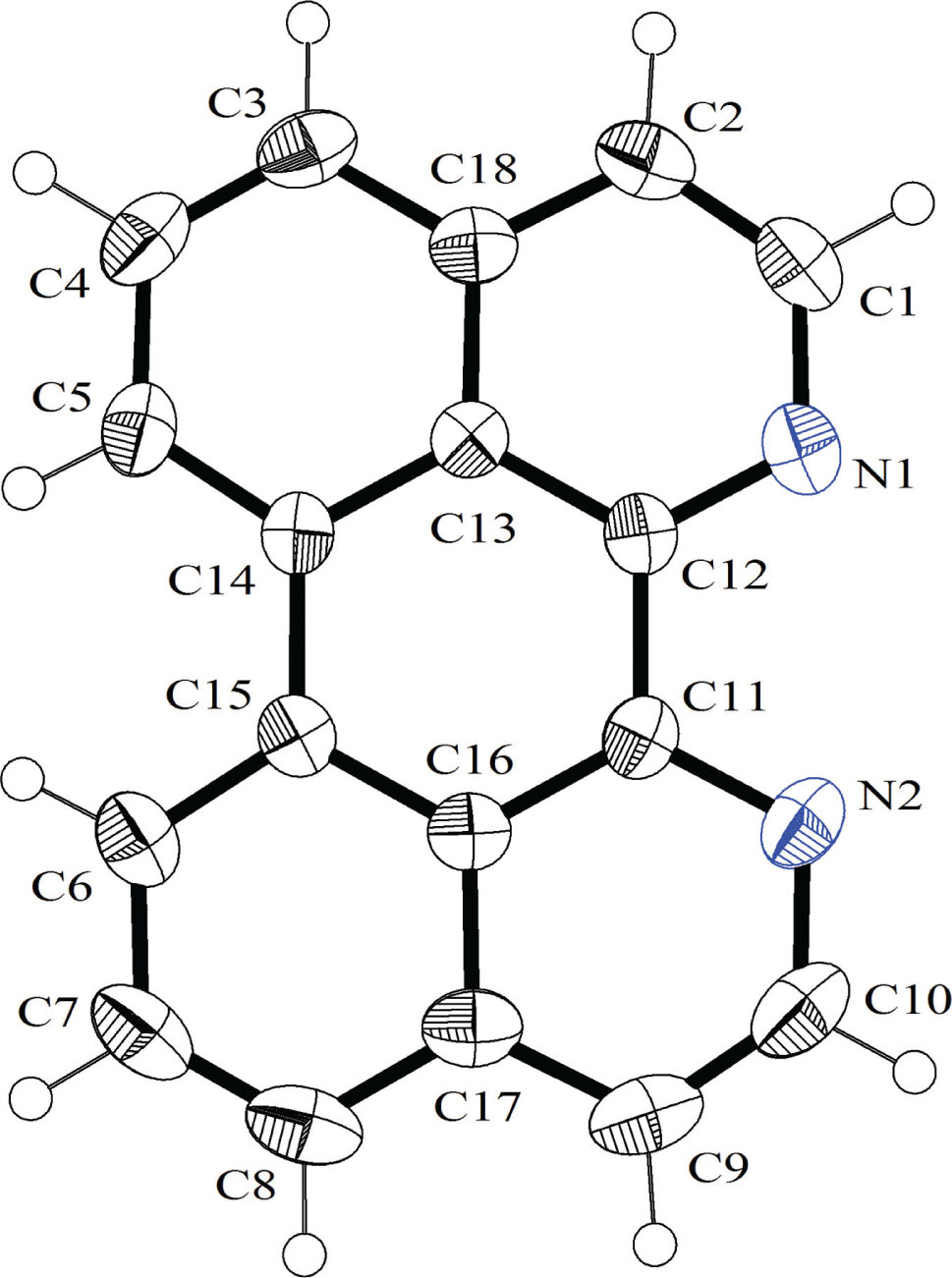
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow flat needle |
| Size: | 0.30 × 0.08 × 0.03 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | STOE StadiVari, ω scans |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 20040, 2051, 0.055 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1229 |
| N(param)refined: | 192 |
| Programs: | SHELX [1], WinGX/ORTEP [2], PLATON [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.9113(2) | 1.03165(14) | 0.25918(12) | 0.0448(4) |
| H1 | 1.0266 | 1.0505 | 0.2957 | 0.045(4)* |
| C2 | 0.7818(2) | 1.10273(14) | 0.27236(11) | 0.0422(4) |
| H2 | 0.8081 | 1.1692 | 0.3153 | 0.053(5)* |
| C3 | 0.4638(2) | 1.14306(14) | 0.23109(11) | 0.0421(4) |
| H3 | 0.4827 | 1.2116 | 0.2717 | 0.054(5)* |
| C4 | 0.2987(2) | 1.10912(14) | 0.18233(12) | 0.0451(4) |
| H4 | 0.2037 | 1.1539 | 0.1906 | 0.051(5)* |
| C5 | 0.2670(2) | 1.00865(14) | 0.11996(11) | 0.0396(4) |
| H5 | 0.1509 | 0.9865 | 0.0883 | 0.050(5)* |
| C6 | 0.2102(2) | 0.80169(14) | −0.02114(12) | 0.0428(4) |
| H6 | 0.1108 | 0.8418 | −0.0135 | 0.048(5)* |
| C7 | 0.1888(2) | 0.70620(15) | −0.08899(13) | 0.0520(5) |
| H7 | 0.0757 | 0.6849 | −0.1275 | 0.054(5)* |
| C8 | 0.3279(2) | 0.64386(15) | −0.10020(12) | 0.0507(5) |
| H8 | 0.3108 | 0.5797 | −0.1460 | 0.057(5)* |
| C9 | 0.6489(2) | 0.61343(14) | −0.04973(13) | 0.0495(4) |
| H9 | 0.6392 | 0.5471 | −0.0927 | 0.060(5)* |
| C10 | 0.8073(2) | 0.65045(15) | 0.00631(12) | 0.0499(5) |
| H10 | 0.9055 | 0.6074 | 0.0007 | 0.054(5)* |
| C11 | 0.69583(18) | 0.80484(12) | 0.07775(10) | 0.0313(3) |
| C12 | 0.72404(17) | 0.90830(12) | 0.14686(10) | 0.0302(3) |
| C13 | 0.57669(17) | 0.97427(12) | 0.15639(10) | 0.0284(3) |
| C14 | 0.40181(17) | 0.94130(13) | 0.10382(10) | 0.0296(3) |
| C15 | 0.37367(18) | 0.83850(12) | 0.03483(10) | 0.0310(3) |
| C16 | 0.52216(18) | 0.77376(12) | 0.02340(10) | 0.0308(3) |
| C17 | 0.4982(2) | 0.67485(13) | −0.04340(11) | 0.0390(4) |
| C18 | 0.60749(19) | 1.07544(13) | 0.22087(10) | 0.0338(4) |
| N1 | 0.88720(15) | 0.93566(12) | 0.19737(9) | 0.0406(3) |
| N2 | 0.83589(16) | 0.74497(11) | 0.07003(10) | 0.0419(3) |
Source of materials
The title compound was synthesized by a reductive cyclization reaction of 1,1′-bisisoqulinoline [4], [5] in dimethoxyethane using potassium as reducing agent [6]. After synthesis, the residue was chromatographed on aluminium oxide with THF and concentrated under reduced pressure. For a further purification the product was dissolved in dichloromethane. This layer was washed by shaking with concentrated sodium hydroxide solution and additionally with sodium chloride brine. The clear yellow organic layer was dried with MgSO4, concentrated under reduced pressure and recrystallized from DMF. Yellow crystals (Mp 258–260 °C) suitable for single crystal X-ray diffraction were obtained by slow evaporation of DMF in an airstream within 3 days.
Experimental details
The hydrogen atoms were calculated in their expected positions and refined as riding atoms with the exception of the temperature factors, which were free refined.
Comment
1,12-Diazaperylene (dap) is an established bidentate ligand. The complexes are characterized by π-π stacking interactions, which often generate supramolecular assemblies, like in iridium(III) complexes with dap and 2,11-dialkylated-dap [7]. Metalla-supramolecular assemblies with honeycomb structures supported by π–π stacking of octahedral Ni(II) and Fe(II) complexes with dap are formed, containing nanochannels [8]. Mononuclear ruthenium(II) complexes show optoelectronic properties and can bind to DNA through intercalation [9]. A further mononuclear Ru(II) complex is formed with dap and the tetradentate ligand N,N′-dimethyl-2,11-diaza-[3.3](2,6)-pyridinophane to complete the octahedral coordination sphere [10]. In contrast, dinuclear Ru(II) complexes are being observed with the bridging ligand 1,6,7,12-tetraazaperylene. Mononuclear ruthenium(II) complexes with several polypyridine-type ligands – including dap – were examined to act as catalysts for water oxidation [11]. Tetrahedral copper(I) complexes with 2,11-dialkylated dap exhibit low-energy MLCT transitions [12]. A dirhodium dap complex with bridging acetato groups was found to have the dual capability of intercalative and coordinative binding to DNA [13]. Formamidinat-bridged dirhodium complexes exhibit broad, strong absorption throughout the UV-visible range [14] well suited for photophysical applications. More recently, absorption and emission spectra of N-derivatives of perylene – including dap – were simulated using a time-dependent approach based on correlation functions determinated by density functional theory. The N-substitution can be used for fine-tuning the optical properties [15]. The complex formation of dap with transition metal ions (Fe, Co, Ni, Cu, Zn, Ru, Os, Re, Pd, Pt, Ag, Cd) in the gas phase has been studied by electrospray ionization mass spectrometry [16], [17]. Heteroleptic polymetallic Ag(I) and Cu(I) complexes were obtained and a multidentate ligand containing a large N,P,N,P,N core yielding interesting structures with π–π stacked columns or discrete sixfold stacks [18].
The title compound is closely related to 1,1′-bisisoquinoline. Both compounds only differ by one C—C bond, which is present in dap additionally and responsible to held the second quinoline unit in a fixed position. As a result a “large-surfaced” ligand is formed. The ring system is nearly full planar with a maximal deviation from the best plane of 0.0612(13) Å (C5). The C—C bond lengths ranges from 1.349(2) Å to 1.478(2) Å. The longest bonds occur between that carbon atoms, which connect both quinoline rings. In comparison with 1,1′-bisisoquinoline (biq) the bond between the carbon atoms, adjacent to the nitrogen atoms is longer (dap: 1.478(2) Å; biq: 1.496(2) Å), resulting from the full inclusion of all C atoms into the aromatic ring system. The distance between both donor N atoms is 2.742(2) Å. The crystal packing is characterized by a large number of π–π interactions. Furthermore, C—H⋯π interactions are observed [C9—H9⋯C1—C5/C12—C14/C18/N1 ring and C3—H3⋯Cg(multiple)].
Acknowledgements
We acknowledge the support of Deutsche Forschungsgemeinschaft (German Research Foundation) and Open Access Publication Fund of Potsdam University.
References
1. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
2. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
3. Spek, A. L.: PLATON – a multipurpose crystallographic tool. Acta Crystallogr. D65 (2011) 148–155.10.1107/S090744490804362XSuche in Google Scholar PubMed PubMed Central
4. Case, F. H.: The preparation of 1,1′- and 3,3′-bisisoquinoline. J. Org. Chem. 17 (1952) 471–472.10.1021/jo01137a021Suche in Google Scholar
5. Grunwald, N.; Kelling, A.; Holdt, H.-J.; Schilde, U.: The crystal structure of 1,1′-bisisoquinoline, C18H12N2. Z. Kristallogr. NCS 232 (2017) 839–841.10.1515/ncrs-2017-0088Suche in Google Scholar
6. Schmelz, O.; Mews, A.; Basché, T.; Herrmann, A.; Müllen, K.: Supramolecular complexes from CdSe nanocrystals and organic fluorophors. Langmuir 17 (2001) 2861–2865.10.1021/la0016367Suche in Google Scholar
7. Kammer, S.; Starke, I.; Pietrucha, A.; Kelling, A.; Mickler, W.; Schilde, U.; Dosche, C.; Kleinpeter, E.; Holdt, H.-J.: 1,12-Diazaperylene and 2,11-dialkylated-1,12-diazaperylene iridium(III) complexes [Ir(C^N)2(N^N)]PF6: new supramolecular assemblies. Dalton Trans. 41 (2012) 10219–10227.10.1039/c2dt30412kSuche in Google Scholar PubMed
8. Kammer, S.; Müller, H.; Grunwald, N.; Bellin, A.; Kelling, A.; Schilde, U.; Mickler, W.; Dosche, C.; Holdt, H.-J.: Supramolecular assemblies with honeycomb structures by π-π stacking of octahedral metal complexes of 1,12-diazaperylene. Eur. J. Inorg. Chem. 2006 (2006) 1547–1551.10.1002/ejic.200600092Suche in Google Scholar
9. Chouai, A.; Wicke, S. E.; Turro, C.; Bacsa, J.; Dunbar, K. R.; Wang, D.; Thummel, R. P.: Ruthenium(II) complexes of 1,12-diazaperylene and their interactions with DNA. Inorg. Chem. 44 (2005) 5996–6003.10.1021/ic0485965Suche in Google Scholar PubMed
10. Brietzke, T.; Mickler, W.; Kelling, A.; Schilde, U.; Krüger, H.-J.; Holdt, H.-J.: Mono- and dinuclear ruthenium(II)-1,6,7,12-tetraazaperylene complexes of N,N′-dimethyl-2,11-diaza[3.3](2,6)-pyridinophane. Eur. J. Inorg. Chem. 2012 (2012) 4632–4643.10.1002/ejic.201200667Suche in Google Scholar
11. Tseng, H.-W.; Zong, R.; Muckerman, J. T.; Thummel, R.: Mononuclear ruthenium(II) complexes that catalyze water oxidation. Inorg. Chem. 47 (2008) 11763–11773.10.1021/ic8014817Suche in Google Scholar PubMed
12. Kammer, S.; Kelling, A.; Baier, H.; Mickler, W.; Dosche, C.; Rurack, K.; Kapp, A.; Lisdat, F.; Holdt, H.-J.: 2,11-Dialkylated 1,12-diazaperylene copper(I) complexes: first supramolecular column assemblies by π-π stacking between homoleptic tetrahedral metal complexes, exhibiting low-energy MLCT transitions. Eur. J. Inorg. Chem. 2009 (2009) 4648–4659.10.1002/ejic.200900695Suche in Google Scholar
13. Kang, M.; Chouai, A.; Chifotides, H. T.; Dunbar, K. R.: 2D NMR spectroscopic evidence for unprecedented interactions of cis-[Rh2(dap)(μ-O2CCH3)2(η1-O2CCH3)(CH3OH)] (O2CCH3) with a DNA oligonucleotide: combination of intercalative and coordinative binding. Angew. Chem. Int. Ed. 45 (2006) 6148–6151.10.1002/anie.200600938Suche in Google Scholar PubMed
14. White, T. A.; Duunbar, K. R.; Thummel, R. P.; Turro, C.: Electronic influences of bridging and chelating diimine ligand coordination in formamidinate-bridged Rh2(II,II) dimers. Polyhedron 103 (2016) 172–177.10.1016/j.poly.2015.10.015Suche in Google Scholar
15. Xiong, T.; Wlodarczyk, R.; Saalfrank, P.: Vibrationally resolved absorption and fluorescence spectra of perylene and N-substituted derivatives from autocorrelation function approaches. Chem. Phys. 515 (2018) 728–736.10.1016/j.chemphys.2018.06.011Suche in Google Scholar
16. Starke, I.; Kammer, S.; Grunwald, N.; Schilde, U.; Holdt, H.-J.; Kleinpeter, E.: Complexation of diazaperylene and bisisoquinoline with transition metal ions in the gas phase studied by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 22 (2008) 665–671.10.1002/rcm.3412Suche in Google Scholar PubMed
17. Starke, I.; Koch, A.; Kammer, S.; Holdt, H.-J.; Möller, H. M.: Electrospray mass spectrometry and molecular modeling study of formation and stability of silver complexes with diazaperylene and bisisoquinoline. J. Mass Spectrom. 53 (2018) 408–418.10.1002/jms.4071Suche in Google Scholar PubMed
18. Attenberger, B.; El Sayed Moussa, M.; Brietzke, T.; Vreshch, V.; Holdt, H.-J.; Lescop, C.; Scheer, M.: Discrete polymetallic arrangements of AgI and CuI ions based on multiple bridging phosphane ligands and π-π interactions. Eur. J. Inorg. Chem. 2015 (2015) 2934–2938.10.1002/ejic.201500445Suche in Google Scholar
©2019 Matthias Kirste et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3


