Abstract
C60H80Cl8N6Pd2, triclinic, P1̄ (no. 2), a = 10.8703(10) Å, b = 17.0284(15) Å, c = 18.9595(17) Å, α = 83.1960(10)°, β = 88.909(2)°, γ = 71.523(2)°, V = 3304.5(5) Å3, Z = 2, Rgt(F) = 0.0488, wRref(F2) = 0.1213, T = 130 K.
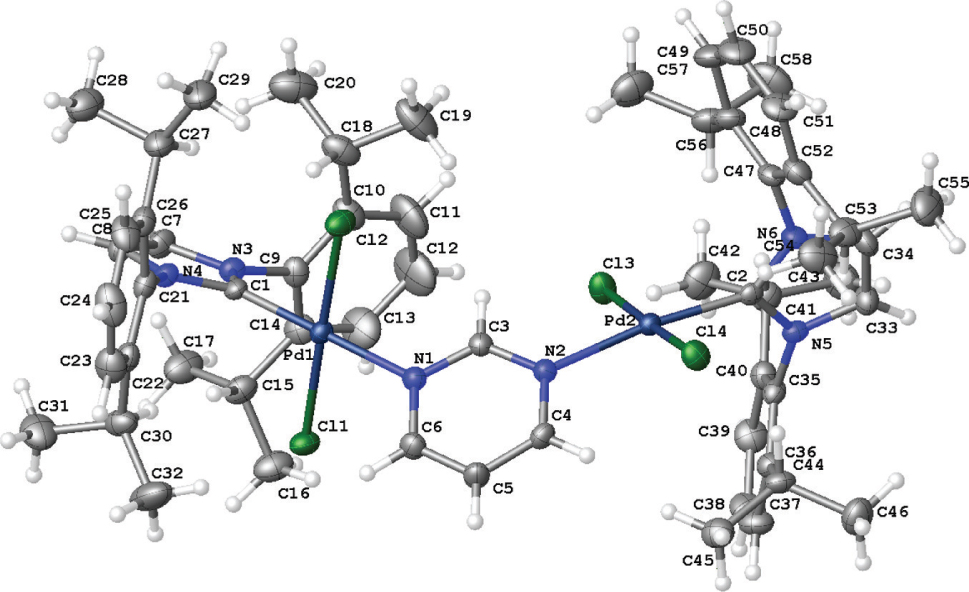
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.08 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.91 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 30.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 33348, 20057, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 11824 |
| N(param)refined: | 701 |
| Programs: | Olex2 [1], SHELX [2], [3], Spek [4], Bruker [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Pd1 | 0.32675(3) | 0.80614(2) | 0.78631(2) | 0.01931(7) |
| Pd2 | 0.46962(3) | 0.45234(2) | 0.73397(2) | 0.01896(7) |
| Cl1 | 0.30881(9) | 0.79747(5) | 0.90750(4) | 0.0278(2) |
| Cl2 | 0.35011(9) | 0.81176(6) | 0.66556(5) | 0.0299(2) |
| Cl3 | 0.25522(9) | 0.52398(6) | 0.75020(5) | 0.0296(2) |
| Cl4 | 0.68336(9) | 0.38385(5) | 0.71236(5) | 0.02754(19) |
| N1 | 0.4663(3) | 0.68818(17) | 0.79806(15) | 0.0208(6) |
| N2 | 0.5260(3) | 0.54846(17) | 0.77164(14) | 0.0198(6) |
| N3 | 0.0635(3) | 0.93120(17) | 0.78850(15) | 0.0209(6) |
| N4 | 0.2070(3) | 0.99135(17) | 0.76020(15) | 0.0201(6) |
| N5 | 0.3872(3) | 0.30146(17) | 0.75799(14) | 0.0205(6) |
| N6 | 0.4342(3) | 0.32303(18) | 0.64774(15) | 0.0232(6) |
| C1 | 0.1920(3) | 0.9155(2) | 0.77674(17) | 0.0203(7) |
| C2 | 0.4243(3) | 0.3554(2) | 0.71040(17) | 0.0190(7) |
| C3 | 0.4484(4) | 0.6277(2) | 0.76470(18) | 0.0212(7) |
| H3 | 0.375019 | 0.642006 | 0.733674 | 0.025* |
| C4 | 0.6312(3) | 0.5291(2) | 0.81383(18) | 0.0230(7) |
| H4 | 0.690021 | 0.473875 | 0.818406 | 0.028* |
| C5 | 0.6560(4) | 0.5876(2) | 0.8508(2) | 0.0272(8) |
| H5 | 0.730602 | 0.573310 | 0.880868 | 0.033* |
| C6 | 0.5698(3) | 0.6671(2) | 0.84285(19) | 0.0252(8) |
| H6 | 0.583196 | 0.707861 | 0.869251 | 0.030* |
| C7 | −0.0010(4) | 1.0165(2) | 0.77892(19) | 0.0266(8) |
| H7 | −0.091404 | 1.043114 | 0.783632 | 0.032* |
| C8 | 0.0880(4) | 1.0537(2) | 0.76188(19) | 0.0271(8) |
| H8 | 0.073053 | 1.112050 | 0.752580 | 0.033* |
| C9 | 0.0006(3) | 0.8674(2) | 0.8043(2) | 0.0254(8) |
| C10 | −0.0207(4) | 0.8269(3) | 0.7473(2) | 0.0339(9) |
| C11 | −0.0806(5) | 0.7657(3) | 0.7634(3) | 0.0554(14) |
| H11 | −0.094790 | 0.735777 | 0.726791 | 0.067* |
| C12 | −0.1191(6) | 0.7481(4) | 0.8306(3) | 0.0672(16) |
| H12 | −0.160733 | 0.706681 | 0.839936 | 0.081* |
| C13 | −0.0984(5) | 0.7900(3) | 0.8861(3) | 0.0520(13) |
| H13 | −0.125875 | 0.776835 | 0.932560 | 0.062* |
| C14 | −0.0378(4) | 0.8510(2) | 0.8736(2) | 0.0294(8) |
| C15 | −0.0186(4) | 0.8976(2) | 0.9337(2) | 0.0304(9) |
| H15 | 0.053796 | 0.920689 | 0.921006 | 0.036* |
| C16 | 0.0166(4) | 0.8420(3) | 1.0047(2) | 0.0407(11) |
| H16A | −0.057164 | 0.823771 | 1.020839 | 0.061* |
| H16B | 0.037874 | 0.873568 | 1.040012 | 0.061* |
| H16C | 0.091758 | 0.793201 | 0.998609 | 0.061* |
| C17 | −0.1420(4) | 0.9709(3) | 0.9422(2) | 0.0434(11) |
| H17A | −0.159182 | 1.009944 | 0.898475 | 0.065* |
| H17B | −0.129902 | 0.999647 | 0.982054 | 0.065* |
| H17C | −0.215633 | 0.949894 | 0.951426 | 0.065* |
| C18 | 0.0162(4) | 0.8487(3) | 0.6717(2) | 0.0388(10) |
| H18 | 0.093433 | 0.868254 | 0.673870 | 0.047* |
| C19 | 0.0531(5) | 0.7739(3) | 0.6290(3) | 0.0576(14) |
| H19A | 0.116456 | 0.726306 | 0.656326 | 0.086* |
| H19B | 0.091085 | 0.788333 | 0.583948 | 0.086* |
| H19C | −0.024594 | 0.759195 | 0.619292 | 0.086* |
| C20 | −0.0957(5) | 0.9214(3) | 0.6342(2) | 0.0568(14) |
| H20A | −0.174338 | 0.905048 | 0.633863 | 0.085* |
| H20B | −0.071806 | 0.934915 | 0.585200 | 0.085* |
| H20C | −0.111867 | 0.970428 | 0.659674 | 0.085* |
| C21 | 0.3300(3) | 1.0043(2) | 0.74054(19) | 0.0235(8) |
| C22 | 0.4183(4) | 0.9997(2) | 0.7951(2) | 0.0268(8) |
| C23 | 0.5369(4) | 1.0083(3) | 0.7747(2) | 0.0367(10) |
| H23 | 0.599721 | 1.004723 | 0.810103 | 0.044* |
| C24 | 0.5655(4) | 1.0218(3) | 0.7042(2) | 0.0415(11) |
| H24 | 0.648278 | 1.026055 | 0.691501 | 0.050* |
| C25 | 0.4747(4) | 1.0292(2) | 0.6520(2) | 0.0363(10) |
| H25 | 0.494803 | 1.040731 | 0.603627 | 0.044* |
| C26 | 0.3534(4) | 1.0202(2) | 0.66863(19) | 0.0268(8) |
| C27 | 0.2554(4) | 1.0288(2) | 0.6106(2) | 0.0338(9) |
| H27 | 0.195709 | 0.997172 | 0.628860 | 0.041* |
| C28 | 0.1732(5) | 1.1216(3) | 0.5922(2) | 0.0461(12) |
| H28A | 0.129783 | 1.143228 | 0.634899 | 0.069* |
| H28B | 0.108014 | 1.126246 | 0.555582 | 0.069* |
| H28C | 0.229904 | 1.153978 | 0.574439 | 0.069* |
| C29 | 0.3165(5) | 0.9941(3) | 0.5434(2) | 0.0447(12) |
| H29A | 0.359929 | 1.031577 | 0.518755 | 0.067* |
| H29B | 0.248939 | 0.989448 | 0.512213 | 0.067* |
| H29C | 0.380152 | 0.938844 | 0.555943 | 0.067* |
| C30 | 0.3837(4) | 0.9927(2) | 0.8726(2) | 0.0337(9) |
| H30 | 0.310508 | 0.968848 | 0.877441 | 0.040* |
| C31 | 0.3358(5) | 1.0801(3) | 0.8961(2) | 0.0465(12) |
| H31A | 0.404197 | 1.106251 | 0.888976 | 0.070* |
| H31B | 0.313955 | 1.076110 | 0.946504 | 0.070* |
| H31C | 0.258643 | 1.113953 | 0.867857 | 0.070* |
| C32 | 0.4959(5) | 0.9357(3) | 0.9208(3) | 0.0558(14) |
| H32A | 0.534359 | 0.883107 | 0.900611 | 0.084* |
| H32B | 0.463154 | 0.924220 | 0.968169 | 0.084* |
| H32C | 0.561819 | 0.963136 | 0.924520 | 0.084* |
| C33 | 0.3782(4) | 0.2348(2) | 0.7254(2) | 0.0282(8) |
| H33 | 0.355882 | 0.188197 | 0.747415 | 0.034* |
| C34 | 0.4068(4) | 0.2484(2) | 0.6571(2) | 0.0291(8) |
| H34 | 0.408071 | 0.213383 | 0.621444 | 0.035* |
| C35 | 0.3506(3) | 0.3110(2) | 0.83094(18) | 0.0214(7) |
| C36 | 0.4490(4) | 0.2922(2) | 0.88326(18) | 0.0243(8) |
| C37 | 0.4103(4) | 0.2944(2) | 0.9533(2) | 0.0325(9) |
| H37 | 0.474201 | 0.281820 | 0.990049 | 0.039* |
| C38 | 0.2811(4) | 0.3146(3) | 0.9708(2) | 0.0381(10) |
| H38 | 0.257179 | 0.314850 | 1.019297 | 0.046* |
| C39 | 0.1862(4) | 0.3345(2) | 0.9180(2) | 0.0344(9) |
| H39 | 0.097584 | 0.348574 | 0.930825 | 0.041* |
| C40 | 0.2182(4) | 0.3342(2) | 0.84649(19) | 0.0254(8) |
| C41 | 0.1120(4) | 0.3572(2) | 0.7897(2) | 0.0301(8) |
| H41 | 0.151232 | 0.368450 | 0.742951 | 0.036* |
| C42 | 0.0010(4) | 0.4361(3) | 0.8020(3) | 0.0437(11) |
| H42A | −0.045264 | 0.424741 | 0.844924 | 0.066* |
| H42B | −0.059188 | 0.452722 | 0.761077 | 0.066* |
| H42C | 0.036399 | 0.481194 | 0.807950 | 0.066* |
| C43 | 0.0572(5) | 0.2846(3) | 0.7848(3) | 0.0474(12) |
| H43A | 0.126420 | 0.236153 | 0.771446 | 0.071* |
| H43B | −0.012841 | 0.301744 | 0.748947 | 0.071* |
| H43C | 0.023370 | 0.269933 | 0.831071 | 0.071* |
| C44 | 0.5919(4) | 0.2685(2) | 0.86557(19) | 0.0273(8) |
| H44 | 0.598157 | 0.283376 | 0.813292 | 0.033* |
| C45 | 0.6605(4) | 0.3178(2) | 0.9035(2) | 0.0343(9) |
| H45A | 0.612251 | 0.377474 | 0.894527 | 0.051* |
| H45B | 0.748677 | 0.307682 | 0.885622 | 0.051* |
| H45C | 0.664502 | 0.299927 | 0.954723 | 0.051* |
| C46 | 0.6594(4) | 0.1745(2) | 0.8825(2) | 0.0420(11) |
| H46A | 0.650755 | 0.157528 | 0.933050 | 0.063* |
| H46B | 0.751558 | 0.161282 | 0.871120 | 0.063* |
| H46C | 0.619121 | 0.144462 | 0.854002 | 0.063* |
| C47 | 0.4543(4) | 0.3619(2) | 0.57822(18) | 0.0242(8) |
| C48 | 0.3477(4) | 0.4261(2) | 0.54612(19) | 0.0309(9) |
| C49 | 0.3665(5) | 0.4588(3) | 0.4766(2) | 0.0400(11) |
| H49 | 0.298491 | 0.503327 | 0.452720 | 0.048* |
| C50 | 0.4813(5) | 0.4275(3) | 0.4425(2) | 0.0431(11) |
| H50 | 0.491649 | 0.450980 | 0.395635 | 0.052* |
| C51 | 0.5815(4) | 0.3625(3) | 0.4754(2) | 0.0368(10) |
| H51 | 0.658991 | 0.340536 | 0.450265 | 0.044* |
| C52 | 0.5711(4) | 0.3284(2) | 0.54488(19) | 0.0297(8) |
| C53 | 0.6825(4) | 0.2560(2) | 0.5797(2) | 0.0346(9) |
| H53 | 0.663949 | 0.247913 | 0.631450 | 0.041* |
| C54 | 0.8132(4) | 0.2718(3) | 0.5730(2) | 0.0442(11) |
| H54A | 0.835913 | 0.277058 | 0.522808 | 0.066* |
| H54B | 0.880106 | 0.225016 | 0.599096 | 0.066* |
| H54C | 0.807428 | 0.323368 | 0.592890 | 0.066* |
| C55 | 0.6904(5) | 0.1755(3) | 0.5488(2) | 0.0491(12) |
| H55A | 0.609736 | 0.162308 | 0.558150 | 0.074* |
| H55B | 0.763648 | 0.129735 | 0.570951 | 0.074* |
| H55C | 0.702819 | 0.182986 | 0.497369 | 0.074* |
| C56 | 0.2174(4) | 0.4539(3) | 0.5811(2) | 0.0356(10) |
| H56 | 0.233430 | 0.439914 | 0.633548 | 0.043* |
| C57 | 0.1476(5) | 0.5486(3) | 0.5668(3) | 0.0516(13) |
| H57A | 0.204779 | 0.578659 | 0.580983 | 0.077* |
| H57B | 0.068002 | 0.563238 | 0.594342 | 0.077* |
| H57C | 0.125761 | 0.564086 | 0.516084 | 0.077* |
| C58 | 0.1322(5) | 0.4051(3) | 0.5598(3) | 0.0557(13) |
| H58A | 0.117021 | 0.415708 | 0.508205 | 0.084* |
| H58B | 0.049078 | 0.422589 | 0.583856 | 0.084* |
| H58C | 0.175425 | 0.345388 | 0.573376 | 0.084* |
| Cl5 | 0.09977(16) | 0.56877(10) | 0.97597(10) | 0.0889(6) |
| Cl6 | 0.37155(14) | 0.54195(9) | 0.94423(7) | 0.0637(4) |
| C59 | 0.2112(5) | 0.5988(3) | 0.9193(2) | 0.0522(13) |
| H59A | 0.194887 | 0.590249 | 0.870026 | 0.063* |
| H59B | 0.197951 | 0.658917 | 0.920213 | 0.063* |
| Cl7 | 0.3030(2) | 0.21462(14) | 0.43612(11) | 0.1153(8) |
| Cl8 | 0.22549(17) | 0.36718(10) | 0.33731(8) | 0.0739(4) |
| C60 | 0.3448(6) | 0.2885(4) | 0.3872(5) | 0.116(3) |
| H60A | 0.412098 | 0.261285 | 0.354161 | 0.139* |
| H60B | 0.385976 | 0.315192 | 0.419234 | 0.139* |
Source of material
The title product was obtained by the previous methods reported by Lu and Shao’s groups [6], [7], [8], [9], [10], [11], [12], [13]. Under N2 atmosphere, a mixture of 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride (0.275 mmol), PdCl2 (0.25 mmol), K2CO3 (0.375 mmol), THF (2.0 mL) and pyrimidine (0.5 mmol) was stirred at 80 °C for 12 h. The solvent was then removed under reduced pressure, and the residue was purified by careful flash chromatography on silica gel to give the title product in good yield as a yellow solid. Crystals of the title compound were obtained by recrystallization from dichloromethane and ethyl acetate.
Experimental details
All hydrogen atoms attached to C atoms were introduced using the HFIX command in the Olex 21.2 program [1], [2], [3]. The C—H distances in CH3 were restrained to 0.98 Å with Uiso values to be 1.5Ueq(C). Vinylic and aromatic C—H distances were restrained to 0.95 Å with Uiso values to be 1.2Ueq(C). The C—H distances in CH in the isopropyl groups were restrained to 1.00 Å with Uiso values to be 1.2Ueq(C) [4], [5].
Comment
During the past years, the groups of Lu and Shao have developed some N-heterocyclic carbene (NHC)-palladium(II) complexes having different N-containing ancillary ligands. In addition, they were found to be excellent catalysts in the cross-coupling of aryl chlorides, implying that subtle changing of the ancillary ligands, various efficient catalysts can be achieved [6], [7], [8], [9], [10], [11], [12], [13]. In 2012, Wang’s group reported the synthesis of a linear dinuclear NHC-Pd(II) complex using bidentate pyrazine as the ancillary ligand [14]. This result thus prompted to further synthesis similar NHC-Pd(II) complex using other bidentate ligand. Herein, we report the crystal structure of the title compound using pyrimidine as the ancillary ligand.
In the crystal structure, two nearly square-planar Pd(II) centers were found bridged by the bidentate pyrimidine ligand. Both palladium centers are coordinated by four ligands such as NHC, pyrimidine, and two chloro ligands in a slightly distorted square-planar geometry. Both palladium centers have the similar bond lengthes and bond angles. For example, the bond lengthes around the palladium centers are: Pd(1)—Cl(1) = 2.2934(9); Pd(1)—Cl(2) = 2.2927(9); Pd(1)—N(1) = 2.088(3); Pd(1)—C(1) = 1.961(3); Pd(2)—Cl(3) = 2.2965(9); Pd(2)—Cl(4) = 2.2991(9); Pd(2)—N(2) = 2.120(3); Pd(2)—C(2) = 1.966(3). The bond angles around the palladium centers are: Cl(1)—Pd(1)—Cl(2) = 178.38(4); N(1)—Pd(1)—Cl(1) = 89.04(8); N(1)—Pd(1)—Cl(2) = 89.40(8); C(1)—Pd(1)—Cl(1) = 90.08(10); C(1)—Pd(1)—Cl(2) = 91.49(10); Cl(3)—Pd(2)—Cl(4) = 177.29(4); N(2)—Pd(2)—Cl(3) = 90.57(8); N(2)—Pd(2)—Cl(4) = 89.43(8); C(2)—Pd(2)—Cl(3) = 91.04(10); C(2)—Pd(2)—Cl(4) = 89.29(10); C(2)—Pd(2)—N(2) = 173.02(12)°. In addition, the bond lengthes and bond angles are all comparable to those found in the pyrazine derived analogue [14], [15].
Acknowledgements
We acknowledge the financial support from Wenzhou University for the publication fee.
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
2. Sheldrick, G. M.: SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSuche in Google Scholar PubMed PubMed Central
5. Bruker. Bruker AXS SADABS, Version 2.03, SAINT, Version 6.02, Bruker AXS Inc. Madison, Winsonsin, USA (2002).Suche in Google Scholar
6. Zhu, L.; Gao, T.-T.; Shao, L.-X.: Well-defined NHC-Pd(II)-Im (NHC = N-heterocyclic carbene; Im = 1-methylimidazole) complexes catalyzed amination of aryl chlorides. Tetrahedron 67 (2011) 5150–5155.10.1016/j.tet.2011.05.057Suche in Google Scholar
7. Wang, Z.-Y.; Ma, Q.-N.; Li, R.-H.; Shao, L.-X.: Palladium-catalyzed Suzuki-Miyaura coupling of aryl sulfamates with arylboronic acids. Org. Biomol. Chem. 11 (2013) 7899–7906.10.1039/c3ob41382aSuche in Google Scholar PubMed
8. Huang, P.; Wang, Y.-X.; Yu, H.-F.; Lu, J.-M.: N-Heterocyclic carbene-palladium(II)-4,5-dihydrooxazole complexes: synthesis and catalytic activity toward amination of aryl chlorides. Organometallic 33 (2014) 1587–1593.10.1021/om401028dSuche in Google Scholar
9. Liu, F.; Zhu, Y.-R.; Song, L.-G.; Lu, J.-M.: Synthesis of N-heterocyclic carbene-PdCl2-(iso)quinoline complexes and their application in arylamination at low catalyst loadings. Org. Biomol. Chem. 14 (2016) 2563–2571.10.1039/C6OB00013DSuche in Google Scholar PubMed
10. Zhao, X.-Y.; Zhou, Q.; Lu, J.-M.: Synthesis and characterization of N-heterocyclic carbene-palladium(II) chlorides-1-methylindazole and -1-methylpyrazole complexes and their catalytic activity toward C-N coupling of aryl chlorides. RSC Adv. 6 (2016) 24484–24490.10.1039/C6RA02556KSuche in Google Scholar
11. Zhang, Z.-M.; Gao, Y.-J.; Lu, J.-M.: Synthesis of N-heterocyclic carbene-Pd(II) complexes and their catalytic activity in the Buchwald-Hartwig amination of aryl chlorides. Tetrahedron 73 (2017) 7308–7314.10.1016/j.tet.2017.11.026Suche in Google Scholar
12. Liu, F.; Hu, Y.-Y.; Li, D.; Zhou, Q.; Lu, J.-M.: N-Heterocyclic carbene-palladacyclic complexes: synthesis, characterization and their applications in the C-N coupling and α-arylation of ketones using aryl chlorides. Tetrahedron 74 (2018) 5683–5690.10.1016/j.tet.2018.07.052Suche in Google Scholar
13. Sun, K.-X.; He, Q.-W.; Xu, B.-B.; Wu, X.-T.; Lu, J.-M.: Synthesis of N-heterocyclic carbene-Pd(II)-2-methyl-4,5-dihydrooxazole complexes and their application toward highly chemoselective mono-Suzuki-Miyaura coupling of dichlorobenzenes. Asian J. Org. Chem. 7 (2018) 781–787.10.1002/ajoc.201800001Suche in Google Scholar
14. Yang, J.; Wang, L.: Synthesis and characterization of dinuclear NHC-palladium complexes and their applications in the Hiyama reactions of aryltrialkyoxysilanes with aryl chlorides. Dalton Trans. 41 (2012) 12031–12037.10.1039/c2dt31174gSuche in Google Scholar PubMed
15. Yang, J.: Mono- and dinuclear N-heterocyclic carbene palladium complexes with diazine ligands and their catalytic activities toward the Mizoroki–Heck reaction. J. Coord. Chem. 70 (2017) 3749–3758.10.1080/00958972.2017.1395416Suche in Google Scholar
©2019 Xiao-Yun Zhao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3


