Abstract
C51H38Cl2F2N6O14S2, triclinic, P1̄ (no. 2), a = 11.8987(6) Å, b = 14.0939(8) Å, c = 16.1915(9) Å, α = 67.590(5)°, β = 75.402(5)°, γ = 77.522(4)°, V = 2407.3(2) Å3, Z = 2, Rgt(F) = 0.0584, wRref(F2) = 0.1645, T = 100.0(1) K.
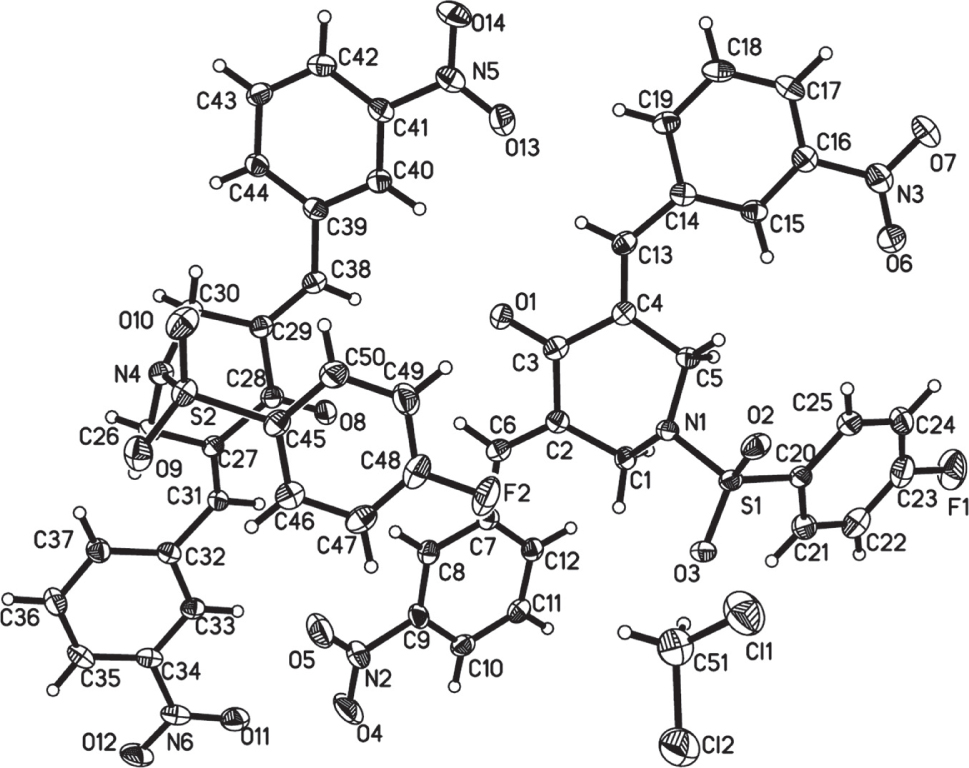
The asymmetric unit of the title molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.12 × 0.11 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.31 mm−1 |
| Diffractometer, scan mode: | SuperNova, |
| θmax, completeness: | 29.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 20754, 11248, 0.028 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 8621 |
| N(param)refined: | 694 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.3285(2) | 0.59236(19) | 0.62774(17) | 0.0171(5) |
| H1A | 0.406713 | 0.557480 | 0.613515 | 0.020* |
| H1B | 0.273239 | 0.542990 | 0.647586 | 0.020* |
| C2 | 0.3219(2) | 0.63555(19) | 0.70138(17) | 0.0158(5) |
| C3 | 0.2338(2) | 0.72859(19) | 0.70366(18) | 0.0170(5) |
| C4 | 0.1496(2) | 0.76505(19) | 0.63950(18) | 0.0163(5) |
| C5 | 0.1757(2) | 0.7245(2) | 0.56201(18) | 0.0164(5) |
| H5A | 0.126611 | 0.671592 | 0.575997 | 0.020* |
| H5B | 0.158350 | 0.780313 | 0.507114 | 0.020* |
| C6 | 0.3921(2) | 0.59859(19) | 0.76293(18) | 0.0167(5) |
| H6 | 0.382410 | 0.634951 | 0.802138 | 0.020* |
| C7 | 0.4828(2) | 0.50708(19) | 0.77592(17) | 0.0157(5) |
| C8 | 0.5844(2) | 0.5122(2) | 0.80113(18) | 0.0172(5) |
| H8 | 0.593025 | 0.570908 | 0.810904 | 0.021* |
| C9 | 0.6723(2) | 0.4286(2) | 0.81140(18) | 0.0187(5) |
| C10 | 0.6615(2) | 0.3368(2) | 0.80315(18) | 0.0199(6) |
| H10 | 0.721193 | 0.281203 | 0.811996 | 0.024* |
| C11 | 0.5585(2) | 0.3309(2) | 0.78125(18) | 0.0190(5) |
| H11 | 0.548005 | 0.269839 | 0.776401 | 0.023* |
| C12 | 0.4709(2) | 0.4150(2) | 0.76648(17) | 0.0168(5) |
| H12 | 0.403396 | 0.410330 | 0.750076 | 0.020* |
| C13 | 0.0548(2) | 0.83261(19) | 0.65495(18) | 0.0172(5) |
| H13 | 0.049903 | 0.851452 | 0.705019 | 0.021* |
| C14 | −0.0418(2) | 0.88096(19) | 0.60503(17) | 0.0162(5) |
| C15 | −0.0682(2) | 0.8488(2) | 0.54095(18) | 0.0176(5) |
| H15 | −0.023425 | 0.792140 | 0.527102 | 0.021* |
| C16 | −0.1622(2) | 0.9030(2) | 0.49853(18) | 0.0190(5) |
| C17 | −0.2337(2) | 0.9871(2) | 0.5168(2) | 0.0208(6) |
| H17 | −0.295272 | 1.022347 | 0.486330 | 0.025* |
| C18 | −0.2096(2) | 1.0163(2) | 0.5819(2) | 0.0215(6) |
| H18 | −0.257034 | 1.071198 | 0.597151 | 0.026* |
| C19 | −0.1157(2) | 0.9650(2) | 0.62506(19) | 0.0182(5) |
| H19 | −0.100992 | 0.986762 | 0.668396 | 0.022* |
| C20 | 0.2633(2) | 0.5944(2) | 0.43145(17) | 0.0166(5) |
| C21 | 0.2977(2) | 0.4880(2) | 0.45398(19) | 0.0207(6) |
| H21 | 0.366107 | 0.457178 | 0.476839 | 0.025* |
| C22 | 0.2287(3) | 0.4293(2) | 0.4418(2) | 0.0261(6) |
| H22 | 0.250149 | 0.358288 | 0.455516 | 0.031* |
| C23 | 0.1275(3) | 0.4777(3) | 0.4090(2) | 0.0274(7) |
| C24 | 0.0912(3) | 0.5828(3) | 0.3868(2) | 0.0276(7) |
| H24 | 0.021818 | 0.612626 | 0.365150 | 0.033* |
| C25 | 0.1608(2) | 0.6423(2) | 0.39769(19) | 0.0225(6) |
| H25 | 0.139532 | 0.713452 | 0.382660 | 0.027* |
| C26 | 0.6508(2) | 0.77958(19) | 0.99781(18) | 0.0166(5) |
| H26A | 0.644556 | 0.769370 | 1.061441 | 0.020* |
| H26B | 0.733265 | 0.776675 | 0.970130 | 0.020* |
| C27 | 0.6019(2) | 0.6920(2) | 0.99134(17) | 0.0156(5) |
| C28 | 0.4854(2) | 0.7147(2) | 0.96240(18) | 0.0166(5) |
| C29 | 0.4122(2) | 0.81629(19) | 0.96007(17) | 0.0157(5) |
| C30 | 0.4627(2) | 0.8900(2) | 0.98407(18) | 0.0170(5) |
| H30A | 0.427253 | 0.960309 | 0.955797 | 0.020* |
| H30B | 0.444176 | 0.873815 | 1.049433 | 0.020* |
| C31 | 0.6539(2) | 0.59328(19) | 1.01301(17) | 0.0153(5) |
| H31 | 0.615877 | 0.548453 | 1.003232 | 0.018* |
| C32 | 0.7614(2) | 0.54555(19) | 1.04973(17) | 0.0155(5) |
| C33 | 0.8092(2) | 0.44626(19) | 1.04741(17) | 0.0165(5) |
| H33 | 0.773522 | 0.413815 | 1.022385 | 0.020* |
| C34 | 0.9094(2) | 0.3968(2) | 1.08246(18) | 0.0181(5) |
| C35 | 0.9648(2) | 0.4394(2) | 1.12283(19) | 0.0216(6) |
| H35 | 1.031483 | 0.403905 | 1.146765 | 0.026* |
| C36 | 0.9175(2) | 0.5370(2) | 1.1263(2) | 0.0231(6) |
| H36 | 0.952992 | 0.567959 | 1.152760 | 0.028* |
| C37 | 0.8177(2) | 0.5890(2) | 1.09093(19) | 0.0191(5) |
| H37 | 0.787260 | 0.654219 | 1.094454 | 0.023* |
| C38 | 0.3091(2) | 0.83801(19) | 0.93303(18) | 0.0172(5) |
| H38 | 0.289568 | 0.788308 | 0.916321 | 0.021* |
| C39 | 0.2232(2) | 0.93215(19) | 0.92683(18) | 0.0164(5) |
| C40 | 0.1564(2) | 0.9673(2) | 0.85843(18) | 0.0174(5) |
| H40 | 0.165495 | 0.931887 | 0.818536 | 0.021* |
| C41 | 0.0766(2) | 1.0559(2) | 0.85170(18) | 0.0183(5) |
| C42 | 0.0588(2) | 1.1118(2) | 0.90948(19) | 0.0203(6) |
| H42 | 0.005800 | 1.172217 | 0.901918 | 0.024* |
| C43 | 0.1227(2) | 1.0746(2) | 0.97870(19) | 0.0194(6) |
| H43 | 0.111660 | 1.109531 | 1.019223 | 0.023* |
| C44 | 0.2034(2) | 0.9851(2) | 0.98795(18) | 0.0175(5) |
| H44 | 0.244768 | 0.960199 | 1.035374 | 0.021* |
| C45 | 0.6177(2) | 0.8921(2) | 0.77609(18) | 0.0187(5) |
| C46 | 0.6962(2) | 0.8088(2) | 0.76228(18) | 0.0198(5) |
| H46 | 0.760629 | 0.783261 | 0.790874 | 0.024* |
| C47 | 0.6767(3) | 0.7642(2) | 0.70494(19) | 0.0222(6) |
| H47 | 0.727432 | 0.707831 | 0.694857 | 0.027* |
| C48 | 0.5812(3) | 0.8049(2) | 0.6634(2) | 0.0253(6) |
| C49 | 0.5014(3) | 0.8866(2) | 0.6773(2) | 0.0272(6) |
| H49 | 0.436530 | 0.910984 | 0.649180 | 0.033* |
| C50 | 0.5207(2) | 0.9317(2) | 0.73432(19) | 0.0240(6) |
| H50 | 0.469207 | 0.987761 | 0.744461 | 0.029* |
| C51 | 0.6996(3) | 0.7302(3) | 0.3190(3) | 0.0393(8) |
| H51A | 0.638414 | 0.688575 | 0.357481 | 0.047* |
| H51B | 0.718445 | 0.765757 | 0.353578 | 0.047* |
| Cl1 | 0.64805(8) | 0.82089(7) | 0.22466(7) | 0.0446(2) |
| Cl2 | 0.82525(8) | 0.64819(7) | 0.28940(7) | 0.0454(2) |
| F1 | 0.06066(17) | 0.41963(16) | 0.39576(14) | 0.0409(5) |
| F2 | 0.56466(17) | 0.76282(15) | 0.60517(13) | 0.0364(5) |
| N1 | 0.29952(18) | 0.68065(16) | 0.54724(15) | 0.0157(4) |
| N2 | 0.7835(2) | 0.43892(19) | 0.82920(17) | 0.0250(5) |
| N3 | −0.1891(2) | 0.86664(19) | 0.43283(18) | 0.0257(5) |
| N4 | 0.59040(19) | 0.88264(16) | 0.95335(15) | 0.0166(4) |
| N5 | 0.0046(2) | 1.09108(18) | 0.78081(17) | 0.0232(5) |
| N6 | 0.9613(2) | 0.29491(17) | 1.07519(16) | 0.0213(5) |
| O1 | 0.22913(17) | 0.77193(15) | 0.75744(13) | 0.0219(4) |
| O2 | 0.33192(17) | 0.77294(14) | 0.38099(13) | 0.0215(4) |
| O3 | 0.46790(15) | 0.61664(14) | 0.44778(13) | 0.0188(4) |
| O4 | 0.8588(2) | 0.36308(18) | 0.84322(19) | 0.0411(6) |
| O5 | 0.79632(19) | 0.52369(17) | 0.82756(17) | 0.0329(5) |
| O6 | −0.13104(19) | 0.78789(15) | 0.42119(15) | 0.0289(5) |
| O7 | −0.2704(2) | 0.91683(19) | 0.39251(17) | 0.0386(6) |
| O8 | 0.45046(17) | 0.65123(14) | 0.94342(14) | 0.0233(4) |
| O9 | 0.76788(17) | 0.93781(16) | 0.84201(14) | 0.0253(4) |
| O10 | 0.57626(19) | 1.04971(14) | 0.82985(14) | 0.0259(5) |
| O11 | 0.91975(18) | 0.26238(15) | 1.03116(15) | 0.0276(5) |
| O12 | 1.04559(18) | 0.24864(16) | 1.11218(15) | 0.0305(5) |
| O13 | 0.02287(19) | 1.04271(16) | 0.72889(15) | 0.0280(5) |
| O14 | −0.0694(2) | 1.16705(18) | 0.77640(17) | 0.0391(6) |
| S1 | 0.35120(5) | 0.67091(5) | 0.44594(4) | 0.01573(15) |
| S2 | 0.64384(6) | 0.94941(5) | 0.84852(4) | 0.01867(15) |
Source of material
The raw material 3-nitrobenzaldehyde (1.51 mol, 10.0 mmol) and 4-piperidone hydrate hydrochloride (0.77 g, 5.0 mmol) were added to a solution with 25 mL acetic acid. Dry HCL gas was passed through the solution about 25 min. After stirring at room temperature overnight, the mixture produced a precipitate. The precipitate was filtered and washed by acetone to get a yellow intermediate. Then, the intermediate, 4-fluorobenzenesulfonylchloride (0.62 g, 5.0 mol) and potassium carbonate (2.76 g, 0.02 mol) were added to 50 mL of dichloromethane. The mixture was stirred for about 12 h at room temperature. The precipitate was collected, washed with water and recrystallized from dichloromethane/methanol (2:3, v/v) to get light yellow crystals of the title compound.
Experimental details
All H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C—H) = 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C) and d(C—H) = 0.93 Å (aromatic), Uiso(H) = 1.2Ueq(C).
Comment
Curcumin, is a yellow pigment extracted from the rhizome of turmeric, which has been extensively investigated for its anti-tumor, anti-inflammatory activity, anti-oxidation and other activities. However, its clinical application was limited due to its poor aqueous solubility, relatively low bioactivities and bioavailability [4]. In order to improve these weakness, many curcumin analogs were previously synthesized as better antitumor and anti-inflammatory agents, such as 3E,5E)-3,5-bis(arylidene)-4-piperidones (BAPs) [5], [6]. In our previous study, we found BAPs have greater predilection or sequential interaction for bio-thiols resulting in a greater activity to tumors rather than with normal cells [7].
In order to improve its biological activity, strong electron-withdrawing substituent groups (Such as -NO2, -CN, -CF3) were introduced on both sides of BAPs. While some electron-withdrawing trifluoromethyl groups (Such as -F, -NO2, -CN, -CF3) were introduced into N-benzenesulfonyl substituents of BAPs. By introducing these groups, the antitumor and anti-inflammatory activity of BAPs should be improved, while toxicity should be reduced to different degrees [8].
Crystal structure and bioactivity of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile (BAP-1) and (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one (BAP-2) were reported by our group [9], [10]. Recently, we found that the antitumor and anti-inflammatory activity were improved when fluorine was substituted on N-benzenesulfonyl substituents of BAPs. Herein, the crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one-dichloromethane(2/1) will be reported.
There are two BAP molecules and a dichloromethane molecule in the asymmetric unit of the title compound (cf. the figure). Bond lengths and angles are all in the expected ranges. Single-crystal structure analysis reveals that configurations of the two BAPs molecules are different. In a molecule, two 3-nitro phenyl groups on both sides of BAP take the trans-configuration compared with the central piperidin-4-one. And the two 3-nitro phenyl groups adopt the E stereochemistry of olefinic double bonds, which was the same as those reported in literature [10]. In the other molecule, two 3-nitro phenyl groups are symmetric compared with central piperidin-4-one, which also adopt the E stereochemistry of the double bond. The dihedral angles between two 4-nitro phenyl groups in both molecules are 32.61(6)° and 26.36(3)°, respectively. Interestingly, the 4-trifluoromethylphenyl group bend towards the side of carbonyl group in one molecule, which looks like an “organic clip” [10], however, 4-trifluoromethylphenyl group stretch in the other direction of carbonyl group in another molecule. The peripheric heteroatoms (Such as F, N, O, S) can act as hydrogen bonding acceptors for bioactive molecules with the aim of creating more potent biological activity [11], [12].
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81601049
Funding source: Key R & D project of Shandong Province
Award Identifier / Grant number: 2018YYSP009
Funding source: Shandong Provincial Medicine and Health Technology Development Plan
Award Identifier / Grant number: 2017WSB290215
Funding statement: This work was supported by the National Natural Science Foundation of China (no. 81601049), Key R & D project of Shandong Province (No. 2018YYSP009) and Shandong Provincial Medicine and Health Technology Development Plan (No. 2017WSB290215).
References
1. Rigaku. CrysAlisPRO. Rigaku Oxford Diffraction Ltd, Yarnton, Oxfordshire, England (2017).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Nelson, K. M.; Dahlin, J. L.; Bisson, J.; Graham, J.; Pauli, G. F.; Walters, M. A.: The essential medicinal chemistry of curcumin. J. Med. Chem. 60 (2017) 1620–1637.10.1021/acs.jmedchem.6b00975Suche in Google Scholar PubMed PubMed Central
5. Zhang, L. S.; Chen, Q.; Hou, G. G.; Zhao, W.; Hou, Y.: Hydroxyl-substituted double Schiff-base condensed 4-piperidone/cyclohexanones as potential anticancer agents with biological evaluation. J. Enzyme Inhib. Med. Chem. 34 (2019) 264–271.10.1080/14756366.2018.1501042Suche in Google Scholar PubMed PubMed Central
6. Li, N.; Xin, W. Y.; Yao, B. R.; Cong, W.; Wang, C. H.; Hou, G. G.: N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 155 (2018) 531–544.10.1016/j.ejmech.2018.06.027Suche in Google Scholar PubMed
7. Yao, B. R.; Li, N.; Wang, C. H.; Hou, G. G.; Meng, Q. G.; Yan, K.: Novel asymmetric 3,5-bis(arylidene)piperidin-4-one derivatives: synthesis, crystal structures and cytotoxicity. Acta Crystallogr. C74 (2018) 659–665.10.1107/S2053229618006605Suche in Google Scholar PubMed
8. Yao, B. R.; Sun, Y.; Chen, S. L.; Suo, H. D.; Zhang, Y. L.; Wei, H.; Wang, C. H.; Zhao, F.; Cong, W.; Xin, W. Y.; Hou, G. G.: Dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones as anti-hepatoma agents by inhibiting NF-kB pathway activation. Eur. J. Med. Chem. 167 (2019) 187–199.10.1016/j.ejmech.2019.02.020Suche in Google Scholar PubMed
9. Liu, L. D.; Liu, S. L.; Hou, G. G.: Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S. Z. Kristallogr. NCS 233 (2018) 1063–1065.10.1515/ncrs-2018-0174Suche in Google Scholar
10. Li, X. Y.; Meng, Q. G.; Hou, G. G.: Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S. Z. Kristallogr. NCS 234 (2019) 771–773.10.1515/ncrs-2019-0097Suche in Google Scholar
11. Li, N.; Xin, W. Y.; Yao, B. R.; Wang, C. H.; Cong, W.; Zhao, F.; Li, H. J.; Hou, Y.; Meng, Q. G.; Hou, G. G.: Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 147 (2018) 21–33.10.1016/j.ejmech.2018.01.088Suche in Google Scholar PubMed
12. Li, N.; Bai, X. Y.; Zhang, L. S.; Hou, Y.: Synthesis, crystal structures and anti-inflammatory activity of four 3,5-bis(arylidene)-N-benzenesulfonyl-4-piperidone derivatives. Acta Crystallogr. C74 (2018) 1171–1179.10.1107/S2053229618013232Suche in Google Scholar PubMed
©2019 Qin Zhao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3


