Abstract
C20H14N2O2, monoclinic, C2/c (no. 15), a = 17.8410(5) Å, b = 15.3081(4) Å, c = 11.3881(3) Å, β = 104.280(1)°, V = 3014.12(14) Å3, Z = 8, Rgt(F) = 0.0364, wRref(F2) = 0.0997, T = 100(2) K.
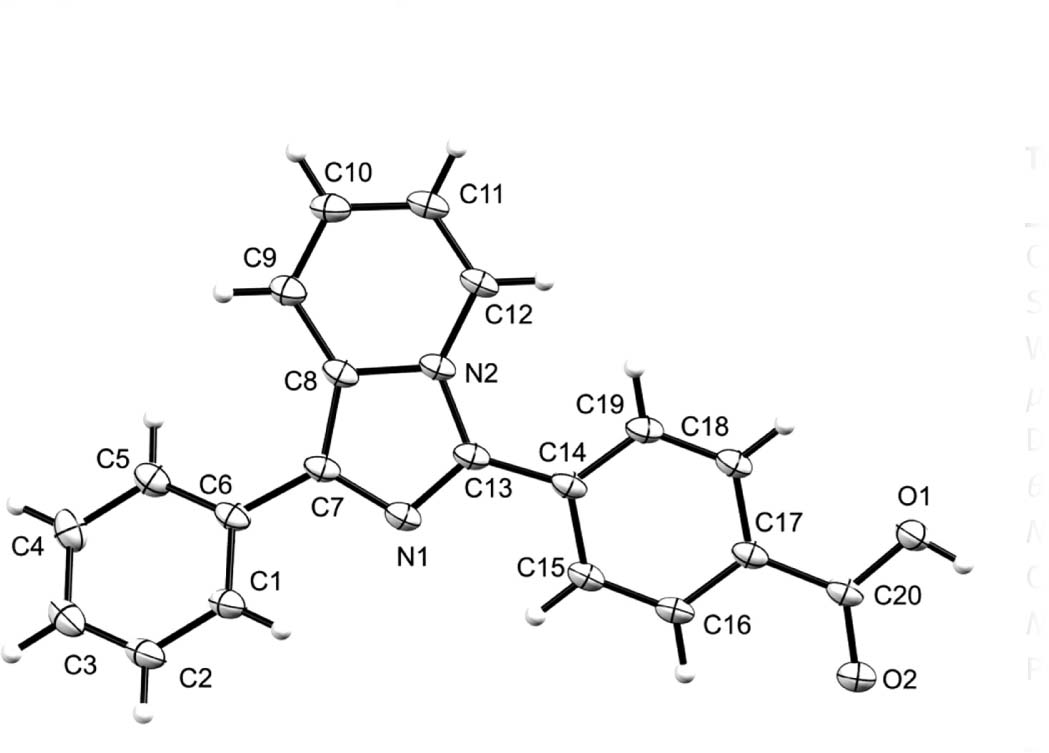
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow needle |
| Size: | 0.27 × 0.16 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX-II, φ and ω |
| θmax, completeness: | 26.0°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 9187, 2897, 0.018 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2410 |
| N(param)refined: | 219 |
| Programs: | Bruker [1], SHELX [2], [3], Mercury [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.45803(6) | 0.58344(6) | 0.89839(9) | 0.0332(2) |
| H1a | 0.485522 | 0.571311 | 0.967603 | 0.050* |
| O2 | 0.45394(6) | 0.43778(6) | 0.88330(10) | 0.0336(3) |
| H2a | 0.479068 | 0.442588 | 0.955813 | 0.050* |
| N1 | 0.20104(7) | 0.46362(7) | 0.31121(11) | 0.0273(3) |
| N2 | 0.22653(6) | 0.60197(7) | 0.27783(11) | 0.0261(3) |
| C1 | 0.08324(8) | 0.34730(8) | 0.17026(15) | 0.0323(3) |
| H1A | 0.089667 | 0.344332 | 0.255496 | 0.039* |
| C2 | 0.04545(8) | 0.28042(9) | 0.09727(16) | 0.0383(4) |
| H2A | 0.025986 | 0.232030 | 0.132816 | 0.046* |
| C3 | 0.03580(8) | 0.28345(10) | −0.02699(16) | 0.0398(4) |
| H3 | 0.010928 | 0.236780 | −0.076569 | 0.048* |
| C4 | 0.06266(8) | 0.35498(10) | −0.07829(15) | 0.0389(4) |
| H4 | 0.055527 | 0.357907 | −0.163668 | 0.047* |
| C5 | 0.09999(8) | 0.42253(9) | −0.00583(14) | 0.0333(3) |
| H5 | 0.117576 | 0.471745 | −0.042280 | 0.040* |
| C6 | 0.11203(7) | 0.41922(8) | 0.11944(13) | 0.0274(3) |
| C7 | 0.15896(8) | 0.48505(8) | 0.19842(13) | 0.0266(3) |
| C8 | 0.17283(8) | 0.57220(8) | 0.17395(13) | 0.0270(3) |
| C9 | 0.14577(8) | 0.63081(9) | 0.07735(14) | 0.0317(3) |
| H9 | 0.106713 | 0.613206 | 0.008572 | 0.038* |
| C10 | 0.17598(8) | 0.71284(9) | 0.08326(15) | 0.0357(4) |
| H10 | 0.157382 | 0.752973 | 0.019050 | 0.043* |
| C11 | 0.23537(8) | 0.73849(9) | 0.18550(14) | 0.0333(3) |
| H11 | 0.258019 | 0.794774 | 0.187020 | 0.040* |
| C12 | 0.25989(8) | 0.68422(8) | 0.28002(14) | 0.0294(3) |
| H12 | 0.299695 | 0.701931 | 0.347744 | 0.035* |
| C13 | 0.24128(8) | 0.53362(8) | 0.35861(13) | 0.0261(3) |
| C14 | 0.29179(8) | 0.53397(8) | 0.48083(13) | 0.0264(3) |
| C15 | 0.32061(8) | 0.45291(8) | 0.52923(13) | 0.0285(3) |
| H15 | 0.308096 | 0.401752 | 0.481162 | 0.034* |
| C16 | 0.36645(8) | 0.44626(8) | 0.64448(13) | 0.0285(3) |
| H16 | 0.385193 | 0.390681 | 0.675557 | 0.034* |
| C17 | 0.38587(7) | 0.52053(8) | 0.71677(13) | 0.0267(3) |
| C18 | 0.35693(8) | 0.60152(8) | 0.66987(13) | 0.0288(3) |
| H18 | 0.369701 | 0.652565 | 0.718102 | 0.035* |
| C19 | 0.31004(8) | 0.60802(8) | 0.55434(13) | 0.0291(3) |
| H19 | 0.289904 | 0.663335 | 0.524293 | 0.035* |
| C20 | 0.43548(8) | 0.51214(8) | 0.83985(13) | 0.0276(3) |
aOccupancy: 0.5.
Source of material
To anhydrous ethanol solution (10 mL) of 2-benzoylpyridine (1 mmol, 0.183 g) was added anhydrous ethanol solution (15 mL) of 4-(aminomethyl)benzoic acid (1 mmol, 0.151 g) in the presence of few drops of concentrated hydrochloric acid as a catalyst and refluxed at 80 °C for 48 h using a modified procedure from literature [5], [6]. The resulting off-white solid was isolated by filtration and washed three times with small amounts of ethanol and the white solid was then recrystallized from a solution of ethanol to give a needle crystals. Yield: (55.74%, 0.175 g), m.p.: 186–187 °C; 1H-NMR: (400 MHz, CD3OD) δ ppm = 8.60 (1H, d), 8.37 (2H, d, J = 8.70 Hz), 8.10 (3H, m), 7.89 (2H, m), 7.67 (2H, m), 7.59 (1H, m), 7.40 (1H, m), 7.26 (1H, m), 4.89 (2H, s); 13C-NMR: (400 MHz, CD3OD) δ ppm = 168.24, 135.27, 131.95, 131.03, 130.90, 130.77, 130.68, 129.15, 128.82, 128.14, 127.63, 126.57, 126.45, 123.75, 120.19, 120.10. MS (ESI): m/z Calc. for [C20H14N2O2]: 314.34; found [M+]: 315 (100%), 316 (24%).
Experimental details
The structure was solved by the direct method using the SHELXS [2] program and refined. The visual crystal structure information was performed using Mercury [4] system software. The C—H and O—H distances were restrained to 0.950 Å and 0.84 Å, respectively with Uiso(H) = 1.2Ueq(C) and Uiso(H) = 1.5Ueq(O). The carboxylate hydrogen was found to be disordered over two positions.
Comment
Heterocyclic compounds possessing both the imidazole and pyridines moieties are of great importance as ligands in inorganic synthesis [7]. The title compound is known to be present in the structures of some biologically active compounds, thus giving it some importance. The compound has a great potential to be applied in other fields such as material science [8], organic synthesis [9], in lighting and photovoltaic technologies [8], and in pharmaceutical science where imidazole[1,5-a]pyridines moiety has been shown to be active against HIV protease [10], as a cardiotonic agent [11] and also as an anti-tumour agent [12] among others. They are also known to be used as precursors for synthesis of N-heterocyclic carbenes [13], as ligands in coordination chemistry [14] and as pH-probes [15]. A number of methods have been used in the preparation of an imidazole[1,5-a]pyridine. One is the Vilsmeier-type cyclization reaction along with the variant methods [16], [17]. Other methods involve the usage of organometallic catalysts [18] or an oxidant [19] and also the one-pot condensation reactions of amines and aldehydes [20], [21]. In an attempt to synthesize a Schiff base using the one-pot condensation reaction using 4-(aminomethyl)benzoic acid and 2-benzoylpyridine, imidazole[1,5-a]pyridine moiety was isolated as a product in agreement with Wang et al. [20] and Volpi et al. [21].
The asymmetric unit of the title compound contains one molecule defined by a benzoic acid moiety, an imidazole[1,5-a]pyridine moiety with an attached phenyl ring, all non coplanar. The dihedral angles between the imidazole[1,5-a]pyridine moiety, the benzoic acid moiety, and the phenyl ring moiety are 27.3 and 29.9(9)°, respectively. The Npyridinyl–Cbenzoic acid, Cbenzoic acid–Nimidazole and the Nimidazole–Cphenyl are similar to those of reported analogues [14], [21], [22], [23], [24]. In the crystal, two molecules are centrosymmetrically connected through intermolecular O—H⋯Oi hydrogen bonds (symmetry code: (i) = 1 − x, 1 − y, 2 − z). The centrosymmetric dimers are further connected through Cgpyridine⋯Cgbenzoic acid interaction and the distance between them is 3.9676(8) Å (symmetry code: (i) = x, 1 − y, −1/2 − z).
Funding source: National Research Foundation of South Africa
Award Identifier / Grant number: 119342
Funding statement: We appreciate the University of KwaZulu-Natal and the National Research Foundation of South Africa (Grant number: 119342) for their financial assistance for Ms Adesola A. Adeleke.
References
1. Bruker. APEXII. Bruker AXS Inc, Madison, WI, USA (2009).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.10.1107/S0021889807067908Suche in Google Scholar
5. Abdel-Monem, Y. K.; Abouel-Enein, S. A.; El-Seady, S. M.: Synthesis, characterization and molecular modeling of some transition metal complexes of Schiff base derived from 5-aminouracil and 2-benzoyl pyridine. J. Mol. Struct. 1152 (2018) 115–127.10.1016/j.molstruc.2017.09.038Suche in Google Scholar
6. Njogu, E. M.; Omondi, B.; Nyamori, V. O.: Silver(I)-pyridinyl Schiff base complexes: synthesis, characterization and antimicrobial studies. J. Mol. Struct. 1135 (2017) 118–128.10.1016/j.molstruc.2017.01.061Suche in Google Scholar
7. Anitha, P.; Manikandan, R.; Prakash, G.; Pachiyappan, B.; Viswanathamurthi, P.; Malecki, J. G.: Ruthenium(II) 8-quinolinolates: synthesis, characterization, crystal structure and catalysis in the synthesis of 2-oxazolines. J. Organomet. Chem. 791 (2015) 266–273.10.1016/j.jorganchem.2015.06.005Suche in Google Scholar
8. Tavasli, M.; Moore, T. N.; Zheng, Y.; Bryce, M. R.; Fox, M. A.; Griffiths, G. C.; Jankus, V.; Al-Attar, H. A.; Monkman, A. P.: Colour tuning from green to red by substituent effects in phosphorescent tris-cyclometalated iridium(III) complexes of carbazole-based ligands: synthetic, photophysical, computational and high efficiency OLED studies. J. Mater. Chem. 22 (2012) 6419–6428.10.1039/c2jm15049bSuche in Google Scholar
9. Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernández, R.; Brown, J. M.; Lassaletta, J. M.: Imidazo[1,5-a]pyridine: a versatile architecture for stable N-heterocyclic carbenes. J. Am. Chem. Soc. 127 (2005) 3290–3291.10.1021/ja0423769Suche in Google Scholar PubMed
10. Ganguly, S.; Vithlani, V.; Kesharwani, A.; Kuhu, R.; Baskar, L.; Mitramazumder, P.; Sharon, A.; Dev, A.: Synthesis, antibacterial and potential anti-HIV activity of some novel imidazole analogs. Acta Pharm. 61 (2011) 187–201.10.2478/v10007-011-0018-2Suche in Google Scholar PubMed
11. Kwong, H. C.; Chidan Kumar, C. S.; Mah, S. H.; Mah, Y. L.; Chia, T. S.; Quah, C. K.; Lim, G. K.; Chandraju, S.: Crystal correlation of heterocyclic imidazo[1,2-a]pyridine analogues and their anticholinesterase potential evaluation. Sci Rep. 9 (2019) 926.10.1038/s41598-018-37486-7Suche in Google Scholar PubMed PubMed Central
12. Nassar, I. F.; Atta-Allah, S. R.; Elgazwy, A. S.: A convenient synthesis and molecular modeling study of novel pyrazolo[3,4-d]pyrimidine and pyrazole derivatives as anti-tumor agents. J. Enzyme Inhib. Med. Chem. 30 (2015) 396–405.10.3109/14756366.2014.940936Suche in Google Scholar PubMed
13. Herrmann, W. A.; Köcher, C.; Gooßen, L. J.; Artus, G. R. J.: Heterocyclic carbenes: a high-yielding synthesis of novel, functionalized N-heterocyclic carbenes in liquid ammonia. Chem. Eur. J. 2 (1996) 1627—1636.10.1002/chem.19960021222Suche in Google Scholar
14. Ardizzoia, G. A.; Brenna, S.; Durini, S.; Therrien, B.: Synthesis and characterization of luminescent zinc(II) complexes with a N,N-bidentate 1-pyridylimidazo[1,5-a]pyridine ligand. Polyhedron 90 (2015) 214–220.10.1016/j.poly.2015.02.005Suche in Google Scholar
15. Hutt, J. T.; Jo, J.; Olasz, A.; Chen, C.-H.; Lee, D.; Aron, Z. D.: Fluorescence switching of imidazo[1,5-a]pyridinium ions: pH-sensors with dual emission pathways. Org. Lett. 14 (2012) 3162–3165.10.1021/ol3012524Suche in Google Scholar PubMed
16. Huang, C.; Giokaris, A.; Gevorgyan, V.: Palladium-catalyzed highly regioselective C-3 arylation of imidazo[1,5-a]pyridine. Chem. Lett. 40 (2011) 1053–1054.10.1246/cl.2011.1053Suche in Google Scholar PubMed PubMed Central
17. Wang, H.; Xu, W.; Xin, L.; Liu, W.; Wang, Z.; Xu, K.: Synthesis of 1,3-disubstituted imidazo[1,5-a]pyridines from amino acids via catalytic decarboxylative intramolecular cyclization. J. Org. Chem. 81 (2016) 3681–3687.10.1021/acs.joc.6b00343Suche in Google Scholar PubMed
18. Li, Y.; Chao, A.; Fleming, F. F.: Isonitrile alkylations: a rapid route to imidazo[1,5-a]pyridines. Chem. Commun. 52 (2016) 2111–2113.10.1039/C5CC08724DSuche in Google Scholar
19. Chen, L.; Zhu, H.; Wang, J.; Lui, H.: One-pot NBS-promoted synthesis of imidazoles and thiazoles from ethylarenes in water. Molecules 24 (2019) 893–90510.3390/molecules24050893Suche in Google Scholar PubMed PubMed Central
20. Wang, J.; Mason, R.; VanDerveer, D.; Feng, K.; Bu, X. R.: Convenient preparation of a novel class of imidazo[1,5-a]pyridines: decisive role by ammonium acetate in chemoselectivity. J. Org. Chem. 68 (2003) 5415–5418.10.1021/jo0342020Suche in Google Scholar PubMed
21. Volpi, G.; Garino, C.; Priola, E.; Diana, E.; Gobetto, R.; Buscaino, R.; Viscardi, G.; Barolo, C.: Facile synthesis of novel blue light and large Stoke shift emitting tetradentate polyazines based on imidazo[1,5-a] pyridine. Dyes Pigments 143 (2017) 284–290.10.1016/j.dyepig.2017.04.034Suche in Google Scholar
22. Sheng, H.; Hu, Y.; Zhou, Y.; Fan, S.; Cao, Y.; Zhao, X.; Yang, W.: A highly selective ESIPT-based fluorescent probe with a large Stokes shift for the turn-on detection of cysteine and its application in living cells. Dyes Pigments 160 (2019) 48–57.10.1016/j.dyepig.2018.07.036Suche in Google Scholar
23. Volpi, G.; Garino, C.; Conterosito, E.; Barolo, C.; Gobetto, R.; Viscardi, G.: Facile synthesis of novel blue light and large Stoke shift emitting tetradentate polyazines based on imidazo[1,5-a]pyridine. Dyes Pigments 128 (2016) 96–100.10.1016/j.dyepig.2015.12.005Suche in Google Scholar
24. Volpi, G.; Magistris, C.; Garino, C.: FLUO-SPICES: natural aldehydes extraction and one-pot reaction to prepare and characterize new interesting fluorophores. Educ. Chem. Eng. 24 (2018) 1–6.10.1016/j.ece.2018.06.002Suche in Google Scholar
©2019 Adeleke A. Adesola et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Artikel in diesem Heft
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3

