Abstract
C21H14ClN, orthorhombic, Pca21 (No. 29), a = 7.6860(5) Å, b = 10.1610(5) Å, c = 19.8990(5) Å, V = 1554.1(13) Å3, Z = 4, Rgt(F) = 0.0318, wRref(F2) = 0.0783, T = 100(2) K.
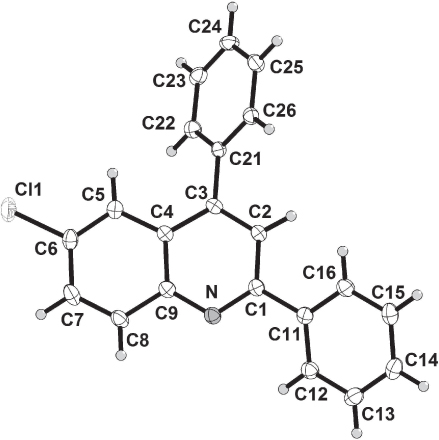
The crystal structure is shown in the figure, Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow, cuboid, size 0.389×0.47×0.651 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.44 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II CCD, φ and ω scans |
| 2θmax: | 55.99° |
| N(hkl)measured, N(hkl)unique: | 26440, 3754 |
| N(param)refined: | 208 |
| Programs: | Bruker data collection and reduction software [21], SHELX [22], Diamond [23] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(5) | 4a | 0.6766 | −0.1534 | 0.6398 | 0.023 |
| H(26) | 4a | 0.7630 | 0.2657 | 0.6155 | 0.022 |
| H(2) | 4a | 0.5213 | 0.2496 | 0.5412 | 0.021 |
| H(7) | 4a | 0.5322 | −0.4166 | 0.4992 | 0.030 |
| H(24) | 4a | 0.7423 | 0.2323 | 0.8166 | 0.030 |
| H(25) | 4a | 0.8452 | 0.3381 | 0.7211 | 0.028 |
| H(16) | 4a | 0.5853 | 0.3669 | 0.4501 | 0.025 |
| H(15) | 4a | 0.5339 | 0.5228 | 0.3677 | 0.029 |
| H(22) | 4a | 0.4723 | −0.0195 | 0.7004 | 0.024 |
| H(23) | 4a | 0.5519 | 0.0565 | 0.8061 | 0.027 |
| H(13) | 4a | 0.2150 | 0.2696 | 0.2727 | 0.029 |
| H(8) | 4a | 0.4329 | −0.2502 | 0.4308 | 0.026 |
| H(12) | 4a | 0.2606 | 0.1157 | 0.3568 | 0.025 |
| H(14) | 4a | 0.3511 | 0.4733 | 0.2779 | 0.030 |
Atomic displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| C(1) | 4a | 0.4676(2) | 0.1223(2) | 0.46425(9) | 0.0167(8) | 0.017(1) | 0.0179(9) | 0.0005(7) | 0.0026(7) | −0.0009(7) |
| C(3) | 4a | 0.5642(2) | 0.0691(2) | 0.5774(1) | 0.0145(8) | 0.017(1) | 0.0168(8) | −0.0021(7) | 0.0021(7) | −0.0028(7) |
| C(5) | 4a | 0.6280(2) | −0.1710(2) | 0.5980(1) | 0.0192(9) | 0.019(1) | 0.0189(8) | 0.0014(8) | 0.0039(7) | 0.0014(7) |
| N | 4a | 0.4495(2) | −0.0026(2) | 0.44632(8) | 0.0227(8) | 0.0178(9) | 0.0169(7) | −0.0002(7) | 0.0016(6) | −0.0008(6) |
| C(26) | 4a | 0.7223(2) | 0.2223(2) | 0.6536(1) | 0.0188(9) | 0.0169(9) | 0.0205(9) | 0.0011(7) | 0.0000(7) | −0.0004(8) |
| C(9) | 4a | 0.4961(3) | −0.0962(2) | 0.4923(1) | 0.0190(9) | 0.018(1) | 0.0170(9) | −0.0013(7) | 0.0028(7) | −0.0012(8) |
| C(2) | 4a | 0.5187(2) | 0.1607(2) | 0.5301(1) | 0.0181(8) | 0.0154(9) | 0.0180(8) | −0.0005(7) | 0.0016(7) | −0.0019(7) |
| C(11) | 4a | 0.4301(3) | 0.2240(2) | 0.41211(9) | 0.0197(8) | 0.019(1) | 0.0151(8) | 0.0028(7) | 0.0037(7) | −0.0012(7) |
| C(6) | 4a | 0.6191(3) | −0.2973(2) | 0.5748(1) | 0.025(1) | 0.0174(9) | 0.0220(9) | 0.0029(8) | 0.0063(8) | 0.0039(8) |
| C(4) | 4a | 0.5627(2) | −0.0667(2) | 0.55779(9) | 0.0157(8) | 0.0163(9) | 0.0158(9) | −0.0012(7) | 0.0035(7) | −0.0007(7) |
| C(7) | 4a | 0.5424(3) | −0.3293(2) | 0.5126(1) | 0.033(1) | 0.016(1) | 0.026(1) | −0.0017(9) | 0.0057(8) | −0.0031(8) |
| C(21) | 4a | 0.6127(2) | 0.1131(2) | 0.64642(9) | 0.0169(8) | 0.0154(9) | 0.0169(8) | 0.0023(7) | −0.0003(7) | −0.0017(7) |
| C(24) | 4a | 0.7085(3) | 0.2034(2) | 0.7742(1) | 0.029(1) | 0.028(1) | 0.0178(9) | 0.0071(9) | −0.0066(8) | −0.0078(8) |
| C(25) | 4a | 0.7709(3) | 0.2663(2) | 0.7169(1) | 0.023(1) | 0.018(1) | 0.029(1) | 0.0011(8) | −0.0040(8) | −0.0049(8) |
| C(16) | 4a | 0.5106(3) | 0.3473(2) | 0.4147(1) | 0.0226(9) | 0.021(1) | 0.0198(9) | 0.0006(8) | 0.0029(8) | −0.0029(8) |
| C(15) | 4a | 0.4806(3) | 0.4408(2) | 0.3652(1) | 0.028(1) | 0.019(1) | 0.025(1) | 0.0013(8) | 0.0066(9) | 0.0020(8) |
| C(22) | 4a | 0.5477(3) | 0.0517(2) | 0.7043(1) | 0.0202(9) | 0.020(1) | 0.0197(9) | 0.0006(7) | 0.0014(7) | −0.0006(8) |
| C(23) | 4a | 0.5957(3) | 0.0973(2) | 0.7678(1) | 0.027(1) | 0.024(1) | 0.0157(9) | 0.0069(8) | 0.0012(8) | 0.0006(8) |
| C(13) | 4a | 0.2886(3) | 0.2893(2) | 0.3084(1) | 0.025(1) | 0.029(1) | 0.0195(9) | 0.0047(8) | −0.0015(8) | 0.0003(8) |
| C(8) | 4a | 0.4828(3) | −0.2298(2) | 0.4721(1) | 0.029(1) | 0.019(1) | 0.0175(9) | −0.0022(8) | 0.0022(8) | −0.0036(7) |
| C(12) | 4a | 0.3171(3) | 0.1965(2) | 0.3587(1) | 0.023(1) | 0.021(1) | 0.0193(9) | 0.0006(8) | 0.0013(8) | −0.0020(8) |
| C(14) | 4a | 0.3701(3) | 0.4114(2) | 0.3115(1) | 0.029(1) | 0.024(1) | 0.022(1) | 0.0073(8) | 0.0044(9) | 0.0048(8) |
| Cl(1) | 4a | 0.70653(9) | −0.42527(5) | 0.62263(3) | 0.0557(4) | 0.0192(2) | 0.0304(3) | 0.0105(2) | −0.0005(3) | 0.0033(2) |
Source of material
Synthesis protocols toward quinoline derivatives involve the use of a Brønsted acid or Lewis acid catalyst [12–20]. Preparation of these compounds under microwave conditions have also been reported [13–15]. A solution of benzaldehyde (5 mL), phenylacetylene (8 mL) and 4-chloroanaline (6.39 g) and FeCl3 · 6H2O (0.68 mg) was heated (100 °C) overnight. The reaction mixture was cooled to room temperature and diluted with dichloromethane (250 mL). The organic solution was extracted with water (3 × 100 mL), dried over Na2SO4 and evaporated in vacuo to yield a crude product mixture. Purification was afforded with flash chromatography on silica gel (Rf 0.46; hexane:ethyl acetate = 9:1). Recrystallization from a minimum amount of dichoromethane in hexane yielded the product as faint yellow needles (2.40 g, 16%). 1H NMR (600 MHz, CDCl3) δ = 8.22–8.17 (m, 1H), 7.90 (d, J = 2.3 Hz, 1H), 7.85 (s, 1H), 7.67 (dd, J = 9.0, 2.3 Hz, 1H), 7.60–7.53 (m, 2H), 7.51–7.47 (m, 1H); 13C NMR (151 MHz, CDCl3) δ = 157.08, 148.45, 147.27, 139.22, 137.79, 132.25, 131.79, 130.49, 129.66, 129.52, 128.96, 128.88, 128.77, 127.59, 126.52, 124.53, 120.06; EIMS (70 eV) m/z 315 (M+, 100%).
Experimental details
The methyl H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.95 Å and Uiso(H) = 1.5 Ueq(C).
Discussion
Quinoline is an important class of alkaloid of which the basic skeleton is present in numerous natural compounds [1, 2]. A high degree of biological activity, e.g. antimalarial, antibacterial, anticancer and antioxidant, have been attributed to these structures and has found application in the pharmaceutical industry [1–6]. Their unique spectral and photochemical properties have also found application in a variety of fields such as photolytic cleavage of DNA, electrogenerated chemiluminescence and light emitting diodes [7–11].
The asymmetric unit consists of one full molecule of the title compound (see the figure). The structure is stabilized by weak π−π and CH-π interactions. The π−π interaction is observed between the phenyl ring of the quinoline and the phenyl substituent of a neighbouring molecule with the angle between the dihedral planes of 9.9° and the centroid to centroid distance of 3.913(2) Å. The CH-π interaction is observed between the phenyl ring of the quinoline and the pyridine ring of a neighbouring molecule with a CH-centroid distance of 3.493(2) Å.
Acknowledgements:
Financial assistance from the University of the Free State is gratefully acknowledged. We also express our gratitude towards Ntembi, PETLabs Pharmaceuticals, SASOL and the South African National Research Foundation (SA-NRF/THRIP). This work is based on the research supported in part by the National Research Foundations of South-Africa (Grant specific unique reference number (UID) 84913). The Grant holder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the author(s) and that the NRF accepts no liability what so ever in this regard.
References
1. Michael, J. P.: Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 21 (2004) 650–668.10.1039/b310691hSearch in Google Scholar PubMed
2. Michael, J. P.: Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 24 (2007) 223–246.10.1039/b509528jSearch in Google Scholar PubMed
3. Chauhan, P. M.; Srivastava, S. K.: Present trends and future strategy in chemotherapy of malaria. S. Curr. Med. Chem. 8 (2001) 1535–1542.10.2174/0929867013371851Search in Google Scholar PubMed
4. Solomon, V. R.; Haq, W.; Srivastava, K.; Puri, S. K.; Katti, S. B.: Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J. Med. Chem. 50 (2007) 394–398.10.1021/jm061002iSearch in Google Scholar PubMed
5. Marella, A.; Tanwar, O. P.; Saha, R.; Ali, M. R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M. M.: Quinoline: A versatile heterocyclic. Saudi Pharma. J. 21 (2013) 1–12.10.1016/j.jsps.2012.03.002Search in Google Scholar PubMed PubMed Central
6. Oliveri, V.; Grasso, G. I.; Bellia, F.; Attanasio, F.; Viale, M.; Vecchio, G.: Soluble sugar-based quinoline derivatives as new antioxidant modulators of metal-induced amyloid aggregation. Inorg. Chem. 54 (2015) 2591–2602.10.1021/ic502713fSearch in Google Scholar PubMed
7. Van der Loop, T. H.; Ruesink, F.; Amirjalayer, S.; Sanders, H. J.; Buma, W. J.; Woutersen, S.: Unraveling the mechanism of a reversible photoactivated molecular proton crane. J. Phys. Chem. B 118 (2014) 12965–12971.10.1021/jp508911vSearch in Google Scholar PubMed
8. Chowdhury, N.; Gangopadhyay, M.; Karthik, S.; Pradeep Singh, N. D.; Baidya, M.; Ghosh, S. K.: Synthesis, photochemistry, DNA cleavage/binding and cytotoxic properties of fluorescent quinoxaline and quinoline hydroperoxides. J. Photochem. Photobiol. B: Biol. 130 (2014) 188–198.10.1016/j.jphotobiol.2013.11.010Search in Google Scholar PubMed
9. Sharma, A.; Khare, R.; Kumar, V.; Beniwal, V.: Synthesis, characterisation and DNA photocleavage activity of new 2-(Thioxo/Oxo) quinoline-4,6-dimethyl pyrimidinyl hydrazones. Int. J. Pharm. Pharm. Sci. 6 (2014) 166–169.Search in Google Scholar
10. Kim, J. I.; Shin, I.; Kim, H.; Lee, J.: Efficient electrogenerated chemiluminescence from cyclometalated iridium(III) complexes. J. Am. Chem. Soc. 127 (2005) 1614–1615.10.1021/ja043721xSearch in Google Scholar PubMed
11. Mori, T.; Itoh, T. J.: EL Behavior for blue-emitting aluminum quinoline- based organic light-emitting diodes. Photopolym. Sci. Tec. 22 (2009) 515–520.10.2494/photopolymer.22.515Search in Google Scholar
12. Nasseri, M. A.; Alavi, S. A.; Zakerinasab, B.: PEG-SO3H as a catalyst in aqueous media: A simple, proficient and green approach for the synthesis of quinoline derivatives. J. Chem. Sci. 125 (2013) 109–116.10.1007/s12039-012-0353-ySearch in Google Scholar
13. Kulkarni, A.; Török, B.: Microwave-assisted multicomponent domino cyclization–aromatization: An efficient approach for the synthesis of substituted quinolines. Green Chem. 12 (2010) 875–878.10.1039/c001076fSearch in Google Scholar
14. Muscia, G. C.; Hautmann, S.; Buldain, G. Y.; Asís, S. E.; Gütschow, M.: Synthesis and evaluation of 2-(1H-indol-3-yl)-4-phenylquinolines as inhibitors of cholesterol esterase. Bioorgan. Med. Chem. Lett. 24 (2014) 1545–1549.10.1016/j.bmcl.2014.01.081Search in Google Scholar PubMed
15. Praveen, C.; DheenKumar, P.; Muralidharan, D.; Perumal, P. T.: Synthesis, antimicrobial and antioxidant evaluation of quinolines and bis(indolyl)methanes. Bioorgan. Med. Chem. Lett. 20 (2010) 7292–7296.10.1016/j.bmcl.2010.10.075Search in Google Scholar PubMed
16. Tang, J.; Wang, L.; Mao, D.; Wang, W.; Zhang, L.; Wu, S.; Xie, Y.: Ytterbium pentafluorobenzoate as a novel fluorous lewis acid catalyst in the synthesis of 2,4-disubstituted quinolines. Tetrahedron 67 (2011) 8465–8469.10.1016/j.tet.2011.09.004Search in Google Scholar
17. Liu, P.; Li, Y.; Wang, H.; Wang, Z.; Hu, X.: Synthesis of substituted quinolines by iron-catalyzed oxidative coupling reactions. Tetrahedron Lett. 53 (2012) 6654–6656.10.1016/j.tetlet.2012.09.090Search in Google Scholar
18. Wang, Y.; Chen, C.; Peng, J.; Li, M.: Copper(II)-catalyzed three-component cascade annulation of diaryliodoniums, nitriles, and alkynes: A regioselective synthesis of multiply substituted quinolines. Angew. Chem. Int. Ed. 52 (2013) 5323–5327.10.1002/anie.201300586Search in Google Scholar PubMed
19. Yao, C.; Qin, B.; Zhang, H.; Lu, J.; Wang, D.; Tu, S.: One-pot solvent-free synthesis of quinolines by C–H activation/C–C bond formation catalyzed by recyclable iron(III) triflate. RSC Adv. 2 (2012) 3759–3764.10.1039/c2ra20172kSearch in Google Scholar
20. Cao, K.; Zhang, F.; Tu, Y.; Zhuo, X.; Fan, C.: Iron(III)-catalyzed and air-mediated tandem reaction of aldehydes, alkynes and amines: An efficient approach to substituted quinolines. Chem. Eur. J. 15 (2009) 6332–6334.10.1002/chem.200900875Search in Google Scholar PubMed
21. Bruker. APEX2 (Version 2012.10-0), SAINT (version 8.27B), SADABS (version 2012/1), BrukerAXS Inc, Madison, Wisconsin, USA. (2012).Search in Google Scholar
22. SHELX: Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
23. DIAMOND: Brandenburg, K.: DIAMOND. Visual crystal structure information system. Version 3.0c Crystal Impact, Bonn, Germany (2005) 470.Search in Google Scholar
©2016 Johannes Van Tonder et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of rac-4,4,4-trifluoro-3-hydroxy-3-methylbutanoic acid, C5H7O2F3
- Crystal structure of 5-methyl-2-phenyl-1,3-dioxane-5-carboxylic acid, C12H14O4
- Crystal structure of ethyl 2-(2-(2-(4-chlorophenyl)-3-methylbutanamido)thiazol-4-yl)acetate, C18H21ClN2O3S
- The crystal structure of (4E,11E,31E,38E)-1,4,12,15,18,26,31,39-Octaaza-7,21,24-trihydroxy-penta-cyclo[13·13·13·16,10·120,24·133,37]tetratetraconta-4,6(44),7,9,11,18,20(43),21,23,25,31,33(42),34,36,38-pentadecaene, C36H42N8O3
- Crystal structure of poly[diaqua-μ5-4-(3,5-dicarboxylato-κ3O1:O2:O3-phenoxy)phthalato-κ3O5,O7:O8)(μ2-4-(1H-pyrazol-5-yl)pyridine-κ2N:N′)dicobalt(II)] C24H16N3O11Co2
- Crystal structure of catena-poly[(μ2-acetamido-benzoato-κ2O:O′)triphenyltin(IV)], C27H23NO3Sn
- Crystal structure of chlorido(2,2′-((1E,1′E)-(((1R,2R)-cyclohexane-1,2-diyl)bis(azanylylidene))bis(methylylidene))diphenolato-κ4N,N′,O,O′)iron(III), C20H20ClFeN2O2
- Crystal structure of (E)-3-(4-tert-butyl)phenyl)-1-(3-chlorophenyl)prop-2-en-1-one, C18H17CIO
- Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy-4-methylbenzoate (1/2), C30H26N2O8
- Crystal structure of poly[aqua-ethylenediamine-tetraacetatolead(II)zinc(II)]
- Crystal structure of (E)-2-((2-(2,4-dinitrophenyl)hydrazono)methyl)-4-nitrophenol — triethylamine (2/1), C32H33N11O14
- Crystal structure of catena-poly[diaqua-bis-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)cadmium(II)] dihydrate, C26H24N2O12Cd
- Crystal structure of 2-(ethoxycarbonyl)-2-(2-nitro-1-phenylethyl)-3-oxopyrrolidinium chloride, C15H19N2O5Cl
- Crystal structure of 4-((pyridin-4-ylmethyl)sulfinyl)pyridine, C11H10N2OS
- Crystal structure of 2,5-diethoxy-1,4-bis[2-(quinoline)ethenyl]benzene, C32H28N2O2
- Crystal structure of diaqua(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)tetrakis(3-carboxy-5-ethylpyridine-2-carboxylato-κ2N,O)dizinc(II), C54H50N8O18Zn2
- Crystal structure of a poly[bis(3,4,5,6-tetrachlorophthalato)neodym(III)potassium(I)] — 4,4′-bipyridine — water (1/1/5.5)
- The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3
- Crystal structure of 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid dihydrate, C14H16N2O6
- Crystal structure of the catena-poly[bis(1H-imidazole-κN)-(μ2-furan-2,5-dicarboxylato-κ2O1:O4)manganese(II)]monohydrate, C12H12MnN4O6
- Crystal structure of (acetylacetonato-κ2O:O′)bis-((1-(2-hydroxyphenyl)-3-(pyridin-2-yl)prop-2-en-1-one)-κ2C,N)iridium(III), C33H27IrN2O6
- Crystal structure of 1-benzyl-3-(4-methylpyridin-2-yl)-1H-imidazol-3-ium hexafluorophosphate, C16H16F6N3P
- Crystal structure of tetraethylammonium 3,5-dinitrosalicylate, C15H23N3O7
- Crystal structure of 4-[5-(4-fluorophenyl)-3-(4-hydroxyphenyl)-4,5-dihydropyrazol-1-yl] benzenesulfonamide, C21H18FN3O3S
- Crystal structure of 2,4-dichlorobenzene anhydride, C14H6Cl4O3
- Crystal structure of bis(2-hydroxy-2-phenylacetato-κ2O,O′)bis(pyridine-κN)nickel(II), C26H24N2NiO6
- Crystal structure of (E)-4-nitro-2-(((3-(tetrahydro-8λ4-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxaborol-8-yl)phenyl)imino)methyl)phenol – water (1/2), C17H18BN3O5·2H2O
- Crystal structure of 5-(4-carboxyphenoxy)-nicotinic acid, C13H9NO5
- Crystal structure of catena-poly[hexaaquabis(μ2-3-nitrophthalate-κ2O:O′)-(μ2-1,4-bis(4-pyridylmethyl)piperazine-κ2N:N′)dimanganese(II)] dihydrate, C32H42Mn2N6O20
- Crystal structure of diaquabis(bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate-κ4O, O′:/O′′,O′′′)bis-(2,2′-bipyridine-κ2N, N′)dicadmium(II) hydrate
- Crystal structure of (R)-1-(1-(6-fluorobenzo[d]thiazol-2-yl)ethyl)-3-phenylthiourea
- Crystal structure of poly[(μ2-1,4-bis((1H-imidiazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-(1,2-phenylenebis(oxy)dibenzoato-κ4O,O′:O′′,O′′′)nickel(II)], C34H26O6N4Ni
- Crystal structure of 3′,4′,5-trihydroxy-3,7-dimethoxyflavone, C17H14O7
- Crystal structure of n-butyl-chlorido-bis[N-sec-butyl,N-n-propyl-carbamodithioato κ2S,S′]-tin(IV), C20H41ClN2S4Sn
- Crystal structure of poly[bis(μ4-4,4′-(1,2-phenylenebis(oxy))dibenzoato-κ4O:O′:O′′:O′′′)bis(μ3-4,4′-(1,2-phenylenebis(oxy))dibenzoato–κ3O:O′:O′′)(μ2-1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole-κ2N:N′)tetracobalt(II)], C94H62O24N4Co4
- Crystal structure of catenapoly[diaqua-(μ24,4′-bipyridine)-κ2N:N′)-bis(2,6-difluorobenzoate)-κO)nickel(II)] ethanol monosolvate, C28H30F4N2O8Ni
- Crystal structure of 2-(9H-fluoren-9-ylidene)hydrazine-1-carbothioamide, C14H11N3S
- Crystal structure of 2-(4-methoxyphenyl)-2,3-dihydro-1H-perimidine, C18H16N2O
- Crystal structure of catena-poly[diaquabis(μ2-3-carboxybenzene-1,2-dicarboxylato-1:2κ2O:O′)-(μ2-1,4-bis(2-ethylbenzimidazol-1-ylmethyl)-benzene-1:1′κ2N:N′)dizinc(II)], [Zn2(C26H26N4)(C9H4O6)2(H2O)2]
- Crystal structure of diaquabis(phenoxyacetato-κ2O,O′)-zinc(II), C16H18O8Zn
- Crystal structure of 4-(1H-imidazol-1-yl)-6-pyrimidinylferrocene, C17H14FeN4
- Crystal structure of [2-(4-methoxyphenyl)pyrazine-κ2C,N) chlorido[N,N′-bis-(2,6-diisopropyl-phenyl)imidazol-2-ylidene-κC)] palladium(II), C38H45ClN4OPd
- Crystal structure of 2-(4-acetyl-2,6-dimethyl-phenyl)-5,6-dichloro-isoindole-1,3-dione, C18H13Cl2NO3
- Crystal structure of 4,4′-bipyridin-1-ium 3,3′,5′-tricarboxy-[1,1′-biphenyl]-2-carboxylate, (C26H18N2O8)
- Crystal structure of catena-poly[diaqua-μ2-4,4′-biphenyl-4,4′-diyldipyridine-κ2N:N′-bis(5-carboxy-2,6-dimethylpyridine-3-carboxylato-κO)nickel(II)] dihydrate, C40H40N4O12Ni
- Crystal structure of poly-[μ2-4,4′-bipyridine-κ2N:N′−μ3-thiophene-2,3-di-carboxylato-κ4O,O′, O′′:O′′′ -cadmium(II)]
- Crystal structure of hexaaquamanganese(II) bis(3-carboxythiophene-2-carboxylate) C12H18MnO14S2
- Crystal structure of 4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoic acid, C14H10N4O2
- Crystal structure of (E)-2-(benzo[d]thiazol-2-yl)-3-(pyridin-3-yl)acrylonitrile)
- Crystal structure of catena-poly[2,2′-bipyridinyl-6,6′-dicarboxylato-κ4N,N′,O,O′)-(μ2-2,2′-bipyridinyl-6′-carboxyl-6-carboxylato-κ5N,N′,O,O′:O′′)samarium(III)] monohydrate, C24H13N4O8Sm · H2O
- Crystal structure of catena-poly[bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato)-κ2N:O)copper(II)] dihydrate, C28H22N8O6Cu
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-zinc(II)] fumarate tetrahydrate, C14H26N2O12Zn
- Crystal structure of triaqua-(1,10-phenanthroline)-(dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoato) cobalt(II)dihydrogen-3,3′,3′′-(2,4,6-trioxo-1,3,5-triazinane-1,3,5-triyl)tripropanoate, C72H82Co2N16O42
- Crystal structure of poly[dibromido-(μ2–4,4′-bis-(pyrid-4-yl)biphenyl-κ2N:N′)lead(II)], C22H16N2PbBr2
- Crystal structure of catena-poly[diaqua-bis(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:N)-cobalt(II)]dihydrate, C26H24N2O12Co
- Crystal structure of 5-(4-pyridyl)pyrimidine–4,4′-bipyridine–1,3,5-benzenetriol–water (1:1:1:1), C25H23N5O4
- Crystal structure of 5-hydroxy-4-((4-hydroxyphenyl)imino)naphthalen-1(4H)-one monohydrate, C16H11NO3 · 0.5H2O
- Crystal structure of 2-amino-N-(4-methoxyphenyl)benzamide, C14H14N2O2
- Crystal structure of (2,5-dihydroxyphenyl)-(4-hydroxy-3,5-dimethoxyphenyl)methanone, C15H14O6
- Crystal structure of dichlorido[bis(2-hydroxyethyl)5′-([2,2′:6′,2′′-terpyridin]-4′-yl)-[1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylato]zinc(II), C39H31Cl2N3O6Zn
- Crystal structure of bis[4-(3-carboxy-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium] benzene-1,4-dicarboxylate (C20H18F2N3O3)2(C8H4O4), C48H40F4N6O10
- Crystal structure of (2,5-dihydroxyphenyl)-(4-methoxyphenyl)methanone, C14H12O4
- Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl] 3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H22F6OS2
- Crystal structure of poly[diaquabis(μ2-biphenyl-2,4′-dicarboxylato-κ2O:O′)tris(μ2-1,1′-biphenyl-4,4′-diylbis(1H-imidazole)-κ2N:N′)dicobalt(II)] monohydrat, C82H64N12O11Co2
- Crystal structure of 2-amino-4-(3,4-difluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12N2O2F2
- Crystal structure of poly[(di-μ2-aqua-κ2O:O)bis(μ5-oxalato-1:2κ2O1; 1κ1O2; 3:4:5κ3O3; 3κ1O4)(μ4-oxalato-1:2κ2O1; 2:3κ2O2; 3:4κ2O3; 4:1κ2O4)dizinc(II)disodium(I)]
- Crystal structure of tetraethylammonium hexachloridotantalate(V), C8H20Cl6NTa
- Crystal structure of (E)-2,4-dibromo-6-(((2-nitrophenyl)imino)methyl)phenol, C13H8Br2N2O3
- The crystal structure of 6-chloro-2,4-diphenylquinoline
- Crystal structure of (E)-2-(((1,10-phenanthrolin-5-yl)imino)methyl)-5-methylphenol monohydrate, C20H15N3O·H2O
- Crystal structure of tris(3-(2-pyridyl)pyrazole)zinc(II)tetrachlorido zincate(II), C24H21Cl4N9Zn2
- Crystal structure of 4-chloro-N,N-diethyl-6-(piperidin-1-yl)-1,3,5-triazin-2-amine, C12H20ClN5
- The crystal structure of 4-allyl-5-benzyl-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H13N3O
- Crystal structure of diethyl 2-(((2-(pyridin-3-ylthio)phenyl)amino)methylene)malonate, C19H20N2O4S
- Crystal structure of fac-tricarbonyl(2-(isopropylimino)methyl-5-methylphenolatido-κ2N,O)(pyridine-κN)rhenium(I), C19H19N2O4Re
- Crystal structure of 1,3,6,8-tetrakis(p-tolylthio)pyrene, C44H34S4
- Crystal structure of catena-poly-(diaqua-(μ2-1,2-bis(4-pyridyl)ethene-κ2N:N′)-(4-methylphthalato-κ2O,O′)-cobalt(II)trihydrate, C21H26CoN2O9
- Crystal structure of tetraethylammonium fac-tricarbonyl(hexafluoroacetylacetonato-κ2O,O′)-(nitrato-κO)rhenium(I), C16H21O8N2F6Re
- Crystal structure of 3-(thiophen-2-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide
- Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]
- Crystal structure of N′-(adamantan-2-ylidene)-isonicotinohydrazide, C16H19N3O
- Crystal structure of trans-tetraaquabis(4-(pyridin-4-ylsulfonyl)pyridine-κN)cobalt(II) diperchlorate dihydrate, C20H28Cl2CoN4O18S2
- Crystal structure of (Z)-4-(furan-2-yl(p-tolylamino)methylene)-3-methyl-1-p-tolyl-1H-pyrazol-5(4H)-one, C23H21N3O2
- Crystal structure of 2-[(4-fluorobenzyl)sulfanyl]-4-(2-methylpropyl)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C16H16FN3OS
- Crystal structure of poly[octaaqua-tris(benzene-1,2,4,5-tetracarboxylato)tetralanthanum(III)] hexahydrate, C30H34La4O38
- Crystal structure of trans-tetraaqua-bis(4,4′-sulfonyldipyridine-κN)zinc(II) diperchlorate dihydrate, C20H28Cl2ZnN4O18S2
- Crystal structure of 4-nitro-thiophene-2-carboxylic acid, a structure with a Z′ = 4, C5H3NO4S
- Crystal structure of dirubidium trimercury(II) tetraselenide, Rb2Hg3Se4
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS
- Crystal structure of hexaaquamagnesium(II) 5,5′-bitetrazole-1,1′-diolate, C2H12N8O8Mg
- The crystal structure of catena-poly[(μ2-1,1′-benzene-1,4-diylbis(1H-benzimidazole-κ2N:N′)silver(I)] nitrate, C20H14N5AgO3
- The crystal structure of 1-(2-(4-chlorophenoxy)-4-chlorophenyl)ethanone, C14H10Cl2O2
- The crystal structure of 3,5-dinitro-1,3,5-oxadiazinane, C3H6N4O5
- Crystal structure of methyl 5-methoxy 1H-indole-2-carboxylate, C11H11NO3
- Crystal structure of (Z)-1-(((3-acetyl-2-hydroxyphenyl)amino)methylene)naphthalen-2(1H)-one, C19H15NO3
- Crystal structure of (Z)-5-(4-chlorobenzylidene)-2-thioxothiazolidin-4-one —dimethylsulfoxide (1:1), C12H12ClNO2S3
- Crystal structure of 5,5′-((4-(trifluoromethyl)phenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) – diethylamine – dichloromethane (1/1/1) C25H32Cl2F3N5O6
- Crystal structure of 2-(dimethylsulfanylidene)-N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide
- Crystal structure of (1,10-phenanthroline-κ2N,N′)bis(thiocyanato-κN)platinum(II), C14H8N4PtS2
- Crystal structure of di(μ2-chlorido)bis[2-(2-pyridyl)phenyl-κ2N,C1]dipalladium(II), C22H16Cl2N2Pd2
- Crystal structure of trans-dibromidodi(pyridine-κN)palladium(II), PdBr2(C5H5N)2

