Abstract
C16H15FN2O2, monoclinic, P21/c (no. 14), a = 9.2751(7) Å, b = 13.1232(7) Å, c = 11.7155(8) Å, β =
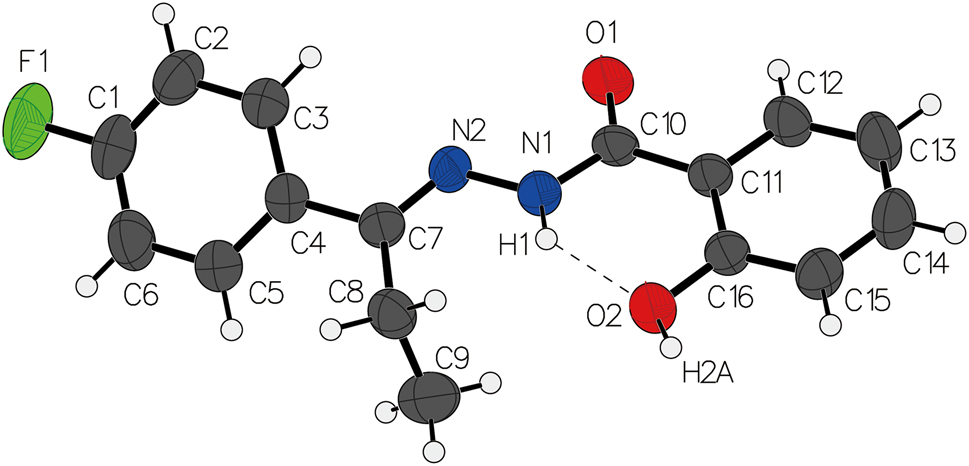
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.46 × 0.43 × 0.37 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, φ and ω |
| θ max, completeness: | 29.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 11,714, 3392, 0.025 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2448 |
| N(param) refined: | 193 |
| Programs: | Bruker [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 1.0138 (3) | 0.47461 (19) | 0.74180 (18) | 0.0621 (6) |
| C2 | 0.8893 (3) | 0.43123 (16) | 0.70312 (18) | 0.0641 (6) |
| H2 | 0.860663 | 0.367323 | 0.728896 | 0.077* |
| C3 | 0.8067 (2) | 0.48401 (15) | 0.62506 (17) | 0.0547 (5) |
| H3 | 0.722544 | 0.454800 | 0.596918 | 0.066* |
| C4 | 0.84766 (19) | 0.58012 (13) | 0.58804 (15) | 0.0447 (4) |
| C5 | 0.9741 (2) | 0.62134 (16) | 0.63075 (17) | 0.0580 (5) |
| H5 | 1.002600 | 0.686048 | 0.607547 | 0.070* |
| C6 | 1.0590 (2) | 0.56774 (18) | 0.70754 (19) | 0.0660 (6) |
| H6 | 1.144805 | 0.595135 | 0.734801 | 0.079* |
| C7 | 0.7588 (2) | 0.63509 (13) | 0.50206 (15) | 0.0441 (4) |
| C8 | 0.7567 (2) | 0.75048 (14) | 0.50179 (16) | 0.0526 (5) |
| H8A | 0.658952 | 0.773642 | 0.488052 | 0.063* |
| H8B | 0.785808 | 0.774961 | 0.576452 | 0.063* |
| C9 | 0.8548 (3) | 0.79586 (18) | 0.4128 (2) | 0.0767 (7) |
| H9A | 0.825323 | 0.773114 | 0.338454 | 0.115* |
| H9B | 0.952155 | 0.774540 | 0.426907 | 0.115* |
| H9C | 0.849287 | 0.868846 | 0.416217 | 0.115* |
| C10 | 0.54761 (19) | 0.57292 (12) | 0.26588 (15) | 0.0427 (4) |
| C11 | 0.46200 (18) | 0.62964 (12) | 0.17896 (14) | 0.0398(4) |
| C12 | 0.3931 (2) | 0.57202 (14) | 0.09568 (17) | 0.0538(5) |
| H12 | 0.402777 | 0.501499 | 0.097119 | 0.065* |
| C13 | 0.3110 (2) | 0.61596 (17) | 0.01124(18) | 0.0648(6) |
| H13 | 0.265243 | 0.575751 | −0.043187 | 0.078* |
| C14 | 0.2975 (2) | 0.72066 (16) | 0.00852 (17) | 0.0608 (6) |
| H14 | 0.242352 | 0.751136 | −0.048322 | 0.073* |
| C15 | 0.3643 (2) | 0.78005 (14) | 0.08844 (16) | 0.0521 (5) |
| H15 | 0.354330 | 0.850520 | 0.085530 | 0.063* |
| C16 | 0.44699 (18) | 0.73583 (12) | 0.17398 (15) | 0.0422 (4) |
| F1 | 1.09534 (17) | 0.42254 (12) | 0.81874 (13) | 0.0963 (5) |
| N1 | 0.60765 (16) | 0.62681 (10) | 0.35036 (12) | 0.0454 (4) |
| H1 | 0.595414 | 0.691702 | 0.353578 | 0.055* |
| N2 | 0.68935 (16) | 0.57799 (10) | 0.43243 (12) | 0.0444 (4) |
| O1 | 0.56368 (17) | 0.48054 (9) | 0.25904 (13) | 0.0685 (5) |
| O2 | 0.51725 (17) | 0.79355 (9) | 0.25231 (12) | 0.0629 (4) |
| H2A | 0.493628 | 0.853386 | 0.244749 | 0.094* |
1 Source of materials
A single crystal of (E)-N′-(1-(4-fluorophenyl) propylidene)-2-hydroxybenzohydrazide was synthesized through a facile method. Initially, salicyl hydrazide (0.15 g, 1 mmol) and 4′-fluoropropiophenone (0.15 g, 1 mmol) were dissolved in 25 mL of absolute alcohol in a round-bottom flask equipped with a stir bar and rubber septum. The mixture was further treated with five drops of glacial acetic acid and stirred for 10 min at 60 °C. Following the addition of acetic acid, the reaction mixture turned yellowish, and stirring was continued for 6 h at the same temperature. The solvent was then evaporated to dryness using a rotary evaporator, yielding the crude product that was then purified to obtain a single crystal of high quality. For crystal growth, the crude product was dissolved in a minimal amount of hot ethanol and slowly cooled to room temperature.
2 Experimental details
The Direct Methods implemented in SHELXT [2] were utilized in order to solve the crystal structure, and it was further refined using the SHELXL program [3]. All hydrogen atoms were placed at calculated positions and refined isotropically. Finally, the OLEX2 software package [4] was employed for generating the graphical representation of the crystal structure.
3 Comment
The N-acylhydrazone moiety has gained considerable attention in the development of biologically active compounds, making it a promising structural motif for drug discovery [5]. An essential step in understanding the properties and behavior of organic compounds is the preparation and study of single crystal structures [6]. Herein, we report the single crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, which provides significant insights into its molecular structure and packing arrangement.
There is one molecule in the asymmetric unit (see the figure). In terms of molecular geometry, the bond lengths and angles were found to be consistent with those that are typically observed for similar compounds. Notably, the C–N bond length was determined to be 1.338(3) Å, while the C=N bond length was observed to be 1.281(3) Å, indicating the presence of partial single and double bond character [7], [8], [9], [10]. The angles between the C7–N2–N1 and C10–N1–N2 atoms were measured to be
The crystal structure of (E)-N′-(1-(4-fluorophenyl) propylidene)-2-hydroxybenzohydrazide displays a complex three-dimensional network of hydrogen bonds, which involve the carbonyl oxygen and amide nitrogen atoms. The crystal structure is composed of layers of molecules, and the hydrogen bonds (N1–H1⋯O2 and O2–H2A⋯O1) facilitate the cohesion of these layers.
The investigation of the crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea can contribute to a better understanding of the structure-property relationships in similar derivatives [13, 14].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Natural Science Foundation of Shaanxi Province (2021JQ-883), The Key Breeding Program by the Collaborative Innovation Center of Green Manufacturing Technology for Traditional Chinese Medicine in Shaanxi Province (2019XT-1-02), Effective Substances of Traditional Chinese Medicine Innovative Team in Shaanxi Institute of International Trade & Commerce (SSY18TD01), and Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City (2021QXNL-PT-0008).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Zhang, B., Zhao, Y., Zhai, X., Wang, L., Yang, J., Tan, Z., Gong, P. Design, synthesis and anticancer activities of diaryl urea derivatives bearing N-acylhydrazone moiety. Chem. Pharm. Bull. 2013, 60, 1046–1054; https://doi.org/10.1248/cpb.c12-00234.Search in Google Scholar PubMed
6. Ajayeoba, T. A., Woods, J. O., Ayeni, A. O., Ajayi, T. J., Akeem, R. A., Hosten, E. C., Akinyele, O. F. Synthesis, crystallographic, computational and molecular docking studies of new acetophenone-benzoylhydrazones. J. Mol. Struct. 2021, 1237, 130275; https://doi.org/10.1016/j.molstruc.2021.130275.Search in Google Scholar
7. Wang, X.-S., Sheng, J., Lu, L., Yang, K., Li, Y.-L. Combinatorial synthesis of 3-arylideneaminoquinazolin-4(1H)-one derivatives catalyzed by iodine in ionic liquids. ACS Comb. Sci. 2011, 13, 196–199; https://doi.org/10.1021/co1000713.Search in Google Scholar PubMed
8. Sreeja, P. B., Sithambaresan, M., Aiswarya, N., Kurup, M. R. P. N′-[(E)-2-Fluorobenzylidene]benzohydrazide. Acta Crystallogr. 2013, E69, o1828; https://doi.org/10.1107/s1600536813031747.Search in Google Scholar PubMed PubMed Central
9. Sivajeyanthi, P., Jeevaraj, M., Edison, B., Balasubramani, K. Crystal structure and Hirshfeld surface analysis of (E)-4-amino-N′-[1-(4-methylphenyl)ethylidene]benzohydrazide. Acta Crystallogr. 2017, E73, 1029–1032; https://doi.org/10.1107/s205698901700857x.Search in Google Scholar
10. Ding, Y.-W., Ni, L.-L. 4-Dimethylamino-N′-(3-pyridylmethylidene) benzohydrazide. Acta Crystallogr. 2010, E66, o2636; https://doi.org/10.1107/s1600536810037670.Search in Google Scholar
11. Mague, J. T., Mohamed, S. K., Akkurt, M., Potgieter, H., Albayati, M. R. 4-Chloro-N′-[(E)-2-chlorobenzylidene]benzohydrazide monohydrate. Acta Crystallogr. 2014, E70, o612; https://doi.org/10.1107/s1600536814008885.Search in Google Scholar
12. Xi-Shi, T., Yi-Min, F. 2-Hydroxy-N′-[1-(3-methylpyrazin-2-yl) ethylidene]benzohydrazide. Acta Crystallogr. 2008, E64, o707; https://doi.org/10.1107/s1600536808006697.Search in Google Scholar
13. Biswas, N., Bera, S., Sepay, N., Pal, A., Halder, T., Ray, S., Acharyya, S., Biswas, A. K., Drew, M. G. B., Ghosh, T. Simultaneous formation of non-oxidovanadium(iv) and oxidovanadium(v) complexes incorporating phenol-based hydrazone ligands in aerobic conditions. New J. Chem. 2020, 44, 3700–3716; https://doi.org/10.1039/c9nj06114b.Search in Google Scholar
14. Tang, C.-B. 2-Methyl-N′-[1-(2-pyridyl)ethylidene]benzohydrazide. Acta Crystallogr. 2011, E67, o271; https://doi.org/10.1107/s1600536810054267.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

