Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
-
Lamya H. Al-Wahaibi
and Ali A. El-Emam
Abstract
C25H31F3N4OS, monoclinic, P21/c (no. 14), a = 6.7323(1) Å, b = 15.4999(2) Å, c = 23.7905(4) Å, β = 93.037(2)°, V = 2479.05(6) Å3, Z = 4, R gt(F) = 0.0473, wR ref(F 2) = 0.1394, T = 293 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.23 × 0.14 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.57 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 75.8°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 24,330, 5166, 0.050 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3136 |
| N(param)refined: | 307 |
| Programs: | CrysAlis PRO [1], Shelx [2, 3], WinGX/Ortep [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | −0.09578 (11) | 0.16702 (5) | 0.63594 (4) | 0.0801 (2) |
| F1 | 0.8781 (3) | 0.04924 (17) | 0.25876 (10) | 0.1340 (9) |
| F2 | 0.6426 (3) | −0.03222 (12) | 0.28323 (8) | 0.1013 (6) |
| F3 | 0.7992 (3) | 0.04584 (14) | 0.34408 (8) | 0.1031 (6) |
| O1 | 0.0216 (2) | 0.33007 (10) | 0.62257 (7) | 0.0601 (4) |
| N1 | 0.2281 (3) | 0.23816 (12) | 0.59120 (8) | 0.0559 (5) |
| N2 | 0.3088 (3) | 0.31956 (12) | 0.58235 (8) | 0.0572 (5) |
| N3 | 0.3537 (3) | 0.13038 (12) | 0.52531 (7) | 0.0537 (5) |
| N4 | 0.3135 (3) | 0.10859 (12) | 0.40671 (7) | 0.0526 (5) |
| C1 | 0.0540 (3) | 0.24310 (15) | 0.61580 (10) | 0.0572 (6) |
| C2 | 0.1811 (3) | 0.37180 (15) | 0.60157 (9) | 0.0526 (5) |
| C3 | 0.1950 (3) | 0.46720 (15) | 0.60646 (9) | 0.0520 (5) |
| C4 | 0.3496 (4) | 0.50188 (18) | 0.56737 (12) | 0.0734 (7) |
| H4A | 0.477369 | 0.475005 | 0.576506 | 0.088* |
| H4B | 0.310019 | 0.487802 | 0.528653 | 0.088* |
| C5 | −0.0062 (4) | 0.51011 (18) | 0.59247 (14) | 0.0791 (8) |
| H5A | −0.103612 | 0.489022 | 0.617747 | 0.095* |
| H5B | −0.051896 | 0.495869 | 0.554241 | 0.095* |
| C6 | 0.2612 (5) | 0.49029 (17) | 0.66728 (10) | 0.0700 (7) |
| H6A | 0.164709 | 0.468720 | 0.692704 | 0.084* |
| H6B | 0.388365 | 0.463426 | 0.677169 | 0.084* |
| C7 | 0.3680 (5) | 0.59947 (19) | 0.57391 (14) | 0.0821 (9) |
| H7 | 0.467309 | 0.621228 | 0.548776 | 0.099* |
| C8 | 0.1678 (5) | 0.6414 (2) | 0.55883 (15) | 0.0950 (10) |
| H8A | 0.179591 | 0.703652 | 0.561842 | 0.114* |
| H8B | 0.125268 | 0.627291 | 0.520335 | 0.114* |
| C9 | 0.0155 (5) | 0.60895 (19) | 0.59865 (17) | 0.0908 (10) |
| H9 | −0.113095 | 0.636503 | 0.589386 | 0.109* |
| C10 | 0.0819 (5) | 0.6299 (2) | 0.65882 (17) | 0.0993 (11) |
| H10A | 0.093329 | 0.691904 | 0.663425 | 0.119* |
| H10B | −0.016020 | 0.609059 | 0.684033 | 0.119* |
| C11 | 0.2795 (5) | 0.58811 (19) | 0.67329 (13) | 0.0844 (9) |
| H11 | 0.321910 | 0.602310 | 0.712238 | 0.101* |
| C12 | 0.4324 (5) | 0.6210 (2) | 0.63444 (15) | 0.0898 (10) |
| H12A | 0.560236 | 0.594554 | 0.644131 | 0.108* |
| H12B | 0.446545 | 0.682957 | 0.638684 | 0.108* |
| C13 | 0.3461 (4) | 0.15942 (15) | 0.58170 (9) | 0.0571 (6) |
| H13A | 0.481342 | 0.169777 | 0.596263 | 0.069* |
| H13B | 0.292698 | 0.113096 | 0.603780 | 0.069* |
| C14 | 0.1642 (4) | 0.10507 (17) | 0.49790 (10) | 0.0633 (6) |
| H14A | 0.085192 | 0.155964 | 0.488660 | 0.076* |
| H14B | 0.090878 | 0.069363 | 0.523112 | 0.076* |
| C15 | 0.2020 (4) | 0.05536 (17) | 0.44479 (10) | 0.0636 (6) |
| H15A | 0.276738 | 0.003417 | 0.454313 | 0.076* |
| H15B | 0.076148 | 0.038426 | 0.426267 | 0.076* |
| C16 | 0.4667 (4) | 0.18368 (16) | 0.48814 (9) | 0.0600 (6) |
| H16A | 0.593188 | 0.199348 | 0.506779 | 0.072* |
| H16B | 0.393824 | 0.236267 | 0.479005 | 0.072* |
| C17 | 0.5015 (4) | 0.13404 (16) | 0.43491 (9) | 0.0589 (6) |
| H17A | 0.575578 | 0.169650 | 0.409826 | 0.071* |
| H17B | 0.580129 | 0.083024 | 0.444084 | 0.071* |
| C18 | 0.3455 (4) | 0.06163 (15) | 0.35435 (9) | 0.0553 (6) |
| H18A | 0.224520 | 0.031546 | 0.342110 | 0.066* |
| H18B | 0.449607 | 0.019021 | 0.361118 | 0.066* |
| C19 | 0.4045 (4) | 0.12342 (15) | 0.30845 (8) | 0.0519 (5) |
| C20 | 0.5785 (4) | 0.11742 (16) | 0.27938 (9) | 0.0584 (6) |
| C21 | 0.6175 (5) | 0.1787 (2) | 0.23836 (10) | 0.0770 (8) |
| H21 | 0.734671 | 0.175288 | 0.219452 | 0.092* |
| C22 | 0.4857 (6) | 0.2433 (2) | 0.22579 (11) | 0.0844 (9) |
| H22 | 0.513760 | 0.283883 | 0.198500 | 0.101* |
| C23 | 0.3129 (5) | 0.24902 (19) | 0.25294 (12) | 0.0806 (9) |
| H23 | 0.222076 | 0.292656 | 0.243758 | 0.097* |
| C24 | 0.2737 (4) | 0.19000 (17) | 0.29391 (10) | 0.0672 (7) |
| H24 | 0.156052 | 0.194746 | 0.312471 | 0.081* |
| C25 | 0.7233 (4) | 0.0458 (2) | 0.29104 (12) | 0.0776 (8) |
1 Source of material
To a solution of 5-(adamantan-1-yl)-1,3,4-oxadiazole-2(3H)-thione [5] (1.18 g, 5.0 mmol), in ethanol (10 mL), 1-[2-(trifluoromethyl)benzyl]piperazine (1.22 g, 5.0 mmol) and 37% formaldehyde solution (1.0 mL) were added. The resulting mixture was stirred at room temperature for 1 h and allowed to stand overnight. The precipitated product was filtered, washed with cold water, dried and recrystallised from its ethanol solution to yield 2.09 g (85%) of the title compound as colourless prisms. Melting point: 425–427 K (uncorrected). IR (cm−1): ν 3097 (Aromatic C–H), 2907, 2854 & 2813 (Aliphatic C–H), 1607 & 1440 (C=N), 1359 (C=S), 1312 (CF3) and 1244 (C–O–C). 1 H NMR (CDCl3, 250 MHz): δ 1.76 (q, 6H, adamantane-H), 2.02 (s, 6H, adamantane-H), 2.12 (s, 3H, adamantane-H), 2.52 (t, 4H, piperazine-CH2), 2.85 (t, 4H, piperazine-CH2), 3.67 (s, 2H, benzylic CH2), 4.99 (s, 2H, NCH2N), 7.32 (t, 1H, Ar–H), 7.50 (t, 1H, Ar–H), 7.62 (d, 1H, Ar–H), 7.75 (d, 1H, Ar–H). 13 C NMR (CDCl3, 62.9 MHz): d 27.48, 34.39, 36.11, 39.13 (adamantane-C), 50.31, 53.09 (piperazine-C), 58.12 (benzylic CH2), 70.08 (NCH2N), 123.35 (CF3), 125.52, 126.78, 127.70, 130.26, 131.72, 137.53 (Ar–C), 167.84 (C=N), 178.64 (C=S). EI–MS (m/z (Rel. Int.)): 492 (M+, 1), 257 (100), 236 (6), 214 (15), 176 (5), 159 (46), 135 (30), 98 (17), 91 (9), 70 (51).
2 Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.93–0.98 Å) and refined as riding with U iso(H) = 1.2U eq(C).
3 Comment
The adamantanyl residue was identified quite some time ago as a crucial building block for numerous chemotherapeutic agents [6, 7]. In addition, the 1,3,4-oxadiazole heterocycle represents an essential structural scaffold in a number of drugs [8, 9]. In the present study, the crystal structure of the title adamantane-oxadiazole hybrid molecule, which was reported to possess marked, broad-spectrum anti-bacterial activity [5], is described.
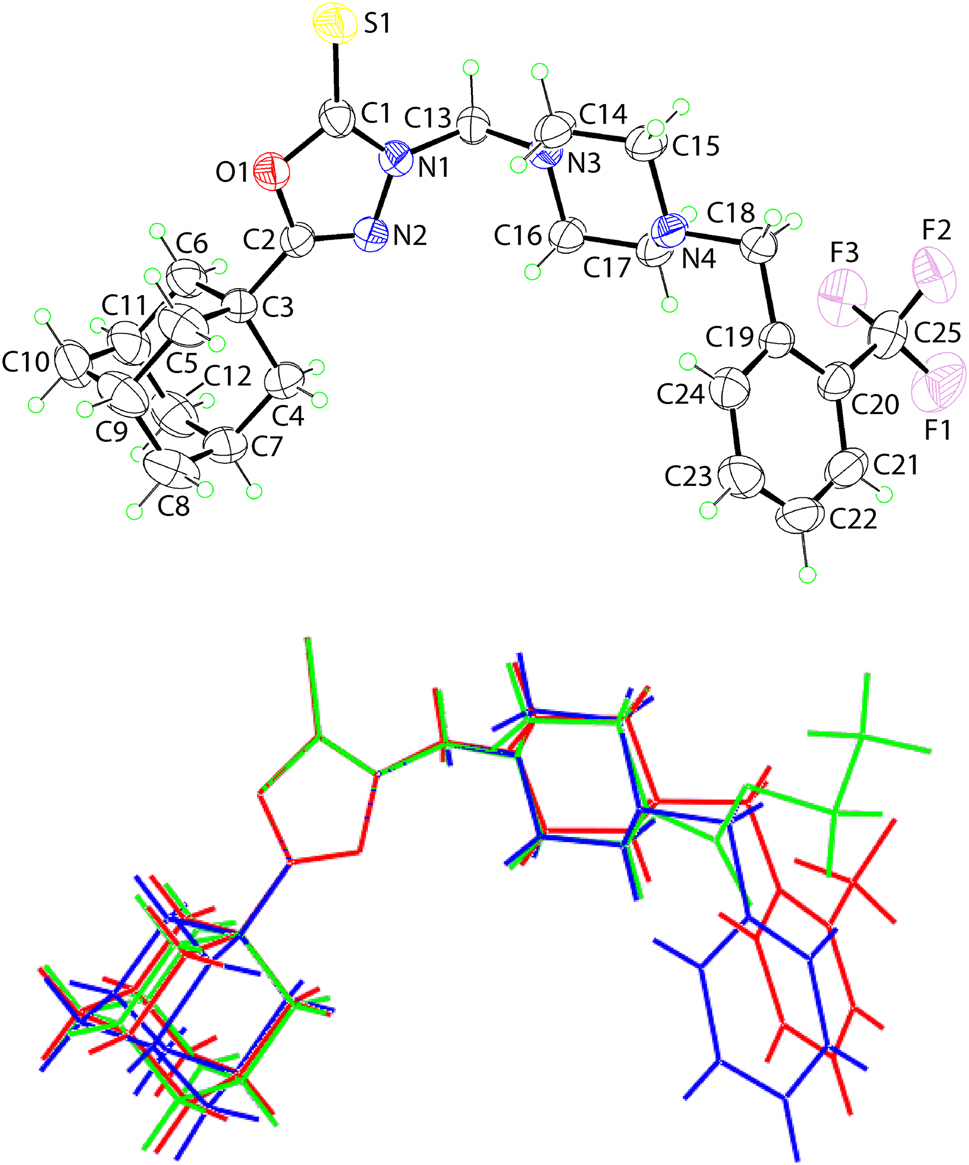
The molecular structure is shown in the upper image of the figure (35% probability ellipsoids). The 1,3,4-oxadiazole ring is strictly planar with the appended S1 [0.031(1) Å], C3 [0.087(2) Å] and C13 [0.189(2) Å] atoms all lying to one side of the plane. Nevertheless, the plane through the heterocyclic ring bisects the adamantanyl residue with three carbon atoms above and three below the plane. The substituted phenyl ring is folded to be directed towards the five-membered ring and forms a dihedral angle of 38.12(13)° with it.
There are two literature precedents for the title compound, hereafter (I). These are the terminal N-bound 4-CH2Ph [10] and 4-C(=O)OEt species [11]; hereafter (II) and (III). An overlay diagram of the three related molecules is shown in the lower view of the figure and shows (I)–(III) as the red, green and blue images, respectively; the molecules have been overlapped so the 1,3,4-oxadiazole rings are coincident. While the adamantanyl substituents are in approximately the same orientation, differences are evident in the relative orientations of the (piperazin-1-yl)methyl residues and, in particular, those of the terminal substituents. For the former, the pairs of Cmethylene–Npiperazin-1-ylCmethylene–Cmethylene torsion angles for (I)–(III) are 70.1(3)° & −62.5(3)°, 61.5(6)° & −66.5(6)° and 62.9(5)° & −67.1(5)°, respectively. For the terminal substituents, the different conformations are best highlighted in the dihedral angles between the least-squares planes through the piperazin-1-yl ring, all of which adopt chair conformations, and the phenyl/ethylacetate residues. Thus, the phenyl ring in (II) adopts a perpendicular orientation [dihedral angle = 89.52(16)°] compared with the equivalent angle in (I) of 82.31(7)°. For (III), it should be noted that the 4-C(=O)OEt residue is planar and adopts an all-trans conformation [torsion angles: N4–C18–O3–C19 = 176.9(5)° and C18–O3–C19–C20 = 177.5(6)°]. This residue forms a dihedral angle of 6.5(2)° with the least-squares plane through the piperazin-1-yl ring, indicating a close to co-planar relationship.
According to an analysis of the molecular packing of (I) employing Platon [12], there are no directional interactions in the crystal. Thus, an analysis of the molecular packing was conducted by calculating the Hirshfeld surfaces with CrystalExplorer [13] using standard procedures [14]. This analysis shows that 98.5% of all contacts involve H with these surface contacts being, in order of significance, H⋯H [55.3%], F⋯H/H⋯F [18.5%], S⋯H/H⋯S [10.0%], C⋯H/H⋯C [8.8%], O⋯H/H⋯O [3.5%] and N⋯H/H⋯N [2.4%]. The next most significant surface contacts are only 0.4%, being for each of the S⋯F/F⋯S and O⋯C/C⋯O contacts.
Acknowledgements
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2023R3), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2023R3), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Rigaku Corporation: Oxford, UK, 2015.Search in Google Scholar
2. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WinGX and Ortep for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. El-Emam, A. A., Al-Deeb, O. A., Al-Omar, M., Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113; https://doi.org/10.1016/j.bmc.2004.07.033.Search in Google Scholar PubMed
6. Spilovska, K., Zemek, F., Korabecny, J., Nepovimova, E., Soukup, O., Windisch, M., Kuca, K. Adamantane – a lead structure for drugs in clinical practice. Curr. Med. Chem. 2016, 23, 3245–3266; https://doi.org/10.2174/0929867323666160525114026.Search in Google Scholar PubMed
7. Wanka, L., Iqbal, K., Schreiner, P. R. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604; https://doi.org/10.1021/cr100264t.Search in Google Scholar PubMed PubMed Central
8. Rana, K., Salahuddin, Sahu, J. K. Significance of 1,3,4-oxadiazole containing compounds in new drug development. Curr. Drug Res. Rev. 2021, 13, 90–100; https://doi.org/10.2174/2589977512666201221162627.Search in Google Scholar PubMed
9. Vaidya, A., Pathak, D., Shah, K. 1,3,4-Oxadiazole and its derivatives: a review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591; https://doi.org/10.1111/cbdd.13795.Search in Google Scholar PubMed
10. El–Emam, A. A., El–Brollosy, N. R., Attia, M. I., Said-Abdelbaky, M., Garcia-Granda, S. 5-(Adamantan-1-yl)-3-[(4-benzylpiperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione. Acta Crystallogr. 2012, E68, o2172–o2173; https://doi.org/10.1107/s1600536812027249.Search in Google Scholar
11. Al-Sanea, M. M., Abdelbaky, M. S. M., Mohamed, A. A. B., Garcia-Granda, S., Tiekink, E. R. T., El-Emam, A. A. Crystal structure of ethyl 4-{[5-(adamantan-1-yl)-2-sulfanylidene-2,3-dihydro-1,3,4-oxadiazol-3-yl]methyl}piperazine-1-carboxylate, C20H30N4O3S. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 403–405; https://doi.org/10.1515/ncrs-2022-0536.Search in Google Scholar
12. Spek, A. L. checkCIF validation alerts: what they mean and how to respond. Acta Crystallogr. 2020, E76, 1–11; https://doi.org/10.1107/s2056989019016244.Search in Google Scholar
13. Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D., Spackman, M. A. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011; https://doi.org/10.1107/s1600576721002910.Search in Google Scholar PubMed PubMed Central
14. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2

