Abstract
C20H26O5, monoclinic, P212121 (no. 19), a = 7.5455(15) Å, b = 12.735(3) Å, c = 18.333(4) Å, V = 1761.6(6) Å3, Z = 4,
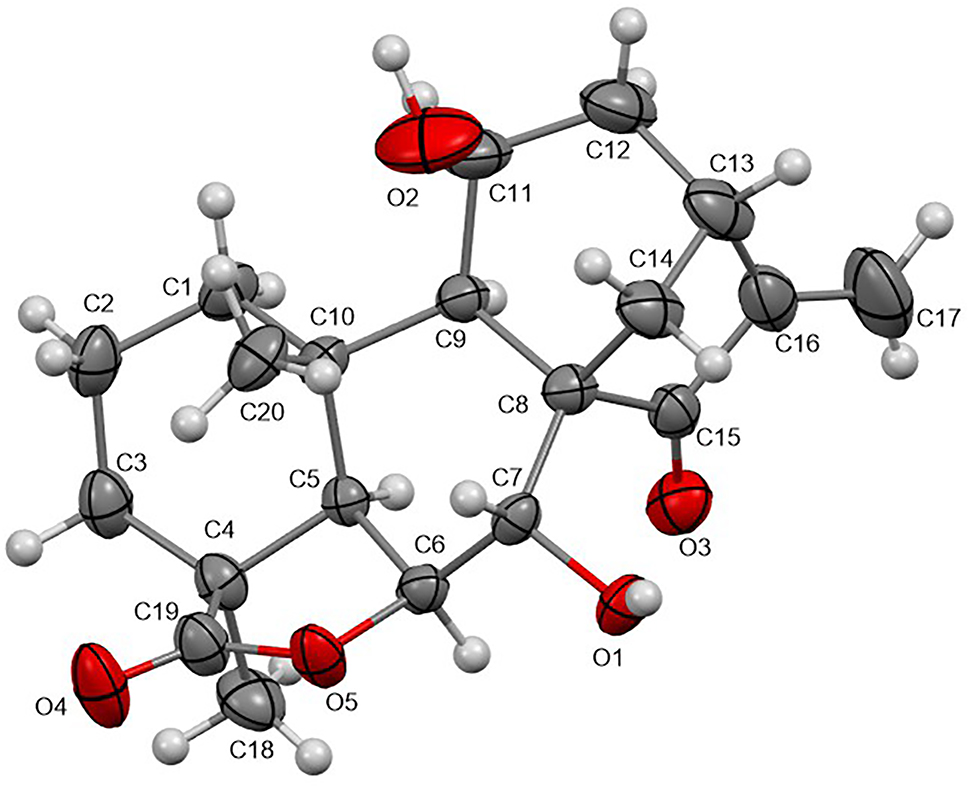
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.24 × 0.21 × 0.19 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 28.3°, >99% |
| N(hkl) measured, N(hkl) unique, R int: | 27,421, 4336, 0.061 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 2967 |
| N(param) refined: | 232 |
| Programs: | Bruker [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.8471 (5) | 0.2771 (3) | 0.4121 (2) | 0.0518 (9) |
| C2 | 0.8452 (5) | 0.1959 (3) | 0.3505 (2) | 0.0569 (10) |
| C3 | 0.6592 (5) | 0.1614 (3) | 0.3310 (2) | 0.0527 (9) |
| C4 | 0.5187 (5) | 0.2482 (2) | 0.32738 (17) | 0.0414 (7) |
| C5 | 0.5469 (4) | 0.3384 (2) | 0.38096 (15) | 0.0321 (6) |
| C6 | 0.4294 (4) | 0.4240 (2) | 0.34983 (16) | 0.0368 (7) |
| C7 | 0.4855 (4) | 0.5357 (2) | 0.36708 (16) | 0.0354 (7) |
| C8 | 0.5843 (4) | 0.5440 (2) | 0.44028 (18) | 0.0371 (7) |
| C9 | 0.7307 (4) | 0.4558 (2) | 0.45429 (17) | 0.0373 (7) |
| C10 | 0.7414 (4) | 0.3755 (2) | 0.39092 (17) | 0.0374 (7) |
| C11 | 0.9037 (5) | 0.5030 (3) | 0.4829 (3) | 0.0613 (11) |
| C12 | 0.8702 (6) | 0.5773 (3) | 0.5468 (3) | 0.0694 (12) |
| C13 | 0.7071 (6) | 0.6488 (3) | 0.5343 (2) | 0.0668 (12) |
| C14 | 0.6623 (5) | 0.6541 (3) | 0.4531 (2) | 0.0545 (9) |
| C15 | 0.4622 (5) | 0.5354 (3) | 0.50565 (17) | 0.0431 (8) |
| C16 | 0.5398 (6) | 0.6022 (3) | 0.5643 (2) | 0.0602 (10) |
| C17 | 0.4570 (9) | 0.6200 (5) | 0.6270 (2) | 0.1008 (19) |
| C18 | 0.3348 (5) | 0.1973 (3) | 0.3375 (2) | 0.0611 (10) |
| C19 | 0.5020 (5) | 0.3094 (3) | 0.25723 (18) | 0.0495 (8) |
| C20 | 0.8288 (5) | 0.4218 (3) | 0.3219 (2) | 0.0524 (9) |
| H3 | 0.663376 | 0.126622 | 0.283979 | 0.063* |
| H5 | 0.499969 | 0.317523 | 0.428649 | 0.039* |
| H5A | 0.852105 | 0.536358 | 0.590738 | 0.083* |
| H6 | 0.767329 | 0.665714 | 0.423659 | 0.065* |
| H7 | 0.576090 | 0.708646 | 0.442898 | 0.065* |
| H8 | 0.727567 | 0.719160 | 0.554172 | 0.080* |
| H9 | 0.974162 | 0.620896 | 0.554102 | 0.083* |
| H10 | 0.980744 | 0.445854 | 0.499507 | 0.074* |
| H11 | 0.796315 | 0.246362 | 0.455747 | 0.062* |
| H12 | 0.913651 | 0.135097 | 0.365330 | 0.068* |
| H14 | 0.244240 | 0.248492 | 0.328137 | 0.092* |
| H16 | 0.322155 | 0.139721 | 0.304160 | 0.092* |
| H17 | 0.564196 | 0.560466 | 0.328185 | 0.043* |
| H17A | 0.346407 | 0.590269 | 0.635595 | 0.121* |
| H17B | 0.509614 | 0.662205 | 0.662257 | 0.121* |
| H18A | 0.307287 | 0.413062 | 0.366314 | 0.044* |
| H18 | 0.323600 | 0.172052 | 0.386668 | 0.092* |
| H20 | 0.901358 | 0.225795 | 0.307694 | 0.068* |
| H21 | 0.968583 | 0.296653 | 0.422828 | 0.062* |
| H22 | 0.955360 | 0.420081 | 0.327170 | 0.079* |
| H23 | 0.794863 | 0.381076 | 0.280111 | 0.079* |
| H24 | 0.790580 | 0.493137 | 0.315459 | 0.079* |
| H25 | 0.684333 | 0.415208 | 0.495478 | 0.045* |
| H33 | 0.621109 | 0.109786 | 0.366628 | 0.063* |
| O1 | 0.3321 (3) | 0.6009 (2) | 0.36854 (13) | 0.0456 (6) |
| H1A | 0.358 (2) | 0.666 (3) | 0.345 (2) | 0.068* |
| O2 | 0.9913 (5) | 0.5581 (4) | 0.4242 (2) | 0.1079 (13) |
| H2A | 1.119 (12) | 0.555 (5) | 0.431 (2) | 0.162* |
| O3 | 0.3277 (3) | 0.4826 (2) | 0.50977 (13) | 0.0564 (7) |
| O4 | 0.5312 (5) | 0.2806 (2) | 0.19502 (13) | 0.0695 (9) |
| O5 | 0.4395 (4) | 0.40646 (18) | 0.27029 (12) | 0.0506 (6) |
1 Source of material
The acrial part of Pteris dispar Kunze collected from Liping County, Guizhou Province were extracted with methanol for three times (once every five days) at room temperature. The combined filtrate was concentrated and extracted with ethyl acetate. The crude product (348 g) was treated by MCI column with methanol/water (volume radio 50–95%) to obtain five fractions of A–F. The title compound was further purified and isolated from D fraction by silica gel column chromatography. The white needle crystal of the title compound (28 mg) suitable for X-ray diffraction was obtained after recrystallization with ethyl acetate for three days.
2 Experimental details
The carbon-bound hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms with d(C–H) = 0.82–0.98 Å, U iso (H) = 1.5times U eq(C) and 1.2times U eq(O).
3 Comment
Ent-kauranediterpene is the main active ingredient of Pteris. In recent years, many scholars have reported that there were a certain amount of ent-kauranediterpenes found in Pteris dispar Kunze. Such as geopyxin B, geopyxin E, neolaxiflorin L and so on [5, 6]. Combined with the literature review, ent-kauranediterpene showed good inhibitory activity against breast cancer (MCF-7,MDA–MB-231 and SK–BR-3), colorectal cancer, nasopharyngeal cancer(CNE-2Z), liver cancer, lung cancer (A549), laryngeal cancer and other tumors [7], [8], [9], [10], [11], [12]. The title compound is a member of ent-kauranediterpene, and we hypothesize that it also has antitumor effects. Therefore, it is significant to investigate further examples of ent-kauranediterpene along with their unambiguous structures.
The title molecular structure consists of three six-membered rings, one five-membered ring and one lactone ring, it contains one keto, one alkenyl, two hydroxyl and two methyl groups (see the figure). The bond lengths and angles derived from the compound are within the normal range. The keto bonds were confirmed by the distance of 1.220(4) Å(C15–O3). The olefinic bond was identified by the distance of 1.327(7) Å(C16–C17). And the hydroxyl groups were confirmed by the distance of 0.95(4) Å(O1–H1A) and 0.97(9) Å(O2–H2A), respectively.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81660723
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: National Natural Science Foundation of China [No. 81660723], the Youth Science and Technology Talent Project of Guizhou Province [No. (2017) 5618], the Science and Technology Tip-top Talent Foundation of Universities in Guizhou Province [No. KY (2021) 034], the Key Projects of Guizhou Basic Research Program [No. Qiankehejichu–ZK (2022) key 046] and the Sinopharm Grou Tongjitang (Guizhou) Pharmaceutical co., Ltd. [Project Contract No. JS-YF-KY-201912014].
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Wijeratne, E. M. K., Bashyal, B. P., Liu, M. X., Rocha, D. D., Gunaherath, G. M. K. B., U’Ren, J. M., Gunatilaka, M. K., Arnold, A. E., Whitesell, L., Gunatilaka, A. A. L. Geopyxins A-E, ent-kaurane diterpenoids from endolichenic fungal strains Geopyxis aff. majalis and Geopyxis sp. AZ0066: structure-activity relationships of geopyxins and their analogues. J. Nat. Prod. 2012, 75, 361–369; https://doi.org/10.1021/np200769q.Search in Google Scholar PubMed PubMed Central
6. Wang, W.-G., Yang, J., Wu, H.-Y., Kong, L.-M., Su, J., Li, X.-N., Du, X., Zhan, R., Zhou, M., Li, Y., Pu, J.-X., Sun, H.-D. Ent-kauranoids isolated from Isodon eriocalyx var.laxiflora, and their structure activity relationship analyses. Tetrahedron 2015, 71, 9161–9171; https://doi.org/10.1016/j.tet.2015.09.066.Search in Google Scholar
7. Wu, J., Meng, L., Long, M., Ruan, Y., Li, X., Huang, Y., Qiu, W. Inhibition of breast cancer cell growth by the Pteris semipinnata extract ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid. Oncol. Lett. 2017, 14, 6809–6814, https://doi.org/10.3892/ol.2017.7113.Search in Google Scholar PubMed PubMed Central
8. Ye, H., Wu, Q., Guo, M., Wu, K., Lv, Y., Yu, F., Liu, Y., Gao, X., Zhu, Y., Cui, L., Liang, N., Yun, T., Li, L., Zheng, X. Growth inhibition effects of ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid on colorectal carcinoma cells and colon carcinoma-bearing mice. Mol. Med. Rep. 2016, 13, 3525–3532, https://doi.org/10.3892/mmr.2016.4950.Search in Google Scholar PubMed
9. Wu, K., Liu, Y., Lv, Y., Cui, L., Li, W., Chen, J., Liang, N. C., Li, L. Ent- 11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis and cell cycle arrest in CNE-2Z nasopharyngeal carcinoma cells. Oncol. Rep. 2013, 29, 2101–2108; https://doi.org/10.3892/or.2013.2375.Search in Google Scholar PubMed PubMed Central
10. Chen, G. G., Leung, J., Liang, N. C., Li, L., Wu, K., Chan, U. P. F., Leung, B. C. S., Li, M., Du, J., Deng, Y. F., Gong, X., Lv, Y., Chak, E. C. W., Lai, P. B. S. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits hepatocellular carcinoma in vitro and in vivo via stabilizing IkBa. Invest. N. Drugs 2012, 30, 2210–2218, https://doi.org/10.1007/s10637-011-9791-5.Search in Google Scholar PubMed
11. Li, L., Chen, G. G., Lu, Y.-N., Liu, Y., Wu, K.-F., Gong, X.-L., Gou, Z.-P., Li, M.-Y., Liang, N.-C. Ent-11α–Hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits growth of human lung cancer A549 cells by arresting cell cycle and triggering apoptosis. Chin. J. Cancer Res. 2012, 24, 109–115; https://doi.org/10.1007/s11670-012-0109-8.Search in Google Scholar PubMed PubMed Central
12. Vlantis, A. C., Lo, C. S., Chen, G. G., Ci Liang, N., Lui, V. W. Y., Wu, K., Deng, Y. F., Gong, X., Lu, Y., Tong, M. C. F., van Hasselt, C. A. Induction of laryngeal cancer cell death by ent-11a-hydroxy-15-oxo-kaur-16-en-19-oic-acid. Head Neck 2010, 32, 1506–1518; https://doi.org/10.1002/hed.21357.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of phenyl(3,3-dichloro-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one)methanone, C7H4Cl2N2O
- Crystal structure of poly[diaqua-bis(μ 2-1,4-diaminobutane-N:N′)cobalt(II)] dichloride, C8H28Cl2CoN4O2
- Synthesis and crystal structure of (4aR,7S)-7-hydroxy-7-isopropyl-1,1-dimethyldecahydro-2H,6H-8a,4a-(epoxymethano)phenanthren-12-one, C20H32O3
- The crystal structure of 1-(2-chlorobenzyl)-3-(3,5-dichlorophenyl)urea, C14H11Cl3N2O
- Crystal structure of tetrapropylammonium-1,3,5-thiadiazole-5-amido-2-carbamate – 1,2,4-thiadiazole-3,5-diamine – water (1/1/1), C17H37N9O3S2
- Tetrabutylammonium 1,3,5-thiadiazole-5-amido-2-carbamate—1,2,4-thiadiazole-3,5-diamine— water (1/1/1), C21H45N9O3S2
- The crystal structure of ((E)-2,4-dichloro-6-(((2-hydroxy-5-nitrophenyl)imino)methyl)phenolato-κ 3 N,O,O′)tris(pyridine-κN)manganese(II), C28H21Cl2MnN5O4
- The crystal structure of aqua-bis{2-bromo-6-((2-(2-phenylacetyl)hydrazineylidene)methyl)phenolato-κ3 N,O,O′}-dimethylformamide-κ1 O-erbium(III) chloride – dimethylformamide – water (1/2/1), C39H49N7O9Br2ClEr
- Crystal structure of (diaqua-bis(phenanthroline-K 2 N,N′)-tetrakis(m 2-3,4,5,6-tetrafluorophthalato-K 4 O,O:O′:O″;K 2 O:O′)dierbium (III) phenanthroline (1/2), C80H38Er2F16N8O18
- Crystal structure of (E)-7-methoxy-2-(4-methoxy-2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C20H17F3O3
- The crystal structure of 4–(4,4,5,5–tetramethyl–1,3,2–dioxaborolan–2–yl)morpholine, C10H20BNO3
- The crystal structure of catena–poly[aqua(1-naphthoato-κ 2 O,O′)-(μ-1-naphthoato-κ 4 O:O,O′:O′)lead(II)], C22H16O5Pb
- The crystal structure of 1-(4-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- The crystal structure of 4-(pyren-1-yl)butyl-4-nitrobenzoate, C27H21NO4
- Crystal structure of cyclo-(bis(µ2-3,3′-(1H-imidazole-3-ium-1,3-diyl)dipropionato-κ4 O,O′:O″,O″′)-dinitrato-κ2 O,O′-tetraoxido-diuranium(VI) C18H22N6O18U2
- The crystal structure of catena-[nitrato-κ 2 O,O′-(μ 3-3-iodobenzene-1,2-dicarboxylato-κ 4 O:O′:O″,O‴)-(2,2′:6′,2″-terpyridine-κ 3 N,N′,N″)lanthanum(III)], C23H14IN4O7La
- Redetermination of crystal structure of [bis(pyridin-2-ylmethyl)amine-κ 3 N,Nʹ,Nʺ]chloridopalladium(II) chloride monohydrate
- Crystal structure of catena-poly[triaqua-[bis(m2-4-(1H-1,2,4-triazol-1-yl)benzoato-k2O:O')-(4-(1H-1,2,4-triazol-1-yl)benzoato-k1O)-praseodymium (III) monohydrate], C27H26N9O10Pr
- Crystal structure of trans-diaqua-bis(methyl methylcarbamohydrazonothioato-κ2 N,N′) nickel(II) iodide semihydrate, C6H22N6O2NiS2I2·0.5H2O
- The crystal structure of 2-(2-fluoro-4-methyl-5-((2,2,2-trifluoroethyl)thio)phenyl)isoindolin-1-one, C17H13F4NOS
- The crystal structure of di-μ-1-naphthylacetato-κ 3 O,O′:O;κ 3 O:O,O′-bis[(1-naphthylacetato-κ 2 O,O′)(2,2′-bipyridine-κ 2 N,N′)lead(II)] monohydrate, C68H54N4O9Pb2
- Crystal structure of tetrapropylammonium guanidinium 4,4′-sulfonyldibenzoate monohydrate, C27H44N4O7S
- Crystal structure of bis(tetrapropylammonium) terephthalate – 1-(diaminomethylene)thiourea – water (1/2/4) C18H40N5O4S
- Crystal structure of (E)-7-fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2
- The crystal structure of poly[diaqua-bis(μ 3-5-bromobenzene-1,3-dicarboxylato-κ 3 O,O,O′)-(μ 2-1,3-bis-(4-pyridyl)-propane-κ 2 N,N′)-dizinc(II))] – 5-bromobenzene-1,3-dicarboxylic acid [2/1], C37H29Br3N2O14Zn2
- The crystal structure of 2-bromo-1,3-phenylene bis(4-methylbenzenesulfonate), C20H17BrO6S2
- Crystal structure of europium dichromium icosaaluminum, EuCr2Al20
- The crystal structure of N′1,N′3-di((E)-benzylidene) isophthalohydrazide dihydrate, C 22 H 22 N 4 O 4
- Crystal structure of 7α,11α-dihydroxy-15-oxo-ent-kauran-16-en-19,6β-olide, C20H26O5
- Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O
- The crystal structure of (Z)-3-(1-(2-((E)-4-isopropylbenzylidene)hydrazinyl)ethylidene) chroman-2,4-dione, C21H20N2O3
- Crystal structure of E-7-fluoro-2-(2-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H12F4O
- Crystal structure of bis(6-aminopyridine-2-carboxylato–k2O,N)-bis(N,N-dimethylformamide-k1 O)zinc(II), C18H24N6O6Zn
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-{[2-(trifluoromethyl)phenyl]-methyl}piperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione, C25H31F3N4OS
- Crystal structure of tetrapropylammonium bicarbonate–1-(diaminomethylene)thiourea – water (2/2/1), C30H72N10O7S2
- Crystal structure of tris(2,2′-bipyridine-κ2 N,N′)iron(II) triiodide – dichloromethane (2/1), C61H50Cl2Fe2I12N12
- Crystal structure of 2-amino-3-[2-(1,3,3-trimethyl-1,3-dihydro-indol-2-ylidene)-ethylideneamino]-but-2-enedinitrile, C17H17N5
- The crystal structure of 1-(2-chlorophenyl)-3-cycloheptylurea, C14H19ClN2O
- Crystal structure of potassium bis(pentaselenido-κ 2 Se 1,Se 5)palladate(II), K2[Pd(Se5)2]
- The crystal structure of 5,10-bis(2-methoxyethyl)-5,10-dihydro-[1,2,3,4]tetrathiocino[5,6-b:8, 7-b′]diindole, C22H22N2O2S4
- The crystal structure of 4-(4-iodophenyl)-5H-1,2,3-dithiazole-5-thione, C8H4INS3
- Crystal structure of bis{μ2-(4-acetyl-phenoxy)acetato-κ2 O:O′}-bis{μ2-(4-acetyl-phenoxy)acetato-κ3 O,O′:O)- bis{(4-acetyl-phenoxy)acetato-κ2 O,O′}-bis(phenanthrolin-κ2 N,N′)didysprosium(III) tetrahydrate, C84H78N4O28Dy2
- Crystal structure of Eu2Pd3.37(1)Zn13.63(1)
- Crystal structure of 2-methoxy-4-(methoxy-carbonyl)phenyl 2-chloro-4-fluorobenzoate, C16H12ClFO5
- Crystal structure of catena-poly[bis(μ2-dicyanamide-κ2 N:N′)-bis(4-vinylpyridine-κN)-copper(II)], C18H14CuN8
- The crystal structure of iguratimod-dimethylformamide (1/1), C17H14N2O6S·C3H7NO
- Synthesis and crystal structure of 1-((3R,10S,13S,17S)-10,13-dimethyl-3-(m-tolylamino)hexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO
- The crystal structure of diaqua-bis(4-bromo-2-formylphenoxy)zinc(II), C14H12Br2O6Zn
- The crystal structure of tetra(1-ethylimidazole-κ 1 N)-[μ 4-imidazole-4,5-dicarboxylato-κ 4 O, N, O′, N′]-trioxido-divanadium, C25H33N10O7V2
- The crystal structure of (E)-N′-(1-(4-fluorophenyl)propylidene)-2-hydroxybenzohydrazide, C16H15FN2O2


