Abstract
C21H15NO6, triclinic, P1̄ (no. 2), a = 9.3591(4) Å, b = 9.4371(5) Å, c = 10.1106(5) Å, α = 102.581(4)°, β = 92.929(4)°, γ = 102.573(4)°, V = 846.09(7) Å3, Z = 2, Rgt(F) = 0.0452, wRref(F2) = 0.1135, T = 288.7(3) K.
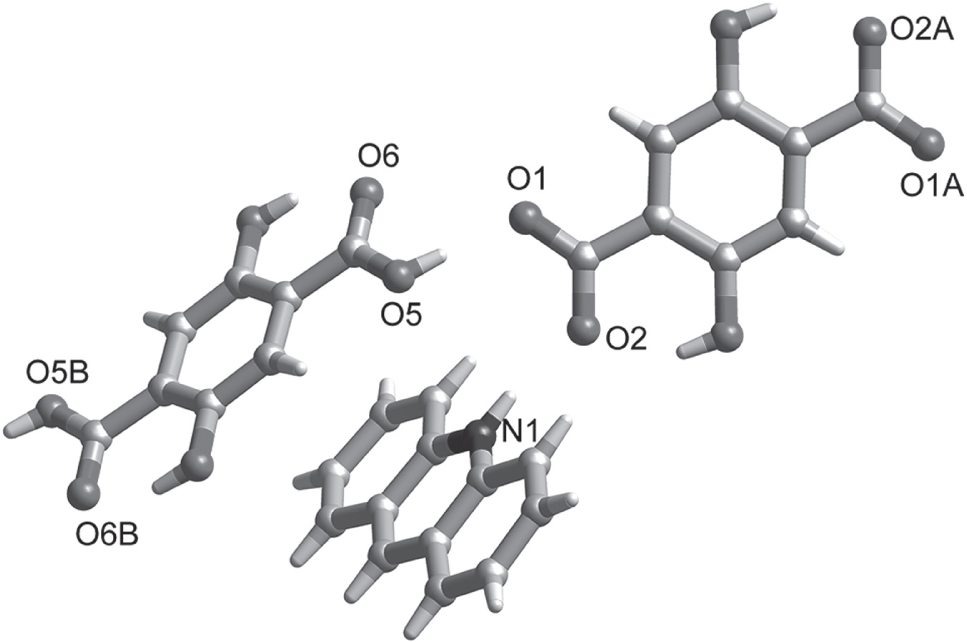
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.26 × 0.23 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.11 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 25.5°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 10752, 3060, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2499 |
| N(param)refined: | 256 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.41853(16) | 0.38719(18) | 0.79478(15) | 0.0292(4) |
| H1 | 0.4915 | 0.3939 | 0.7463 | 0.035* |
| C1 | 0.4759(2) | 0.6554(2) | 0.8570(2) | 0.0345(5) |
| H1A | 0.5537 | 0.6609 | 0.8032 | 0.041* |
| C2 | 0.4447(2) | 0.7815(2) | 0.9303(2) | 0.0420(5) |
| H2 | 0.5022 | 0.8739 | 0.9262 | 0.050* |
| C3 | 0.3272(3) | 0.7760(2) | 1.0126(2) | 0.0433(6) |
| H3 | 0.3092 | 0.8643 | 1.0627 | 0.052* |
| C4 | 0.2404(2) | 0.6430(2) | 1.0191(2) | 0.0385(5) |
| H4 | 0.1628 | 0.6408 | 1.0731 | 0.046* |
| C5 | 0.2671(2) | 0.5063(2) | 0.94356(18) | 0.0296(4) |
| C6 | 0.1825(2) | 0.3661(2) | 0.94405(19) | 0.0326(5) |
| H6 | 0.1010 | 0.3589 | 0.9933 | 0.039* |
| C7 | 0.2163(2) | 0.2362(2) | 0.87274(19) | 0.0309(5) |
| C8 | 0.1334(2) | 0.0897(2) | 0.8707(2) | 0.0430(5) |
| H8 | 0.0504 | 0.0780 | 0.9177 | 0.052* |
| C9 | 0.1739(3) | −0.0322(3) | 0.8013(2) | 0.0479(6) |
| H9 | 0.1182 | −0.1270 | 0.8002 | 0.057* |
| C10 | 0.3009(3) | −0.0164(2) | 0.7303(2) | 0.0451(6) |
| H10 | 0.3284 | −0.1015 | 0.6846 | 0.054* |
| C11 | 0.3831(2) | 0.1201(2) | 0.7276(2) | 0.0371(5) |
| H11 | 0.4662 | 0.1287 | 0.6804 | 0.045* |
| C12 | 0.3410(2) | 0.2495(2) | 0.79748(18) | 0.0287(4) |
| C13 | 0.3882(2) | 0.5152(2) | 0.86381(18) | 0.0277(4) |
| O1 | 0.60291(14) | 0.34549(17) | 0.44697(14) | 0.0433(4) |
| O2 | 0.66247(14) | 0.43920(16) | 0.67028(14) | 0.0384(4) |
| O3 | 0.92840(15) | 0.57414(19) | 0.76347(13) | 0.0462(4) |
| H3A | 0.8400 | 0.5432 | 0.7666 | 0.069* |
| C14 | 0.89348(19) | 0.4251(2) | 0.39358(18) | 0.0268(4) |
| H14 | 0.8218 | 0.3749 | 0.3217 | 0.032* |
| C15 | 0.85271(19) | 0.45881(19) | 0.52453(17) | 0.0236(4) |
| C16 | 0.6944(2) | 0.4114(2) | 0.54846(19) | 0.0282(4) |
| C17 | 0.9612(2) | 0.5355(2) | 0.63228(17) | 0.0264(4) |
| O4 | −0.13293(16) | 0.18322(16) | 0.67900(16) | 0.0454(4) |
| H4A | −0.2104 | 0.1351 | 0.6972 | 0.068* |
| O5 | 0.33512(14) | 0.26655(15) | 0.47314(15) | 0.0378(4) |
| H5 | 0.4230 | 0.2864 | 0.4628 | 0.057* |
| O6 | 0.33634(17) | 0.05419(18) | 0.32518(18) | 0.0578(5) |
| C18 | 0.13156(19) | 0.0637(2) | 0.45407(18) | 0.0267(4) |
| C19 | 0.0605(2) | 0.1523(2) | 0.54319(19) | 0.0284(4) |
| H19 | 0.1009 | 0.2545 | 0.5717 | 0.034* |
| C20 | −0.0696(2) | 0.0910(2) | 0.59015(19) | 0.0290(4) |
| C21 | 0.2764(2) | 0.1284(2) | 0.4111(2) | 0.0320(5) |
Source of material
In a typical experiment, 2,5-dihydroxyterephthalic acid (0.02 g, 0.1 mmol) is dissolved in 5 mL of absolute ethanol, acridine (0.02 g, 0.1 mmol) is dissolved in another 5 mL of absolute ethanol. The two solutions are mixed. The mixture was left at room temperature for 10 minutes to get red block crystals of the title compound.
Experimental details
The hydrogen atoms were placed at calculated positions with the SHELX program (AFIX options: 43 and 147).
Comment
Over the past few decades, the design and assembly of organic cocrystals molecular materials has gained considerable interest [4], [5], [6] due to their potential applications such as luminescence and polarized emission [7], [8], luminescent sensors [9], [10] and tunable mechanical properties [11]. Synthesis methods of organic cocrystals mainly include solution processes [12], [13], vacuum sublimation [14] and external stimuli [15]. The most frequently used method for preparing cocrystals is a solution process and single crystals are grown by slow evaporation of the solvents. But it is still difficult to predict the generation of a new cocrystal. In order to further study the formation conditions of cocrystals, in this article, we use 2,5-dihydroxyterephthalic acid (H2diph) and acridine (AD) to synthesize a cocrystal.
The asymmetric unit of the cocrystal contains one acridinium cation, one half of a 2,5-dihydroxyterephthalic acid molecule and one half 2,5-dihydroxyterephthalate dianion. In the cocrystal, π-π intermolecular interactions between benzene rings of AD molecules and pyridine rings of AD molecules (3.722 Å), benzene rings of H2diph molecules and benzene rings of AD molecules (3.593 Å), and hydrogen bonding from O(5)—H(5)⋯O(1)a (distance: 2.49 Å, angle: 174°), C(6)—H(6)⋯O(3) (distance: 3.16 Å, angle: 133°), and N(1)—H(1)⋯O(2)b (distance: 2.67 Å, angle: 169°) form a three-dimensional structure. The cocrystal structure is similar to the structures of cocrystals reported by Christian Wolf’s group [16]. They reported a series of cocrystals by acridine and dicarboxylic acids. All these cocrystal molecules are stabilized by π-stacking and hydrogen bonding.
Acknowledgements
This work was supported by key scientific research projects of higher education of Henan Province (16 A150016) and Henan Province Natural Science Foundation (182300410166).
References
1. Oxford Diffraction Ltd: CrysAlisPRO. Oxford Diffraction Ltd, Abingdon, Oxfordshire, England (2006).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Yan, D.; Delori, A.; Lloyd, G. O.; Friščić, T.; Day, G. M.; Jones, W.; Lu, J.; Wei, M.; Evans, D. G.; Duan, X.: A cocrystal strategy to tune the luminescent properties of stilbene-type organic solid-state materials. Angew. Chem. Int. Ed. 50 (2011) 12483–12486.10.1002/anie.201106391Search in Google Scholar PubMed
5. Yan, D.: Micro-/nanostructured multicomponent molecular materials: design, assembly, and functionality. Chem. Eur. J. 21 (2015) 4880–4896.10.1002/chem.201405456Search in Google Scholar PubMed
6. Yan, D.; Evans, D. G.: Molecular crystalline materials with tunable luminescent properties: from polymorphs to multi-component solids. Mater. Horiz. 1 (2014) 46–57.10.1039/C3MH00023KSearch in Google Scholar
7. Sagara, Y.; Kato, T.: Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 1 (2009) 605–610.10.1038/nchem.411Search in Google Scholar PubMed
8. Lei, Y. L.; Liao, L. S.; Lee, S. T.: Selective growth of dual-color-emitting heterogeneous microdumbbells composed of organic charge-transfer complexes. J. Am. Chem. Soc. 135 (2013) 3744–3747.10.1021/ja3114278Search in Google Scholar PubMed
9. Li, H.; Chi, Z.; Xu, B.; Zhang, X.; Li, X.; Liu, S.; Zhang, Y.; Xu, J.: Aggregation-induced emission enhancement compounds containing triphenylamine-anthrylenevinylene and tetraphenylethene moieties. J. Mater. Chem. 21 (2011) 3760–3767.10.1039/c0jm02571bSearch in Google Scholar
10. Wang, J.; Zhao, Y.; Zhang, J.; Zhang, J.; Yang, B.; Wang, Y.; Zhang, D.; You, H.; Ma, D.: Assembly of one-dimensional organic luminescent nanowires based on quinacridone derivatives. J. Phys. Chem. C 111 (2007) 9177–9183.10.1021/jp072488xSearch in Google Scholar
11. Karunatilaka, C.; Bučar, D.-K.; Ditzler, L. R.; Friščić, T.; Swenson, D. C.; MacGillivray, L. R.; Tivanski, A. V.: Softening and hardening of macro- and nano-sized organic cocrystals in a single-crystal transformation. Angew. Chem. Int. Ed. 50 (2011) 8642–8646.10.1002/anie.201102370Search in Google Scholar PubMed
12. Roy, S.; Banerjee, R.; Nangia, A.; Kruger, G. J.: conformational, concomitant polymorphs of 4,4-diphenyl-2,5-cyclohexadienone: conformation and lattice energy compensation in the kinetic and thermodynamic forms. Chem. Eur. J. 12 (2006) 3777–3788.10.1002/chem.200501417Search in Google Scholar PubMed
13. Liu, J.; Han, M. L.: Crystal structure of trans-1,2-bis(pyridinium-4-yl)ethylene–2-carboxy- 4-methylbenzoate (1/2), C30H26N2O8. Z. Kristallogr. NCS 231 (2016) 31–33.10.1515/ncrs-2014-9081Search in Google Scholar
14. Zhang, H. Y.; Zhang, Z. L.; Ye, K. Q.; Zhang, J. Y.; Wang, Y.: Organic crystals with tunable emission colors based on a single organic molecule and different molecular packing structures. Adv. Mater. 18 (2006) 2369–2372.10.1002/adma.200600704Search in Google Scholar
15. Davis, R.; Rath, N. P.; Das, S.: Thermally reversible fluorescent polymorphs of alkoxy-cyano-substituted diphenylbutadienes: role of crystal packing in solid state fluorescence. Chem. Commun. (2004) 74–75.10.1039/b311121kSearch in Google Scholar PubMed
16. Mei, X.; Wolf, C.: Neutral and ionic supramolecular structures of unsaturated dicarboxylic acids and acridine: significance of molecular geometry and proton transfer. Eur. J. Org. Chem. 2004 (2004) 4340–4347.10.1002/ejoc.200400396Search in Google Scholar
©2019 Xin-Hong Chang, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Articles in the same Issue
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3


