Abstract
C15H17N3O4, triclinic, P1̄ (no. 2), a = 10.5986(5) Å, b = 11.5298(5) Å, c = 13.5386(6) Å, α = 102.694(1)°, β = 102.999(1)°, γ = 108.918(1)°, V = 1446.03(11) Å3, Z = 4, Rgt(F) = 0.0525, wRref(F2) = 0.1526, T = 100(1) K.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.24 × 0.21 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX-II Duo, φ and ω |
| θmax, completeness: | 33.4°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 73858, 11136, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7872 |
| N(param)refined: | 419 |
| Programs: | Bruker [1], SHELX [2], Spek [3], Mercury [4] |
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1A | 0.65213(11) | 0.41861(11) | 0.48761(8) | 0.0208(2) |
| N2A | 0.75650(10) | 0.41828(10) | 0.56589(8) | 0.0208(2) |
| N3A | 0.27976(11) | 0.23705(11) | 0.36196(8) | 0.0216(2) |
| O1A | 0.43652(10) | 0.39827(10) | 0.33063(8) | 0.0338(2) |
| O2A | 0.31426(9) | 0.10495(9) | 0.50149(7) | 0.02256(18) |
| O3A | 0.48760(9) | 0.10627(9) | 0.63086(7) | 0.02215(18) |
| O4A | 1.05834(9) | 0.24363(8) | 0.93112(7) | 0.02120(18) |
| C1A | 0.52645(12) | 0.32543(11) | 0.46876(9) | 0.0176(2) |
| C2A | 0.54855(12) | 0.25726(11) | 0.54094(9) | 0.0172(2) |
| C3A | 0.69571(12) | 0.32016(11) | 0.59972(9) | 0.0177(2) |
| C4A | 0.78790(12) | 0.29839(11) | 0.68710(9) | 0.0175(2) |
| C5A | 0.78258(12) | 0.17554(11) | 0.68716(9) | 0.0181(2) |
| H5AA | 0.7144 | 0.1015 | 0.6305 | 0.022* |
| C6A | 0.87491(12) | 0.16028(11) | 0.76840(9) | 0.0179(2) |
| H6AA | 0.8700 | 0.0762 | 0.7671 | 0.022* |
| C7A | 0.97529(11) | 0.26827(11) | 0.85228(9) | 0.0172(2) |
| C8A | 0.98506(12) | 0.39121(11) | 0.85215(9) | 0.0200(2) |
| H8AA | 1.0551 | 0.4653 | 0.9078 | 0.024* |
| C9A | 0.89131(12) | 0.40509(12) | 0.76965(9) | 0.0201(2) |
| H9AA | 0.8982 | 0.4894 | 0.7698 | 0.024* |
| C10A | 0.40860(12) | 0.32193(12) | 0.38133(9) | 0.0202(2) |
| C11A | 0.15894(13) | 0.23705(14) | 0.28471(11) | 0.0267(3) |
| H11A | 0.0746 | 0.1620 | 0.2750 | 0.040* |
| H11B | 0.1764 | 0.2326 | 0.2162 | 0.040* |
| H11C | 0.1448 | 0.3166 | 0.3112 | 0.040* |
| C12A | 0.43929(12) | 0.15065(11) | 0.55421(9) | 0.0173(2) |
| C13A | 0.38507(12) | −0.00275(12) | 0.64493(9) | 0.0204(2) |
| H13A | 0.3485 | −0.0796 | 0.5806 | 0.024* |
| H13B | 0.3050 | 0.0181 | 0.6567 | 0.024* |
| C14A | 0.45907(15) | −0.02924(14) | 0.74070(11) | 0.0292(3) |
| H14A | 0.3922 | −0.1010 | 0.7539 | 0.044* |
| H14B | 0.4969 | 0.0482 | 0.8034 | 0.044* |
| H14C | 0.5363 | −0.0522 | 0.7272 | 0.044* |
| C15A | 1.17205(13) | 0.35331(12) | 1.01190(10) | 0.0231(2) |
| H15A | 1.2258 | 0.3237 | 1.0623 | 0.035* |
| H15B | 1.1340 | 0.4091 | 1.0502 | 0.035* |
| H15C | 1.2340 | 0.4020 | 0.9783 | 0.035* |
| N1B | 0.54141(10) | 0.59549(10) | 0.25371(8) | 0.01830(19) |
| N2B | 0.44011(10) | 0.58408(10) | 0.16723(8) | 0.01920(19) |
| N3B | 0.90511(10) | 0.80346(11) | 0.38993(8) | 0.0207(2) |
| O1B | 0.76239(9) | 0.62764(9) | 0.41632(7) | 0.02308(19) |
| O2B | 0.86270(9) | 0.92868(9) | 0.24178(7) | 0.02146(18) |
| O3B | 0.70762(9) | 0.86463(8) | 0.07794(7) | 0.02111(18) |
| O4B | 0.12929(9) | 0.68998(9) | −0.23482(7) | 0.02364(19) |
| C1B | 0.66734(12) | 0.68711(11) | 0.27008(9) | 0.0166(2) |
| C2B | 0.64854(11) | 0.74108(11) | 0.18698(9) | 0.0165(2) |
| C3B | 0.50377(12) | 0.67138(11) | 0.12482(9) | 0.0171(2) |
| C4B | 0.41443(12) | 0.68267(11) | 0.02924(9) | 0.0176(2) |
| C5B | 0.43412(12) | 0.65344(12) | −0.07021(9) | 0.0206(2) |
| H5BA | 0.5122 | 0.6325 | −0.0764 | 0.025* |
| C6B | 0.34120(13) | 0.65437(12) | −0.16076(9) | 0.0214(2) |
| H6BA | 0.3553 | 0.6334 | −0.2282 | 0.026* |
| C7B | 0.22768(12) | 0.68630(11) | −0.15148(9) | 0.0189(2) |
| C8B | 0.20752(12) | 0.71718(11) | −0.05218(9) | 0.0188(2) |
| H8BA | 0.1307 | 0.7402 | −0.0457 | 0.023* |
| C9B | 0.29956(12) | 0.71424(11) | 0.03684(9) | 0.0184(2) |
| H9BA | 0.2844 | 0.7339 | 0.1040 | 0.022* |
| C10B | 0.78378(12) | 0.70430(11) | 0.36456(9) | 0.0176(2) |
| C11B | 1.02207(13) | 0.83072(14) | 0.48354(10) | 0.0279(3) |
| H11D | 1.0994 | 0.9113 | 0.4928 | 0.042* |
| H11E | 1.0543 | 0.7596 | 0.4741 | 0.042* |
| H11F | 0.9914 | 0.8396 | 0.5469 | 0.042* |
| C12B | 0.75130(12) | 0.85248(11) | 0.17349(9) | 0.0172(2) |
| C13B | 0.80215(14) | 0.97189(13) | 0.05564(10) | 0.0249(3) |
| H13C | 0.8943 | 0.9652 | 0.0622 | 0.030* |
| H13D | 0.8176 | 1.0555 | 0.1060 | 0.030* |
| C14B | 0.73009(18) | 0.96135(15) | −0.05726(11) | 0.0345(3) |
| H14D | 0.7908 | 1.0294 | −0.0780 | 0.052* |
| H14E | 0.6407 | 0.9712 | −0.0617 | 0.052* |
| H14F | 0.7118 | 0.8766 | −0.1056 | 0.052* |
| C15B | 0.12541(14) | 0.63066(14) | −0.34055(10) | 0.0274(3) |
| H15D | 0.0405 | 0.6245 | −0.3925 | 0.041* |
| H15E | 0.1240 | 0.5438 | −0.3474 | 0.041* |
| H15F | 0.2090 | 0.6830 | −0.3543 | 0.041* |
| H1NB | 0.5194(16) | 0.5392(16) | 0.2904(12) | 0.024(4)* |
| H1NA | 0.6678(18) | 0.4786(17) | 0.4517(14) | 0.035(5)* |
| H3NB | 0.9094(19) | 0.8547(18) | 0.3466(14) | 0.038(5)* |
| H3NA | 0.270(2) | 0.1825(19) | 0.4080(15) | 0.046(5)* |
Source of material
Hydrazine hydrate (3.3 mmol) was added to a mixture of ethyl 4-hydroxy-2-(4-methoxyphenyl)-1-methyl-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylate (3 mmol) and 15 mL of acetic acid. The mixture was refluxed for 3 h and cooled. The reaction mixture was evaporated under reduced pressure and the crude was purified by column chromatography on silica gel (ethyl acetate/petroleum ether, 80/20) to afford the product [5].
Experimental details
Data collection was performed by using the APEX2 software [1] and the cell refinement and data reduction were performed under the SAINT software [1]. The crystal structure was solved by direct method [2] and refined by full-matrix least squares technique on F2 using SHELXTL [2]. Absorption correction was applied to the final crystal data by using the SADABS software [1]. All geometrical calculations were carried out using the program PLATON [2]. The molecular graphics were drawn using SHELXTL [2] and Mercury [4] program. In this compound, the N-bound H atoms were located in difference Fourier maps and allowed to be refined freely [refined distance: N—H = 0.904(16)−0.976(19) Å]. The remaining hydrogen atoms were positioned geometrically [C—H = 0.95−0.99 Å] and were refined using a riding model, with Uiso(H) = 1.2 Ueq(C) or 1.5 Ueq(methyl C). A rotating-group model was used for the methyl groups.
Comment
Pyrazoles play a vital role in pharmaceuticals, since they possess an interesting pharmacological profile, such as anti-inflammatory [6], antimicrobial [7], antiviral [8], antitumor [9], anticonvulsant [10], antidepressant activities [11] and antihistaminic [12] etc. It commonly synthesized either by condensation of hydrazines with 1,3-dicarbonyl or by intermolecular cycloaddition reaction of alkynes to 3-dipoles [13]. During the course of our study, we have unexpectedly found that substituted pyrazole could be synthesized by condensation of pyrrolidine with hydrazine in acidic condition. To the best of our knowledge; this is the first report that synthesizes substituted pyrazole from pyrrolidine ring transformation.
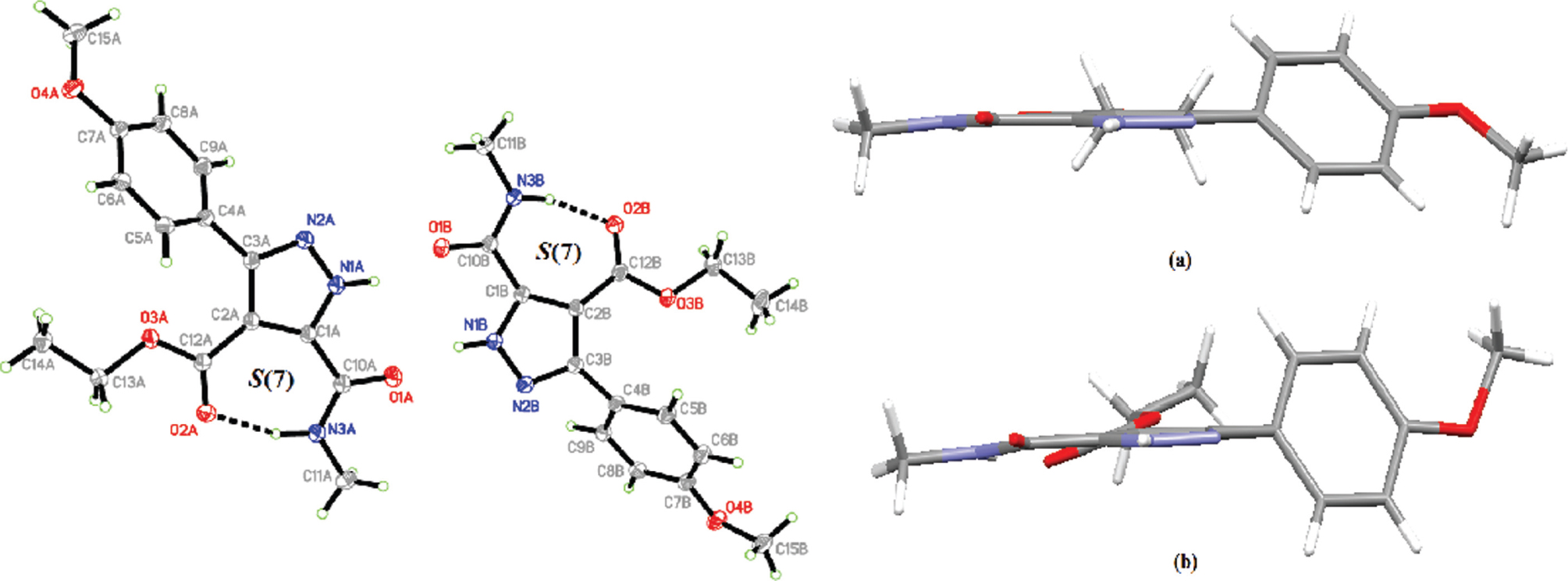
The asymmetric unit of the title compound consists of two crystallographically independent molecules, A and B (left part of the figure). The bond lengths and angles are within the normal ranges and comparable to the related structures [14], [15], [16], [17]. In each molecule (A and B), intramolecular N3A—H3NA⋯O2A and N3B—H3NB⋯O2B hydrogen bond are found, respectively, forming S(7) ring motif [18]. This interaction further stabilizes the molecular structure and locks its atoms in a nearly planar arrangement. In the independent molecule A, the 1-methoxy-4-methylbenzene (C4A—C9A/O4A/C15A) moiety is slightly twisted at C3A—C4A bond with C2A—C3A—C4A—C5A torsion angle of 44.46(19)°. Meanwhile, in molecule B the 1-methoxy-4-methylbenzene (C4B—C9B/O4B/C15B) and ethyl acetate (O2B/O3B/C12B—C14B) moieties are observed to be slightly twisted at C3B—C4B and C2B—C12B bonds, respectively. The twisted angle of C2B—C3B—C4B—C5B is found to be 67.48(18)° while the twisted angle for C1B—C2B—C12B—O2B is −12.4(2)° (right part of the figure). In both molecules, the pyrazole rings (N1A/N2A/C1A—C3A and N1B/N2B/C1B—C3B) form dihedral angles of 41.72(7)° and 62.81(7)° with the phenyl rings (C4A—C9A and C4B—C9B), respectively.
Acknowledgements
The authors would like to acknowledge Universiti Teknologi MARA and Malaysian Government (MOHE) for the financial support (600-IRMI/FRGS 5/3 (0071/2016).
References
1. Bruker. SADABS, APEX2 and SAINT. Bruker AXS Inc, Madison, WI, USA (2009).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
4. Macrae, C. F.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Shields, G. P.; Taylor, R.; Towler, M.; Van de Streek, J.: Mercury: visualization and analysis of crystal structures. J. Appl. Crystallogr. 39 (2006) 453–457.10.1107/S002188980600731XSearch in Google Scholar
5. Gein, V. L.; Kasimova, N. N.; Aliev, Z. G.; Vakhrin, M. I.: Three-component reaction of methyl 2,4-dioxo-4-phenyl- butanoate and methyl 2,4-dioxopentanoate with aromatic aldehydes and propane-1,2-diamine and chemical properties of the products. Russ. J. Org. Chem. 46 (2010) 875–883.10.1134/S1070428010060163Search in Google Scholar
6. Viveka, S.; Dinesha; Shama, P.; Nagaraja, G. K.; Ballav, S.; Kerkar, S.: Design and synthesis of some new pyrazolyl-pyrazolines as potential anti-inflammatory, analgesic and antibacterial agents. J. Med. Chem. 101 (2015) 442–451.10.1016/j.ejmech.2015.07.002Search in Google Scholar PubMed
7. Bouabdallah, I.; M’Barek, L. A.; Zyad, A.; Ramdani, A.; Zidane, I.; Melhaoui, A.: Anticancer effect of three pyrazole derivatives. Nat. Prod. Res. 20 (2006) 1024–1030.10.1080/14786410600921441Search in Google Scholar PubMed
8. Shahavar Sulthana, S.; Arul Antony, S.; Balachandran, C.; Syed Shafi, S.: Thiophene and benzodioxole appended thiazolyl-pyrazoline compounds: microwave assisted synthesis, antimicrobial and molecular docking studies. Bioorg. Med. Chem. Lett. 25 (2015) 2753–2757.10.1016/j.bmcl.2015.05.033Search in Google Scholar PubMed
9. Mohareb, R. M.; El-sayed, N. N. E.; Abdelaziz, M. A.: Uses of cyanoacetylhydrazine in heterocyclic synthesis: novel synthesis of pyrazole derivatives with anti-tumor activities. Molecules 17 (2012) 8449–8463.10.3390/molecules17078449Search in Google Scholar PubMed PubMed Central
10. Abunada, N. M.; Hassaneen, H. M.; Kandile, N. G.; Miqdad, O. A.: Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo[3,4-d]-pyrimidine and pyrazolo[4,3-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives. Molecules 13 (2008) 1501–1517.10.3390/molecules13071501Search in Google Scholar PubMed PubMed Central
11. Bailey, D. M.; Hansen, P. E.; Hlavac, A. G.; Baizman, E. R.; Pearl, J.; DeFelice, A. F.; Feigenson, M. E.: 3,4-diphenyl-1H-pyrazole-1-propanamine antidepressants. J. Med. Chem. 28 (1985) 256–260.10.1021/jm00380a020Search in Google Scholar PubMed
12. Mishra, P. D.; Wahidullah, S.; Kamat, S. Y.: A heteroaromatic acid from marine sponge Suberites vestigium. Indian J. Chem. 37B (1998) 199–200.Search in Google Scholar
13. Kumari, S.; Paliwal, S.; Chauhan, R.: Synthesis of pyrazole derivatives possesing anticancer activity: current status. Synth. Commun. 44 (2014) 1521–1578.10.1080/00397911.2013.828757Search in Google Scholar
14. Wang, D. J.; Zheng, C. Y.; Fan, L.: Synthesis, characterization, and crystal structures of new 3,5-diaryl-1H-pyrazoles. J. Mol. Struct. 938 (2009) 311–315.10.1016/j.molstruc.2009.10.001Search in Google Scholar
15. Sessler, J. L.; Rubin, B. L.; Camiolo, S.; Cho, W. S.; Dan Pantos, G.; Lynch, V. M.: Diamidopyrazoles: a new class of anion receptors. Supramol. Chem. 18 (2006) 103–109.10.1080/10610270500445523Search in Google Scholar
16. Sun, J.; Lv, P. C.; Yin, Y.; Yuan, R. J.; Ma, J.; Zhu, H. L.: Synthesis, structure and antibacterial activity of potent DNA gyrase inhibitors: N′-benzoyl-3-(4-bromophenyl)-1H-pyrazole-5-carbohydrazide derivatives. PLoS One 8 (2013) e69751.10.1371/journal.pone.0069751Search in Google Scholar PubMed PubMed Central
17. Jaćimović Željko, K.; Kosović, M.; Bogdanović Goran, A.; Novaković Sladjana, B.; Giester, G.; Bigović, M.: The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4. Z. Kristallogr. NCS 232 (2017) 651–653.10.1515/ncrs-2016-0393Search in Google Scholar
18. Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N. L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 34 (1995) 1555–1573.10.1002/anie.199515551Search in Google Scholar
©2019 Fatin Nur Ain Abdul Rashid et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3
Articles in the same Issue
- Frontmatter
- Crystal structure of [aqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N3,N3′,O4,O4′] zinc(II)] monohydrate, C16H10N4O9Zn⋅H2O
- Crystal structure of ethyl 3-(4-methoxyphenyl)-5-methylcarbamoyl-1H-pyrazole-4-carboxylate, C15H17N3O4
- 7-(4-Fluorobenzylidene)-3-(4-fluorophenyl)-N-phenyl-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbothioamide–dimethylformamide (2/1), C27H23F2N3S, 0.5(C3H7NO)
- Crystal structure of 4,4′-(hydrazonomethylene)diphenol dihydrate, C13H16N2O4
- Crystal structure of 4-methoxyphenyl-3-phenylpropiolate, C16H12O3
- Crystal Structure of tris(tetrakis{1-vinyl-1H-imidazole-κN}copper(II)) bis[tri-μ2-bromido-tetrabromido-bis(1-vinyl-1H-imidazole-κN)tetracopper(I)], C80H96N32Cu11Br14
- Crystal structure of (E)-2-(3,6-bis(diethylamino)-9H-xanthen-9-yl)-N′-(quinoxalin-2-ylmethylene)benzohydrazide, C37H36N6O2
- Crystal structure of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2)
- Crystal structure of 3-fluoro-3-methyl-1-((2-nitrophenyl)sulfonyl)-5,5-diphenylpiperidine, C24H23FN2O4S
- Crystal structure of dimethyl 3,12-dibenzyl-6,10-diphenyl-3,12-diazapentacyclo [6.3.1.02.7.04.11.05.9]-dodecane-7,11-dicarboxylate — acetone (2/1), C40H38N2O2 ⋅ 0.5C3H6O
- Crystal structure of poly[(μ2-2-(1H-1,2,4-triazol-1-yl)benzoato-κ4O:O′:N:N′)silver(I)] monohydrate, C9H8AgO3N3
- Crystal structure of poly[(μ2-9H-carbazole-3,6-dicarboxylate-κ4O1,O2:O3,O4)(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmium(II)]monohydrate, C27H23N3O5Cd
- The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir
- The crystal structure of 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfonyl)-1H-pyrazole-3-carboxamide, C12H6N4Cl2F6O3S
- Synthesis and crystal structure of poly[(μ2-nitrato-κ4O,O′:O′,O′′)-nitrato-κO-(μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)cadmium(II)], C14H14N6O6Cd
- Crystal structure of ethyl (Z)-(4-oxo-4-phenylbut-2-en-2-yl)glycinate, C14H17NO3
- Halogen bonds in the crystal structure of 5-bromo-3,4′-bipyridine – 1,4-diiodotetrafluorobenzene (2/1), C26H14Br2F4I2N4
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-tetrakis(μ2-3-(phenylsulfonamido)propanoato-κ2O:O′)-bis(3-(phenylsulfonamido)propanoato-κ2O,O′)digadolinium(III) – 2,2′-bipyridine (1/1), C84H84Gd2N12O24S6
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ2O:O′)bis(μ2-pyrazin-κ2N:N′)silver(I)], C14H16Ag2N5O8S2
- The crystal structure of 1,6-di-tert-butyl-1,1,3,3,4,4,6,6-octamethyl-2,2,5,5-tetrakis (trimethylsilyl)hexasilane, C28H78Si10
- Crystal structure of discandium triruthenium tetrasilicide, Sc2Ru3Si4
- Crystal structure of poly[(μ2-4-amino-1,5-naphthalenedisulfonato-κ4O,N:O′, N′)bis(μ2-hexamethylenetetramino-κ2N;N′)silver(I)], {C22H30Ag2N9O6S2}n
- Crystal structure of diaqua[5,5′-dicarboxy-2,2′-(propane-1,3-diyl)bis(1H-imidazole-4-carboxylato-κ4O,O′,N,N′)]zinc(II) dihydrate, C13H18N4O12Zn
- The crystal structure of poly [(μ3-N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide-κ3-O:N:N′)-(p-toluenesulfonato-κ2O,O′)silver(I)], C25H27Ag1N4O5S
- The crystal structure of 1,2-bis(3-bromophenoxy) ethane, C14H12Br2O2
- The crystal structure of 4-(pyren-1-yl)butyl-3-nitrobenzoate, C27H21NO4
- Crystal structure of bis[(2-(4-chlorophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ1O) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Cl2N4NiO8
- The crystal structure of 1,5-dinitro-2,3,4-trichlorobenzene, C6H1Cl3N2O4
- The crystal structure of the solid solution of 3,5-dinitropyrazole and 4-chlorine-3,5-dinitropyrazole, C3H1.24Cl0.76N4O4
- The cocrystal structure of 4-nitropyrazole — acetic acid (1/1), C5H7N3O4
- The crystal structure of propan-2-one O-(2,4,6-trinitrophenyl) oxime, C9H8N4O7
- The crystal structure of ethyl 2-(3-(2-ethoxy-2-oxoethyl)benzo[d] thiazol-2(3H)-ylidene)acetate, C15H17NO4S
- Crystal structure of (acetic acid-κ1O)-bis(μ2-2-chlorobenzoato-κ2O:O′)-(2-chlorobenzoato-κ1O)-(μ2-hydroxy-κ2O:O)-bis(1,10-phenanthroline-κ2N,N′)dimanganese(II) — methanol (1/1), C48H37Cl3Mn2N4O10
- Crystal structure of 3-methyl-2-phenyl-1,8-naphthyridine, C15H12N2
- Crystal structure of chlorido-(5-acetyl-2-(5-methylpyridin-2-yl)benzen-1-ido-κ2C,N)-pyridine-κN-palladium(II), C19H17ClN2OPd
- Crystal structure of (4-methyl-benzoato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C24H45ClN4NiO7
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium) — ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*⋅2 NH3, C22H45K0.3N2O6Rb0.7
- Crystal structure of (3E,5E)-1-((4-fluorophenyl)sulfonyl)-3,5-bis(3-nitrobenzylidene)piperidin-4-one — dichloromethane (2/1), C51H38Cl2F2N6O14S2
- Crystal structure of (E)-N′-((1,6-dihydropyren-1-yl)methylene)isonicotinohydrazide — methanol (1/1), C24H19N3O2
- Crystal structure of poly[aqua(μ2-2-amino-1,4-benzenedisulfonato-κ3N,O:O′)-(μ4-hexamethylenetetramino-κ4N:N′:N′′:N′′′)disilver(I)] monohydrate, C12H21Ag2N5O8S2
- Crystal structure of bis(acridin-10-ium) 2,5-dihydroxyterephthalate — 2,5-dihydroxyterephthalic acid (1/1), C21H15NO6
- The crystal structure of 1,12-diazaperylene, C18H10N2
- Crystal structure of 1-(5-(4-chlorophenyl)-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)ethan-1-one, C17H14N2OFCl
- Crystal structure of (4aR,6aR,6bR,10S,12aR)-10-acetoxy-1,2,3,4, 4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-icosahydro-2,2,4a,6b,9,9,12a-heptamethylpicene-6a-carboxylic acid, C32H50O4
- The crystal structure of tetrachlorido-bis{1,3-bis(2,6-diisopropylphenyl)-1H-3λ4-imidazol-2-yl}-(μ2-pyrimidine-κ2N:N′)dipalladium(IV) — dichloromethane (1/2), C60H80Cl8N6Pd2
- The crystal structure of (E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-yl 4-nitrobenzoate, C22H19NO7
- Crystal structure of 3-methyl-N-(pyrimidin-5-ylmethyl)pyridin-2-amine, C11H12N4
- The crystal structure of 2,5-dichloroterephthalic acid dihydrate, C8H8Cl2O6
- The crystal structure of 2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine — dimethylformamide (1/1), C33H28N10O
- Crystal structure of N-(adamantan-1-yl)-5-(dimethylamino)naphthalene-1-sulfonamide, C22H28N2O2S
- Crystal structure of poly[diaqua-(μ4-4-(3,5-dicarboxy-κ1O-phenoxy)phthalato-κ3O:O′:O′)cadmium(II)], C16H12CdO11
- Crystal structure of poly[diaqua-bis(μ2-3-((1H-imidazol-1-yl)methyl)benzoato-κ2N:O)manganese(II)], C22H22MnN4O6
- Crystal structure of 9-(3-phenoxyphenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione, C25H23NO3
- The crystal structure of poly[(μ3-2,4,6-tris[4-(1H-imidazol-1-yl)phenyl]-1,3,5-triazine-k3N:N′:N′′)-(nitrato-k2O,O)-(nitrato-k1O)zinc(II)] - N,N-dimethylacetamide (1/2), C38H39N13O8Zn
- Crystal structure of poly[(μ7-4-(3,5-dicarboxylatophenoxy)phthalato)-(1,10-phenanthroline-κ2N,N′)dizinc(II)], C28H14N2O9Zn2
- The crystal structure of methyl 2-(benzylamino)-5-(benzyloxy)benzoate, C22H21NO3
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanoplatinate(II), C14H24N8PdPt
- Crystal structure of (pyridine-2-carboxylato-κ2N,O)-[2-(2-pyridyl)phenyl-κ2N,C1]palladium(II), C17H12N2O2Pd
- Crystal structure of (cyclohexane-1,4-diammonium) 4-[(4-carboxylatophenyl)disulfanyl]benzoate dimethylsulphoxide hydrate (1/1/1/1), [C6H16N2]2+[C14H8O4S2]2−⋅C2H6OS⋅H2O
- Crystal structure of the 2:1 co-crystal 2-[(2-carboxyphenyl)disulfanyl]benzoic acid – 3-bromobenzoic acid, 2(C14H10O4S2)⋅C7H5BrO2
- Crystal structure of chlorido-dimethyl-(phenylpiperazine-1-carbodithioato-κ2S,S′)tin(IV), C13H19ClN2S2Sn
- Crystal structure of (N-n-butyl, N-methyl-dithiocarbamato-κ2 S,S′)-chlorido-dimethyl-tin(IV), C8H18ClNS2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-bromobenzyl)dibromidotin(IV), C24H20Br4N2Sn
- Crystal structure of (2,2′-bipyridyl)bis(4-chlorobenzyl)dichloridotin(IV), C24H20Cl4N2Sn
- Crystal structure of N-methyl-N-phenyl(methylsulfanyl)carbothioamide, C9H11NS2
- Crystal structure of 4-phenylpiperazin-1-ium (4-phenylpiperazin-1-yl)carbothioylsulfanide, [C10H15N2][C11H13N2S2]
- Crystal structure of catena-{di-aqua-sodium [n-butyl(methyl)carbamothioyl]sulfanide}n, [C6H16NNaO2S2]n
- Crystal structure of (2-([1,1-bis(hydroxymethyl)-2-oxyethyl]iminomethyl)-5-(n-decyl)phenolato)-dimethyl-tin(IV), C23H39NO5Sn
- Crystal structure of 4-chloro-N′-[(1E)-(3-ethoxy-2-hydroxyphenyl)methylidene]benzohydrazide – a Z′ = 3 structure, C16H15ClN2O3


