Abstract
C18H12N2, tetragonal, I41/a (no. 88), a = 13.8885(6) Å, c = 13.6718(6) Å, V = 2637.2(3) Å3, Z = 8, Rgt(F) = 0.0295, wRref(F2) = 0.0854, T = 210 K.
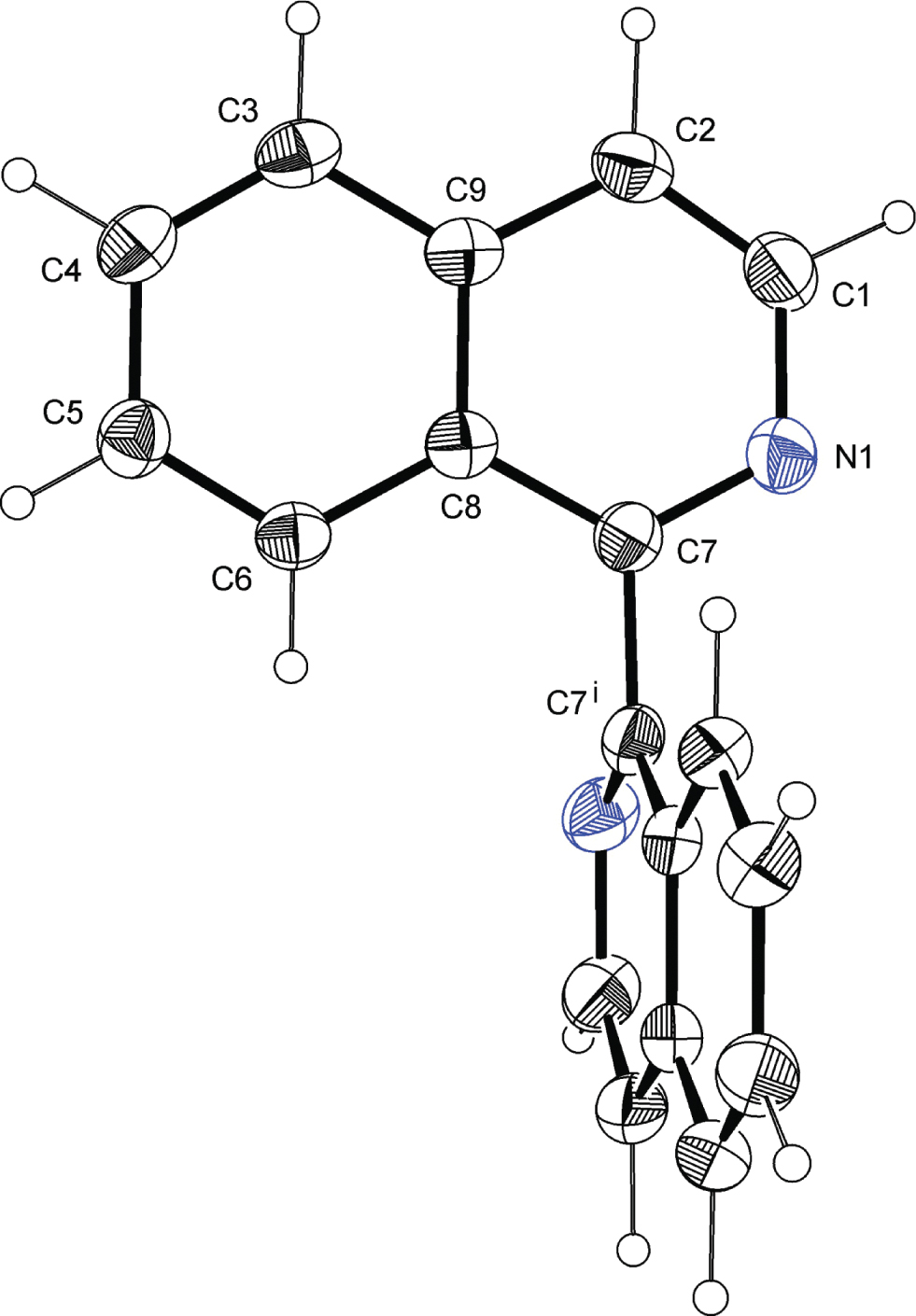
The molecular structure of the title compound is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow prism |
| Size: | 0.64 × 0.58 × 0.32 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.8 cm−1 |
| Diffractometer, scan mode: | STOE IPDS 2, ω scans |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 8390, 1164, 0.055 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 927 |
| N(param)refined: | 116 |
| Programs: | SHELX [1], WinGX [2], DIAMOND [3], PLATON [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.18787(9) | 0.82972(9) | 0.16368(10) | 0.0612(4) |
| H1 | 0.2126(10) | 0.8789(10) | 0.2112(10) | 0.071(4)* |
| C2 | 0.24871(9) | 0.77946(8) | 0.10507(10) | 0.0553(3) |
| H2 | 0.3242(9) | 0.7896(9) | 0.1085(9) | 0.058(3)* |
| C3 | 0.26831(9) | 0.65245(8) | −0.02158(9) | 0.0530(3) |
| H3 | 0.3387(10) | 0.6630(9) | −0.0206(8) | 0.059(3)* |
| C4 | 0.22713(9) | 0.58602(9) | −0.08111(9) | 0.0551(3) |
| H4 | 0.2675(10) | 0.5459(10) | −0.1238(10) | 0.068(4)* |
| C5 | 0.12673(9) | 0.57268(9) | −0.08217(9) | 0.0524(3) |
| H5 | 0.0986(9) | 0.5240(9) | −0.1231(10) | 0.058(3)* |
| C6 | 0.06914(9) | 0.62691(8) | −0.02349(8) | 0.0466(3) |
| H6 | 0.0012(9) | 0.6174(9) | −0.0235(8) | 0.052(3)* |
| C7 | 0.05365(8) | 0.75473(7) | 0.10355(8) | 0.0455(3) |
| C8 | 0.10969(7) | 0.69667(7) | 0.03968(8) | 0.0420(3) |
| C9 | 0.21067(7) | 0.70946(7) | 0.04073(8) | 0.0457(3) |
| N1 | 0.09037(7) | 0.81878(7) | 0.16407(8) | 0.0574(3) |
Source of materials
The title compound has been obtained by nickel-catalyzed C—C coupling of 1-chlorisoquinoline [5], which was synthesized by chlorination of isoquinoline-N-oxid with POCl3 [6]. The N-oxid was prepared by reaction of isoquinoline with H2O2 [7]. Yellow crystals (Mp. 162–163 °C) suitable for single-crystal X-ray diffraction were obtained from diethyl ether by slow evaporation within 3 days.
Experimental details
Coordinates of hydrogen atoms were refined without any constraints or restraints.
Comment
The title compound is a well-known ligand in the coordination chemistry [8], [9], [10], [11], [12], [13]. In most complexes 1,1′-bisisoquinoline acts as bidendate ligand, but can also occur as bridging ligand in binuclear complexes. The latter ones are also observed in luminescent silver(I) complexes [14], whereas in gold(I) complexes both types of complexes are formed [15]. Optically active complexes with high chiral recognition can be formed by reaction of 1,1′-bisisoquinoline with chiral palladium complexes [16]. Pd(II) and Ni(II) complexes of 1,1′-bisisoquinolines can be used as catalysts for Suzuki and Heck reactions [17]. 1,1′-Bisisoquinolinium salts exhibit chemiluminescence on addition of hydrogen peroxide in alkaline solution [18].
The title structure shows C2 symmetry. The asymmetric unit contains half a molecule. Both quinoline moieties are related by a 4̅ axis, located between the bridging atoms (C7 and C7′) running along the crystallographic c direction. The quinoline ring system is nearly full planar with a maximal deviation of the best plane of 0.012(1) Å (C1). The length of the C7—C7′ bond is 1.496(2) Å. The main structural feature is the position of the quinoline subunits being almost perpendicular to each other [88.54(1)°], which is caused by the transannular steric effect of the hydrogen atoms in the 8-position (labelled here with H6). For enhancing the transannular steric hindrance a series of 8,8′-dialkylsubstituted 1,1-bisisoquinolines were synthesized, whereby dihedric angles between both isoquinoline moieties of 77.68° (Me) and 86.27° (Et) can be observed [19]. Introducing ethoxy substituents in the 4,4′-positions leads to a dihedral angle of 66.20° [20]. During complexation reaction the isoquinoline subunits can forced into a more planar position depending on the requirements of the metal centre [13]. In presence of other suitable ligands, which also can complete the coordination sphere the twisted bisisoquinoline structure is only slightly changed and bisisoquinoline often acts as bridging ligand and binuclear complexes are formed [16]. The crystal packing is characterized by a large number of π–π interactions. Furthermore, C—H⋅⋅⋅π interactions are observed [C4—H4⋅⋅⋅C3—C6/C8/C9 ring].
References
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.3.2. Crystal Impact, Bonn, Germany, 2017.Search in Google Scholar
Spek, A. L.: PLATON − a multipurpose crystallographic tool. Acta Crystallogr. D65 (2011) 148–155.10.1107/S090744490804362XSearch in Google Scholar
Case, F. H.: The preparation of 1,1′- and 3,3′-bisisoquinoline. J. Org. Chem. 17 (1952) 471–472.10.1021/jo01137a021Search in Google Scholar
Alcock, N. W.; Brown, J. M.; Hulmes, D. I.: Synthesis and resolution of 1-(2-diphenylphosphino-1-naphthyl)isoquinoline; a P—N chelating ligand for asymmetric catalysis. Tetrahedron Asymmetry 4 (1993) 743–756.10.1016/S0957-4166(00)80183-4Search in Google Scholar
Zhu, C.; Yi, M.; Wei, D.; Chen, X.; Wu, Y.; Cui, X.: Copper-catalyzed direct amination of quinoline N-oxides via C—H bond activation under mild conditions. Org. Lett. 16 (2014) 1840–1843.10.1021/ol500183wSearch in Google Scholar
Starke, I.; Kammer, S.; Grunwald, N.; Schilde, U.; Holdt, H.-J.; Kleinpeter, E.: Complexation of diazaperylene and bisisoquinoline with transition metal ions in the gas phase studied by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 22 (2008) 665–671.10.1002/rcm.3412Search in Google Scholar
Frediani, P.; Giannelli, C.; Salvini, A.; Ianelli, S.: Ruthenium complexes with 1,1′-biisoquinoline as ligand. Synthesis and hydrogenation activity. J. Organomet. Chem. 667 (2003) 197–208.10.1016/S0022-328X(02)02193-9Search in Google Scholar
Yu, W.-Y.; Cheng, W.-C.; Che, C.-M.; Wang, Y.: Synthesis, redox properties and reactivities of ruthenium(II) complexes of 1,1′-biisoquinoline (BIQN) and X-ray crystal structure of [RuII(terpy)(BIQN)(Cl)]ClO4 (terpy = 2,2′:6′,2′′-terpyridine). Polyhedron 13 (1994) 2963–2969.10.1016/S0277-5387(00)83415-4Search in Google Scholar
Yang, R.; Dai, L.: Synthesis and electronic spectra of ruthenium(II)-1,1′-biisoquinoline complexes. Chin. Chem. Lett. 4 (1993) 1021–1024.Search in Google Scholar
Cheng, L. K.; Yeung, K. S.; Che, C. M.; Cheng, M. C.; Wang, Y.: X-ray structure and spectroscopic properties of platinum(II) complexes of 1,1′-biisoquinoline. Polyhedron 12 (1993) 1201–1207.10.1016/S0277-5387(00)88212-1Search in Google Scholar
Ashby, M. T.; Alguindigue, S. S.; Schwane, J. D.; Daniel, T. A.: Regular and inverse secondary kinetic enthalpy effects (KHE) for the rate of inversion of thioether and 1,1′-biisoquinoline complexes of ruthenium and osmium. Inorg. Chem. 40 (2001) 6643–6650.10.1021/ic0105720Search in Google Scholar PubMed
Bardaji, M.; Miguel-Coello, A. B.; Espinet, P.: Predominance of bridging coordination in luminescent 1,1′-bisisoquinoline silver(I) derivatives. Inorg. Chim. Acta 386 (2012) 93–101.10.1016/j.ica.2012.01.059Search in Google Scholar
Bardaji, M.; Miguel-Coello, A. B.; Espinet, P.: Mono- and dinuclear luminescent 1,1′-bisisoquinoline gold(I) complexes. Inorg. Chim. Acta 392 (2012) 91–98.10.1016/j.ica.2012.06.024Search in Google Scholar
Dai, L.-X.; Zhou, Z.-H.; Zhang, Y.-Z.; Ni, C.-Z.; Zhang, Z.-M.; Zhou, Y.-F.: 1,1′-Bi-isoquinoline: a chiral bidentate N-donor ligand with C2-symmetry; formation of optically active complexes with high chiral recognition. J. Chem. Soc. Chem. Commun. (1987) 1760–1762.10.1039/C39870001760Search in Google Scholar
Khrushcheva, N. S.; Bulygina, L. A.; Starikova, Z. A.; Sokolov, V. I.: Synthesis, structure, and catalytic activity of complexes of 1,1′-bisisoquinoline with PdCl2 and NiCl2. Russ. Chem. Bull. 63 (2014) 883–889.10.1007/s11172-014-0524-8Search in Google Scholar
Maeda, K.; Matsuyama, Y.; Isozaki, K.; Yamada, S.; Mori, Y.: Mechanism of the chemiluminescence of biisoquinolinium salts. J. Chem. Soc. Perkin Trans. 2 (1996) 121–126.10.1039/p29960000121Search in Google Scholar
Tsue, H.; Fujinami, H.; Itakura, T.; Tsuchiya, R.; Kobayashi, K.; Takahashi, H.; Hirao, K.: Absolute configuration of 8,8′-dialkyl-1,1′-bisisoquinoline. Chem. Lett. 28 (1999) 17–18.10.1246/cl.1999.17Search in Google Scholar
Kapatsina, E.; Mateescu, M.; Baro, A.; Frey, W.; Laschat, S.: Concise synthesis of [1,1′-biisoquinoline]-4,4′-diol via a protecting group strategy and its application for potential liquid-crystalline compounds. Helvet. Chim. Acta 92 (2009) 2024–2037.10.1002/hlca.200900085Search in Google Scholar
©2017 Nicolas Grunwald et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

