Abstract
C25H24N3O3, monoclinic, P21/c (no. 14), a = 9.4549(11) Å, b = 10.7640(12) Å, c = 21.279(3) Å, β = 98.778(2)°, V = 2140.2(4) Å3, Z = 4, Rgt(F) = 0.0498, wRref(F2) = 0.1408, T = 296(2) K.
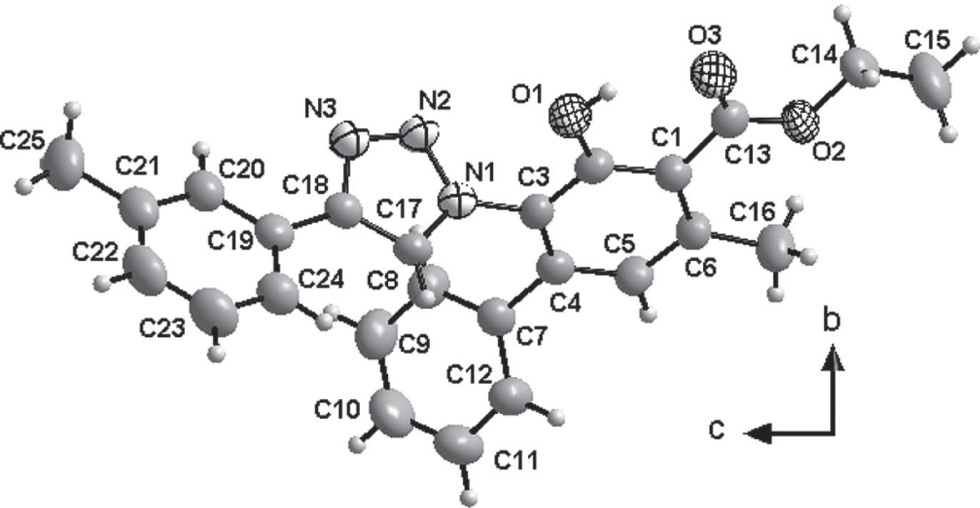
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.2 × 0.2 × 0.1 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Bruker APEXII, ω |
| 2θmax, completeness: | 50.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11223, 3756, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2088 |
| N(param)refined: | 284 |
| Programs: | Bruker [1] , SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.3376(3) | 1.0343(2) | 0.05762(12) | 0.0426(6) |
| C2 | 0.3998(3) | 1.0459(2) | 0.12172(12) | 0.0425(6) |
| C3 | 0.3512(3) | 0.9739(2) | 0.16836(11) | 0.0412(6) |
| C4 | 0.2450(3) | 0.8857(2) | 0.15322(12) | 0.0424(6) |
| C5 | 0.1902(3) | 0.8721(2) | 0.08930(12) | 0.0483(7) |
| H5 | 0.1211 | 0.8111 | 0.0781 | 0.058* |
| C6 | 0.2314(3) | 0.9429(2) | 0.04123(12) | 0.0443(7) |
| C7 | 0.1898(3) | 0.8064(2) | 0.20119(12) | 0.0460(7) |
| C8 | 0.1515(3) | 0.8547(3) | 0.25628(13) | 0.0578(8) |
| H8 | 0.1645 | 0.9389 | 0.2652 | 0.069* |
| C9 | 0.0939(3) | 0.7789(3) | 0.29839(14) | 0.0723(9) |
| H9 | 0.0679 | 0.8123 | 0.3353 | 0.087* |
| C10 | 0.0751(4) | 0.6547(3) | 0.28585(16) | 0.0778(10) |
| H10 | 0.0363 | 0.6041 | 0.3143 | 0.093* |
| C11 | 0.1133(4) | 0.6046(3) | 0.23168(16) | 0.0741(10) |
| H11 | 0.1014 | 0.5201 | 0.2234 | 0.089* |
| C12 | 0.1696(3) | 0.6807(2) | 0.18953(14) | 0.0578(8) |
| H12 | 0.1944 | 0.6468 | 0.1525 | 0.069* |
| C13 | 0.3889(3) | 1.1200(2) | 0.01174(13) | 0.0472(7) |
| C14 | 0.3509(3) | 1.2013(2) | −0.09355(12) | 0.0566(8) |
| H14A | 0.4466 | 1.1842 | −0.1025 | 0.068* |
| H14B | 0.3472 | 1.2866 | −0.0792 | 0.068* |
| C15 | 0.2433(4) | 1.1801(3) | −0.15101(14) | 0.0858(11) |
| H15A | 0.2508 | 1.0962 | −0.1654 | 0.129* |
| H15B | 0.2604 | 1.2366 | −0.1839 | 0.129* |
| H15C | 0.1491 | 1.1938 | −0.1408 | 0.129* |
| C16 | 0.1603(3) | 0.9160(3) | −0.02537(13) | 0.0649(9) |
| H16A | 0.1014 | 0.9852 | −0.0412 | 0.097* |
| H16B | 0.1020 | 0.8430 | −0.0254 | 0.097* |
| H16C | 0.2320 | 0.9026 | −0.0521 | 0.097* |
| N3 | 0.4768(2) | 1.08661(19) | 0.32060(10) | 0.0536(6) |
| C18 | 0.5468(3) | 0.9755(2) | 0.32530(12) | 0.0429(6) |
| C19 | 0.6478(3) | 0.9405(2) | 0.38142(12) | 0.0455(7) |
| C20 | 0.6543(3) | 1.0063(2) | 0.43832(12) | 0.0528(7) |
| H20 | 0.5904 | 1.0713 | 0.4406 | 0.063* |
| C21 | 0.7527(3) | 0.9780(3) | 0.49132(13) | 0.0575(8) |
| C22 | 0.8459(3) | 0.8806(3) | 0.48652(15) | 0.0681(9) |
| H22 | 0.9136 | 0.8596 | 0.5214 | 0.082* |
| C23 | 0.8398(3) | 0.8139(3) | 0.43057(16) | 0.0684(9) |
| H23 | 0.9026 | 0.7482 | 0.4284 | 0.082* |
| C24 | 0.7423(3) | 0.8437(2) | 0.37834(13) | 0.0540(7) |
| H24 | 0.7396 | 0.7987 | 0.3409 | 0.065* |
| C25 | 0.7567(4) | 1.0503(3) | 0.55197(14) | 0.0811(10) |
| H25A | 0.8291 | 1.0165 | 0.5839 | 0.122* |
| H25B | 0.6653 | 1.0450 | 0.5662 | 0.122* |
| H25C | 0.7783 | 1.1357 | 0.5446 | 0.122* |
| N2 | 0.3995(2) | 1.09658(19) | 0.26430(10) | 0.0522(6) |
| N1 | 0.4201(2) | 0.99066(18) | 0.23284(9) | 0.0441(5) |
| C17 | 0.5095(3) | 0.9140(2) | 0.26894(12) | 0.0440(7) |
| H17 | 0.5398 | 0.8357 | 0.2581 | 0.053* |
| O1 | 0.5079(2) | 1.12501(17) | 0.14144(8) | 0.0561(5) |
| H1 | 0.5293 | 1.1628 | 0.1108 | 0.084* |
| O2 | 0.3137(2) | 1.11617(16) | −0.04560(8) | 0.0578(5) |
| O3 | 0.4903(2) | 1.19018(17) | 0.02504(9) | 0.0608(6) |
Source of material
To ethynyl-3-methylbenzene (63.8 mg, 0.55 mmol) and ethyl 4-azido-3-oxobutanoate (85.5 mg, 0.5 mmol) in t-BuOH/H2O (5 mL, V/V = 1/1) were added CuSO4⋅ 5H2O (26 μL, 1M, 5 mmol) and sodium ascorbate (9.9 mg, 10 mmol). The mixture was stirred at room temperature for 12 h. After completion monitoring by TLC, the mixture was charged with 1-buta-2,3-dien-1-one (79.3 mg, 0.55 mmol) and NaOH (40.0 mg, 1.0 mmol), stirred and heated at 80 °C for 1.5h. After cooling to room temperature, the reaction mixture was extracted with dichloromethane (3 × 15 mL), and the combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuum. The resulting residue was purified by flash chromatography (SiO2) using EtOAc/ petroleum ether (v/v = 1/5) as the solvent system to give the title compound (143.5 mg, 63%) as yellow solid. Single crystals were obtained by slow evaporation of its chloroform solution at room temperature.
Experimental details
All H atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C) and to 1.5Ueq(C) for methyl groups.
Discussion
1,2,3-Triazoles are heterocyclic compounds with a wide range of applications in medicinal chemistry, chemical biology, material science, and synthetic organic chemistry [4], [5], [6], [7], [8], [9], [10]. Among them, the 1-aryl-[1,2,3]-triazoles are key building units embedded in the lead compounds currently being developed as σ2 receptor ligands [11], Hsp90 inhibitors [12], suppressors of estrogen-related receptor α [13]. Owing to its larger conjugated system, coplanarity, and capability of hydrogen bonding, 1-aryl-[1,2,3]-triazoles play an important role in ionic recognition and design of chemical sensors [14]. Further, 1-aryl-[1,2,3]-triazoles are also indispensable intermediates for the preparation of a broad range of fine chemicals [15], [16], [17].
All bond lengths and angles of the title structure are in normal ranges. There is a 69.70(9)° dihedral angle between ring (C1—C6) and ring (N1—N3/C17—C18). The dihedral angle between the two benzene rings of biphenyl is 45.75(9)°.
Acknowledgement
This research project was supported by the Natural Science Research Program from Education Department of Henan Province (No. 2011 A150015).
References
Bruker AXS Inc., SAINT, APEX2, SHELXTL, Madison, Wisconsin, USA, 2009.Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Vernekar, S. K. V.; Qiu, L.; Zhang, J.; Kankanala, J.; Li, H.; Geraghty, R. J.; Wang, Z.: 5′-Silylated 3′-1,2,3-triazolyl thymidine analogues as inhibitors of west nile virus and dengue virus. J. Med. Chem. 58 (2015) 4016–4028.10.1021/acs.jmedchem.5b00327Search in Google Scholar PubMed PubMed Central
Zhang, Y.; Ye, X.; Petersen, J. L.; Li, M.; Shi, X.: Synthesis and characterization of bis-N-2-aryl triazole as a fluorophore. J. Org. Chem. 80 (2015) 3664–3669.10.1021/acs.joc.5b00006Search in Google Scholar PubMed
Shi, S.; Kuang, C.: Palladium-catalyzed ortho-alkoxylation of 2-aryl-1,2,3-triazoles. J. Org. Chem. 79 (2014) 6105–6112.10.1021/jo5008306Search in Google Scholar PubMed
Lei, X.; Gao, M.; Tang, Y.: Tandem O–H Insertion/[1,3]-Alkyl shift of rhodium azavinyl carbenoids with benzylic alcohols: a route to convert C–OH bonds into C–C bonds. Org. Lett. 18 (2016) 4998–5001.10.1021/acs.orglett.6b02459Search in Google Scholar PubMed
Man, Z.; Dai, H.; Shi, Y.; Yang, D.; Li, C.: Synthesis of 5-iodo-1,2,3,4-tetrahydropyridines by rhodium-catalyzed tandem nucleophilic attacks involving 1-sulfonyl-1,2,3-triazoles and iodides. Org. Lett. 18 (2016) 4962–4965.10.1021/acs.orglett.6b02428Search in Google Scholar PubMed
Verma, Y. K.; Reddy, B. S.; Pawar, M. S.; Bhunia, D.; Kumar, H. M. S.: Design, synthesis, and immunological evaluation of benzyloxyalkyl-substituted 1,2,3-triazolyl α-GalCer analogues. ACS Med. Chem. Lett. 7 (2016) 172–176.10.1021/acsmedchemlett.5b00340Search in Google Scholar PubMed PubMed Central
Shieh, P.; Bertozzi, C. R.: Design strategies for bioorthogonal smart probes. Org. Biomol. Chem. 12 (2014) 9307–9320.10.1039/C4OB01632GSearch in Google Scholar
Bai, S.; Li, S.; Xu, J.; Peng, X.; Sai, K.; Chu, W.; Tu, Z.; Zeng, C.; Mach, R. H.: Synthesis and structure–activity relationship studies of conformationally flexible tetrahydroisoquinolinyl triazole carboxamide and triazole substituted benzamide analogues as σ2 receptor ligands. J. Med. Chem. 57 (2014) 4239–4251.10.1021/jm5001453Search in Google Scholar PubMed PubMed Central
Taddei, M.; Ferrini, S.; Giannotti, L.; Corsi, M.; Manetti, F.; Giannini, G.; Vesci, L.; Milazzo, F. M.; Alloatti, D.; Guglielmi, M. B.; Castorina, M.; Cervoni, M. L.; Barbarino, M.; Foderà, R.; Carollo, V.; Pisano, C.; Armaroli, S.; Cabri, W.: Synthesis and evaluation of new Hsp90 inhibitors based on a 1,4,5-trisubstituted 1,2,3-triazole scaffold. J. Med. Chem. 57 (2014) 2258–2274.10.1021/jm401536bSearch in Google Scholar PubMed
Xu, S.; Zhuang, X.; Pan, X.; Zhang, Z.; Duan, L.; Liu, Y.; Zhang, L.; Ren, X.; Ding, K.: 1-Phenyl-4-benzoyl-1H-1,2,3-triazoles as orally bioavailable transcriptional function suppressors of estrogen-related receptor α. J. Med. Chem. 56 (2013) 4631–4640.10.1021/jm4003928Search in Google Scholar PubMed
Cheng, C.; Wan, J.; Lin, M.; Liu, Y.; Lu, X.; Liu, J.; Xu, Y.; Chen, J.; Tu, Z.; Cheng, Y. E.; Ding, K.: Design, Synthesis, and in vitro biological evaluation of 1H-1,2,3-triazole-4-carboxamide derivatives as new anti-influenza A agents targeting virus nucleoprotein. J. Med. Chem. 55 (2012) 2144–2153.10.1021/jm2013503Search in Google Scholar PubMed
Stangenberg, R.; Türp, D.; Müllen, K.: Shape persistent hybrid dendrimers from benzene and triazole via click chemistry. Tetrahedron 70 (2014) 3178–3184.10.1016/j.tet.2014.03.037Search in Google Scholar
Li, Y.; Xu, L.; Yang, W. L.; Liu, H. B.; Lai, S. W.; Che, C. M.; Li, Y. L.: Amidetriazole: a versatile building block for construction of oxyanion anion receptors. Chem. Eur. J. 18 (2012) 4782–4790.10.1002/chem.201102760Search in Google Scholar PubMed
Sureshbabu, B.; Venkatachalam, R.; Sankararaman, S.: Substituent effect on the formation of helical to layered hydrogen bond networks in hydroxyl and carboxyl substituted 1-aryl-1H-1,2,3-triazoles. CrystEngComm 16 (2014) 6098–6106.10.1039/C4CE00738GSearch in Google Scholar
©2017 Qiang Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

