Abstract
C49H40BCu2F4Fe2N5O5, triclinic, P1̄ (no. 2), a = 11.526(2) Å, b = 12.902(2) Å, c = 15.469(3) Å, α = 95.632(2)°, β =107.789(2)°, γ = 96.251(2)°, V = 2156.3(7) Å3, Z = 2, Rgt(F) = 0.0443, wRref(F2) = 0.1198, T = 100(2) K.
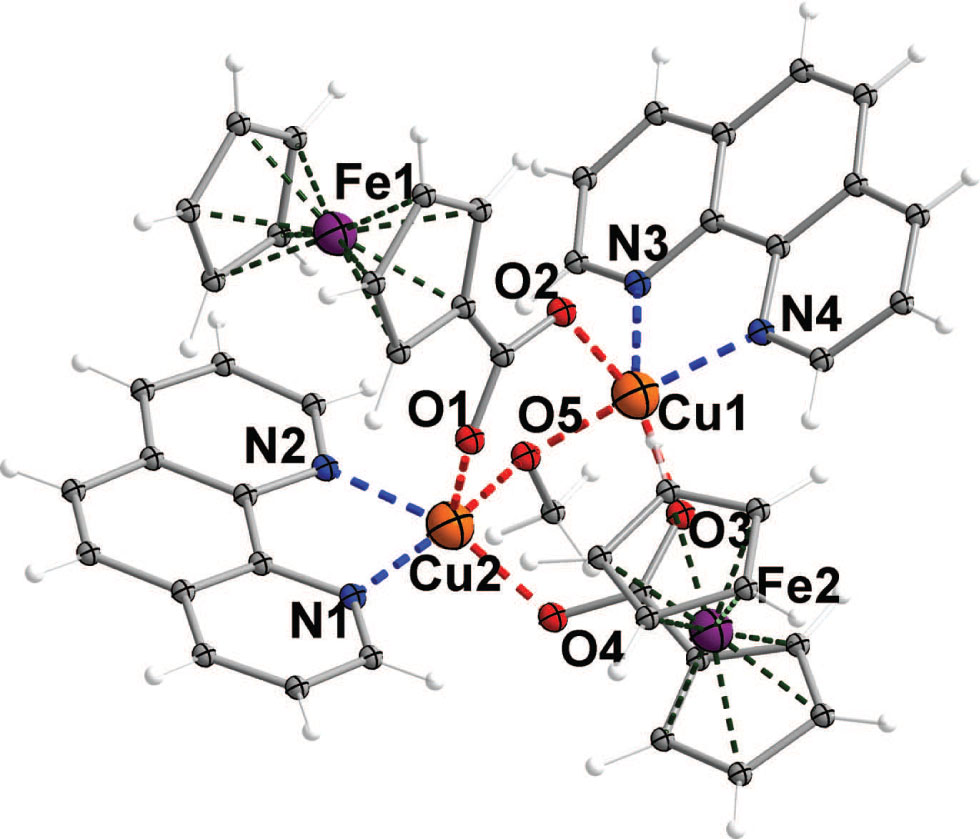
Parts of the title crystal structure are shown using a ball-and-stick scheme in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Blue-green block |
| Size: | 0.21 × 0.20 × 0.19 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 17.1 cm−1 |
| Diffractometer, scan mode: | Bruker CCD, φ and ω |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15093, 7571, 0.037 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5704 |
| N(param)refined: | 637 |
| Programs: | SHELX [1], DIAMOND [2], Bruker programs [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| B1 | 0.6060(6) | 0.4603(4) | 0.6830(4) | 0.0358(14) |
| Cu1 | −0.02525(4) | 0.17999(4) | 0.39136(3) | 0.01835(14) |
| Cu2 | −0.02780(4) | 0.09554(4) | 0.19203(3) | 0.01927(14) |
| Fe2 | −0.36557(5) | −0.15626(4) | 0.26782(4) | 0.01788(15) |
| Fe1 | −0.35154(5) | 0.36527(5) | 0.08624(4) | 0.01868(16) |
| O1 | −0.1984(3) | 0.1446(2) | 0.18177(19) | 0.0276(7) |
| O2 | −0.1781(3) | 0.2560(2) | 0.30717(18) | 0.0234(7) |
| O3 | −0.0881(3) | 0.0299(2) | 0.37014(18) | 0.0220(7) |
| O4 | −0.0700(3) | −0.0375(2) | 0.23497(18) | 0.0215(6) |
| O5 | 0.0727(3) | 0.1671(2) | 0.31094(17) | 0.0214(6) |
| N1 | −0.1151(3) | 0.0307(3) | 0.0588(2) | 0.0221(8) |
| N2 | 0.0438(3) | 0.2055(3) | 0.1291(2) | 0.0223(8) |
| N3 | 0.0791(3) | 0.3172(3) | 0.4637(2) | 0.0200(8) |
| N4 | −0.1007(3) | 0.1950(3) | 0.4935(2) | 0.0205(8) |
| N5a | −0.5445(8) | 0.0991(7) | 0.4620(6) | 0.087(3) |
| N5Ab | −0.6635(17) | 0.1209(15) | 0.3943(13) | 0.082(7) |
| C1 | −0.1991(4) | −0.0546(3) | 0.0271(3) | 0.0276(10) |
| H1A | −0.2137 | −0.0999 | 0.0687 | 0.033* |
| C2 | −0.2674(4) | −0.0796(4) | −0.0671(3) | 0.0306(11) |
| H2A | −0.3271 | −0.1411 | −0.0880 | 0.037* |
| C3 | −0.2476(4) | −0.0155(4) | −0.1281(3) | 0.0296(11) |
| H3A | −0.2934 | −0.0321 | −0.1914 | 0.035* |
| C4 | −0.1593(4) | 0.0745(3) | −0.0964(3) | 0.0249(10) |
| C5 | −0.1286(4) | 0.1470(4) | −0.1536(3) | 0.0284(11) |
| H5A | −0.1689 | 0.1335 | −0.2180 | 0.034* |
| C6 | −0.0440(4) | 0.2338(4) | −0.1182(3) | 0.0301(11) |
| H6A | −0.0254 | 0.2795 | −0.1582 | 0.036* |
| C7 | 0.0183(4) | 0.2585(4) | −0.0215(3) | 0.0282(11) |
| C8 | 0.1031(4) | 0.3494(4) | 0.0205(3) | 0.0310(11) |
| H8A | 0.1230 | 0.3998 | −0.0155 | 0.037* |
| C9 | 0.1574(4) | 0.3651(4) | 0.1142(3) | 0.0305(11) |
| H9A | 0.2159 | 0.4259 | 0.1432 | 0.037* |
| C10 | 0.1255(4) | 0.2907(3) | 0.1667(3) | 0.0268(10) |
| H10A | 0.1639 | 0.3020 | 0.2313 | 0.032* |
| C11 | −0.0090(4) | 0.1881(3) | 0.0360(3) | 0.0196(9) |
| C12 | −0.0955(4) | 0.0953(3) | −0.0015(3) | 0.0202(9) |
| C13 | 0.1709(4) | 0.3753(3) | 0.4490(3) | 0.0236(10) |
| H13A | 0.1990 | 0.3504 | 0.4002 | 0.028* |
| C14 | 0.2290(4) | 0.4720(3) | 0.5021(3) | 0.0269(10) |
| H14A | 0.2968 | 0.5100 | 0.4906 | 0.032* |
| C15 | 0.1876(4) | 0.5112(4) | 0.5704(3) | 0.0309(11) |
| H15A | 0.2238 | 0.5781 | 0.6053 | 0.037* |
| C16 | 0.0901(4) | 0.4508(3) | 0.5885(3) | 0.0241(10) |
| C17 | 0.0380(4) | 0.4842(4) | 0.6581(3) | 0.0295(11) |
| H17A | 0.0683 | 0.5515 | 0.6938 | 0.035* |
| C18 | −0.0535(4) | 0.4217(3) | 0.6736(3) | 0.0296(11) |
| H18A | −0.0845 | 0.4450 | 0.7213 | 0.036* |
| C19 | −0.1044(4) | 0.3213(3) | 0.6201(3) | 0.0241(10) |
| C20 | −0.1996(4) | 0.2519(4) | 0.6309(3) | 0.0296(11) |
| H20A | −0.2354 | 0.2708 | 0.6769 | 0.035* |
| C21 | −0.2416(4) | 0.1572(4) | 0.5758(3) | 0.0313(11) |
| H21A | −0.3051 | 0.1095 | 0.5839 | 0.038* |
| C22 | −0.1891(4) | 0.1312(3) | 0.5067(3) | 0.0260(10) |
| H22A | −0.2186 | 0.0654 | 0.4683 | 0.031* |
| C23 | −0.0569(4) | 0.2882(3) | 0.5498(3) | 0.0198(9) |
| C24 | 0.0395(4) | 0.3532(3) | 0.5341(3) | 0.0215(9) |
| C25 | −0.4173(4) | 0.2109(3) | 0.0797(3) | 0.0245(10) |
| H25A | −0.3990 | 0.1504 | 0.0428 | 0.029* |
| C26 | −0.5161(4) | 0.2691(3) | 0.0489(3) | 0.0289(11) |
| H26A | −0.5796 | 0.2567 | −0.0133 | 0.035* |
| C27 | −0.5093(4) | 0.3479(4) | 0.1224(3) | 0.0273(10) |
| H27A | −0.5665 | 0.4012 | 0.1206 | 0.033* |
| C28 | −0.4049(4) | 0.3385(3) | 0.1984(3) | 0.0236(10) |
| H28A | −0.3761 | 0.3841 | 0.2592 | 0.028* |
| C29 | −0.3473(4) | 0.2541(3) | 0.1716(3) | 0.0202(9) |
| C30 | −0.2314(4) | 0.2168(3) | 0.2259(3) | 0.0175(9) |
| C31 | −0.1732(4) | 0.4161(3) | 0.0966(3) | 0.0242(10) |
| H31A | −0.0988 | 0.3903 | 0.1359 | 0.029* |
| C32 | −0.2423(4) | 0.3737(4) | 0.0042(3) | 0.0264(10) |
| H32A | −0.2250 | 0.3128 | −0.0327 | 0.032* |
| C33 | −0.3400(4) | 0.4328(3) | −0.0259(3) | 0.0251(10) |
| H33A | −0.4040 | 0.4208 | −0.0878 | 0.030* |
| C34 | −0.3320(4) | 0.5108(3) | 0.0475(3) | 0.0214(9) |
| H34A | −0.3896 | 0.5637 | 0.0461 | 0.026* |
| C35 | −0.2292(4) | 0.5011(3) | 0.1233(3) | 0.0215(9) |
| H35A | −0.2010 | 0.5460 | 0.1845 | 0.026* |
| C36 | −0.2229(4) | −0.1664(3) | 0.3820(3) | 0.0221(9) |
| H36A | −0.2014 | −0.1214 | 0.4427 | 0.027* |
| C37 | −0.3036(4) | −0.2632(3) | 0.3540(3) | 0.0254(10) |
| H37A | −0.3492 | −0.2983 | 0.3914 | 0.030* |
| C38 | −0.3084(4) | −0.3019(3) | 0.2637(3) | 0.0256(10) |
| H38A | −0.3588 | −0.3685 | 0.2262 | 0.031* |
| C39 | −0.2319(4) | −0.2286(3) | 0.2348(3) | 0.0226(9) |
| H39A | −0.2183 | −0.2348 | 0.1738 | 0.027* |
| C40 | −0.1787(4) | −0.1435(3) | 0.3082(3) | 0.0191(9) |
| C41 | −0.1071(4) | −0.0430(3) | 0.3045(3) | 0.0175(9) |
| C42 | −0.5282(4) | −0.1585(4) | 0.1660(3) | 0.0411(13) |
| H42A | −0.5651 | −0.2135 | 0.1112 | 0.049* |
| C43 | −0.4483(4) | −0.0661(4) | 0.1715(3) | 0.0345(12) |
| H43A | −0.4176 | −0.0438 | 0.1213 | 0.041* |
| C44 | −0.4189(4) | −0.0103(4) | 0.2601(3) | 0.0329(11) |
| H44A | −0.3634 | 0.0585 | 0.2838 | 0.039* |
| C45 | −0.4810(5) | −0.0675(4) | 0.3095(3) | 0.0389(12) |
| H45A | −0.4777 | −0.0466 | 0.3743 | 0.047* |
| C46 | −0.5479(4) | −0.1594(4) | 0.2519(4) | 0.0443(14) |
| H46A | −0.6013 | −0.2154 | 0.2684 | 0.053* |
| C47 | 0.1791(5) | 0.1234(5) | 0.3500(4) | 0.0591(17) |
| H47A | 0.2251 | 0.1637 | 0.4101 | 0.089* |
| H47B | 0.2306 | 0.1257 | 0.3100 | 0.089* |
| H47C | 0.1562 | 0.0502 | 0.3573 | 0.089* |
| C48a | −0.5099(10) | 0.1810(8) | 0.4407(8) | 0.068(3) |
| C48Ab | −0.581(2) | 0.1862(16) | 0.3960(18) | 0.064(6) |
| C49a | −0.478(2) | 0.264(2) | 0.397(2) | 0.059(3) |
| H49Aa | −0.3991 | 0.2578 | 0.3870 | 0.089* |
| H49Ba | −0.4722 | 0.3313 | 0.4345 | 0.089* |
| H49Ca | −0.5421 | 0.2616 | 0.3371 | 0.089* |
| C49Ab | −0.474(4) | 0.257(5) | 0.408(5) | 0.059(3) |
| H49Db | −0.4311 | 0.2789 | 0.4733 | 0.089* |
| H49Eb | −0.4972 | 0.3193 | 0.3784 | 0.089* |
| H49Fb | −0.4199 | 0.2233 | 0.3796 | 0.089* |
| F1 | 0.5053(3) | 0.4827(3) | 0.7045(3) | 0.0878(14) |
| F2 | 0.6804(3) | 0.4135(2) | 0.7527(2) | 0.0635(10) |
| F3 | 0.5736(3) | 0.3913(2) | 0.6026(2) | 0.0543(8) |
| F4 | 0.6735(3) | 0.5536(2) | 0.67552(19) | 0.0501(8) |
aOccupancy: 0.683(11); bOccupancy: 0.317(11).
Source of materials
Blue-green crystals of [Cu(II)2(FcCOO)2(phen)2(CH3O)]BF4⋅ CH3CN were prepared by treating ferrocenecarboxylic acid (FcCOOH) with Cu(CH3CN)4BF4 in methanol in molar ratio 1:1. To the resulting solution, equivalent amounts of triethylamine and 1,10-phenantroline (phen) was added. The brown suspension was sealed in a vial that was heated and kept at 343 K for 20 h. Then the solution was cooled to room temperature and filtered, slow evaporation afforded blue-green block crystals of title complex.
Experimental details
All hydrogen atoms were positioned geometrically and allowed to ride on their parent atoms. The acetonitrile molecule shows a positional disorder.
Comment
Heterometallic complexes have attracted great attention in catalysis [4], magnetic materials [5], sensing [6], medical usage [7], etc. metalloligands are the best building blocks for the construction of heterometallic complexes [8]. Since its discovery, ferrocene has proven to be a versatile backbone for various ligands because of its rich chemistry, stability and redox properties [9]. Ferrocenecarboxylic acid is the mono-carboxylated ferrocene [10] and widely used in the modification of electrode for biosensors and electrocatalytic oxidation [11], [12], [13]. As a versatile functionalized ligand, ferrocenecarboxylate is widely used for the construction of heterometallic complexes with other metals. Among them, most of heterodimetallic complexes, such as mononuclear complexes [14], [15], [16], polymers [17], clusters [18], and MOFs [19] are reported in the last few years.
The title complex is a dinuclear copper(II) complex with two ferrocenecarboxylato ligands, two 1,10-phenanthroline ligands and one methanolato ligand. Each Cu(II) adopts square pyramid configuration by two nitrogen atoms of the phen ligand, one oxygen atom from the methanolate and the other two from each ferrocenecarboxylato ligand. The phen ligand coordinates in bidentate fashion with an average d(Cu—N) = 2.036(3) Å [range from 2.026(3) Å to 2.043(3) Å]. Each ferrocenecarboxylato ligand bridges two copper ions (cf. the figure) with Cu1—O2 = 2.233(3) Å, Cu1—O3 = 1.951(3) Å, Cu2—O1 = 2.095(3) Å, Cu2—O4 = 1.966(3) Å, respectively. The methanolato ligand bridges two copper ions with Cu1—O5 = 1.926(3) Å, Cu2—O5 = 1.926(3) Å, respectively. Those distances are in a reasonable range [20].
Acknowledgement
We acknowledge support for the publication fee by the National Natural Science Foundation of China (grant no. 21601097 and 21571143), the Research Starting Funds for Imported Talents, Ningxia University (grant no. BQD2015002) and the Doctor’s scientific research foundation of Hezhou University (grant no. HZUBS201507).
References
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Version 3.2i. Crystal Impact, Bonn, Germany (2012).Search in Google Scholar
Bruker. APEX3 and SAINT. Bruker AXS Inc., Madison, WI (2012).Search in Google Scholar
Buchwalter, P.; Rosé, J.; Braunstein, P.: Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 115 (2015) 28–126.10.1021/cr500208kSearch in Google Scholar PubMed
Piquer, L. R.; Sanudo, E. C.: Heterometallic 3d-4f single-molecule magnets. Dalton Trans. 44 (2015) 8771–8780.10.1039/C5DT00549CSearch in Google Scholar PubMed
Ma, D. L.; Ma, V. P. Y.; Chan, D. S. H.; Leung, K. H.; He, H. Z.; Leung, H.: Recent advances in luminescent heavy metal complexes for sensing. Coord. Chem. Rev. 256 (2012) 3087–3113.10.1016/j.ccr.2012.07.005Search in Google Scholar
Livramento, J. B.; Toth, E.; Sour, A.; Borel, A.; Merbach, A. E.; Ruloff, R.: High relaxivity confined to a small molecular space: a metallostar-based, potential MRI contrast agent. Angew. Chem., Int. Ed. 44 (2005) 1480–1484.10.1002/anie.200461875Search in Google Scholar PubMed
Srivastava, S.; Gupta, R.: Metalloligands to material: design strategies and network topologies. CrystEngComm 18 (2016) 9185–9208.10.1039/C6CE01869FSearch in Google Scholar
Astruc, D.: Why is ferrocene so exceptional? Euro. J. Inorg. Chem. 2017 (2017) 6–29.10.1002/ejic.201600983Search in Google Scholar
Cotton, F. A.; Reid, A. H. J.: Solid-state structure of ferrocenecarboxylic acid, [Fe(C5H4CO2H)(C5H5)]. Acta Crystallogr. C41 (1985) 686–688.10.1107/S010827018500511XSearch in Google Scholar
Ndamanisha, J. C.; Guo, L.: Electrochemical determination of uric acid at ordered mesoporous carbon functionalized with ferrocenecarboxylic acid-modified electrode. Biosens. Bioelectron. 23 (2008) 1680–1685.10.1016/j.bios.2008.01.026Search in Google Scholar PubMed
Raoof, J. B.; Ojani, R.; Kolbadinezhad, M.: Electrocatalytic characteristics of ferrocenecarboxylic acid modified carbon paste electrode in the oxidation and determination of L-cysteine. Electroanalysis 17 (2005) 2043–2051.10.1002/elan.200403332Search in Google Scholar
Ojani, R.; Raoof, J. B.; Alinezhad, A.: Catalytic oxidation of sulfite by ferrocenemonocarboxylic acid at the glassy carbon electrode. Application to the catalytic determination of sulfite in real sample. Electroanalysis 14 (2002) 1197–1202.10.1002/1521-4109(200209)14:17<1197::AID-ELAN1197>3.0.CO;2-ASearch in Google Scholar
Jankolovits, J.; Kampf, J. W.; Maldonado, S.; Pecoraro V. L.: Voltammetric characterization of redox-inactive guest binding to LnIII[15-metallacrown-5] hosts based on competition with a redox probe. Chem. Eur. J. 16 (2010) 6786–6796.10.1002/chem.200903015Search in Google Scholar
Costa, R.; Lopez, C.; Molins, E.; Espinosa, E.; Perez, J.: Heterodimetallic copper(II) compounds containing ferrocenecarboxylato(-1) and triamines as ligands. Dalton Trans. 19 (2001) 2833–2837.10.1039/b102030gSearch in Google Scholar
Collison, D.; Mabbs, F. E.; Turner, S. S.; Powell, A. K.; Mclnnes, E. J. L.; Yellowlees, L. J.: Synthesis, characterization and crystal structure of [(Hdmpz){HB(dmpz)3}VO(μ-η5-C5H4CO2)Fe(η5-C5H5)] (Hdmpz = 3,5-dimethylpyrazole). Dalton Trans. 7 (1997) 1201–1204.10.1039/a606707gSearch in Google Scholar
Li, G.; Li, Z. F.; Hou, H. W.; Meng, X. R.; Fan, Y. T.; Chen, W. H.: A novel helical chain zinc(II) coordination polymer derived from both ferrocenecarboxylato and bibenzimidazolyl ligands: synthesis, crystal structure and properties. J. Mol. Struct. 694 (2004) 179–183.10.1016/j.molstruc.2004.03.020Search in Google Scholar
Prokopuk, N.; Shriver, D. F.: A one-dimensional array of clusters: Na2Mo6Cl8(O2CC5H4FeCp)6⋅CH3OH. Inorg. Chem. 36 (1997) 5609–5613.10.1021/ic970816+Search in Google Scholar
Hou, H. W.; Li, L. K.; Li, G.; Fan, Y. T.; Zhu, Y.: Self-assembly of a series of novel metal-organic compounds containing ferrocenecarboxylate components. Inorg. Chem. 42 (2003) 3501–3508.10.1021/ic034031hSearch in Google Scholar
Koman, M.; Melník, M.; Glowiak, T.: Crystal and molecular structure of bis{2,6-bis(hydroxymethyl)pyridine-O,O,N}{μ-bis(2-hydroxymethylpyridyl)methanolate-O,N}dicopper(II)di(propionate). First example of non-coordinate propionate anoins. Cryst. Res. Technol. 37 (2002) 119–124.10.1002/1521-4079(200202)37:1<119::AID-CRAT119>3.0.CO;2-ESearch in Google Scholar
©2017 Kuanguan Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

